Abstract
Few studies have prospectively examined lipid changes across the menopause transition or in relation to menopausal changes in endogenous hormones. The relative independent contributions of menopause and age to lipid changes are unclear. Lipid changes were examined in relation to changes in menopausal status and in levels of estradiol and follicle-stimulating hormone in 2,659 women followed in the Study of Women's Health Across the Nation (1995–2004). Baseline age was 42–52 years, and all were initially pre- or perimenopausal. Women were followed annually for up to 7 years (average, 3.9 years). Lipid changes occurred primarily during the later phases of menopause, with menopause-related changes similar in magnitude to changes attributable to aging. Total cholesterol, low density lipoprotein cholesterol, triglycerides, and lipoprotein(a) peaked during late peri- and early postmenopause, while changes in the early stages of menopause were minimal. The relative odds of low density lipoprotein cholesterol (≥130 mg/dL) for early postmenopausal, compared with premenopausal, women were 2.1 (95% confidence interval: 1.5, 2.9). High density lipoprotein cholesterol also peaked in late peri- and early postmenopause. Results for estradiol and follicle-stimulating hormone confirmed the results based on status defined by bleeding patterns. Increases in lipids were smallest in women who were heaviest at baseline.
Keywords: body weight, lipids, menopause
Numerous studies have shown associations between postmenopausal status and elevated levels of total cholesterol and low density lipoprotein cholesterol (1–5). In contrast, evidence regarding the relation between menopause and either high density lipoprotein cholesterol or triglycerides is inconsistent (1, 2–4, 6–10). Although lipoprotein(a) is an established coronary risk factor (11, 12), the association of the menopause transition with lipoprotein(a) is unclear (13–15). Furthermore, because both menopause and lipids are highly correlated with age, it remains unclear whether menopausal lipid changes are independent of age effects (2, 6, 7, 10, 16).
Few studies have examined whether demographic variables and measures of health status and behaviors modulate menopausal changes in lipids. Obesity has been associated with adverse lipid profiles (17), and body mass index has been related to endogenous estradiol and follicle-stimulating hormone levels during menopause (18). Smoking is associated with decreased high density lipoprotein cholesterol (19) and earlier menopause (20). Physical activity has a favorable effect on lipids (19), and maintenance of activity during menopause may prevent or attenuate weight gain (21). Whether these factors affect lipid changes during menopause is potentially important for identifying women at risk for adverse postmenopausal lipid profiles.
The Study of Women's Health Across the Nation (SWAN) provided the opportunity to examine longitudinal changes in serum lipids during the stages of the menopause transition in a multiethnic cohort. The goals of the present analysis were 1) to examine changes in serum lipids related to the stages of the menopause transition as defined by menstrual patterns, 2) to confirm these status-related changes by examining lipid changes in relation to changes in follicle-stimulating hormone and estradiol, 3) to examine the magnitude of these effects in relation to the magnitude of lipid changes attributable solely to age, and 4) to assess whether lipid changes during menopause differ by levels of demographic factors and indicators of health status and behaviors.
MATERIALS AND METHODS
Analyses include data from the baseline and annual evaluations of SWAN, a longitudinal study of the menopause transition (22). SWAN is conducted at 7 clinical sites: Boston, Massachusetts, Chicago, Illinois, Detroit, Michigan, Los Angeles, California, Newark, New Jersey, Oakland, California, and Pittsburgh, Pennsylvania. Each site recruited at least 430 participants, including non-Hispanic Caucasian women and women from 1 other designated minority group (African American, Chinese, Hispanic, or Japanese). (New Jersey data were truncated at visit 5, because of incomplete subsequent follow-up). During 1995–1997, 16,065 women were screened for eligibility, and 3,302 were enrolled. Eligible women were required to be aged 42–52 years at screening, to have an intact uterus and at least 1 intact ovary, to not be pregnant or breastfeeding, to have not used exogenous hormones affecting ovarian or pituitary function within 3 months, and to have reported menstrual bleeding within the past 3 months.
Analyses excluded 222 women with any type of surgical menopause, 84 with prevalent heart disease or stroke at baseline, 15 using lipid-lowering medications at all visits, and 322 missing outcome or covariate data. Analyses included 2,659 women with up to 7 observations (average, 3.9). At visit 7, 1,058 women provided data, 537 were omitted because of prior initiation of hormone therapy with no subsequent untreated observations, and 1,064 were omitted because of missing data or loss to follow-up. At visit 7, the distribution of menopausal status in the analytical sample was similar to that in all participants (omitting hormone therapy users and surgically menopausal women).
At each visit, data for women reporting hormone therapy use in the previous year were omitted from analyses. For subsequent visits, women were included if use had stopped at least 6 months prior. Reflecting in part the exclusion of women with prevalent cardiovascular disease, at baseline the excluded women were more likely to be African American or Hispanic, smokers, diabetic, treated hypertensives, less educated, heavier, and less physically active.
All sites utilized common protocols, training, and standardization procedures. Informed consent was obtained following procedures approved by each institution's human subjects review board.
Menopausal status was based on responses to questions regarding menstrual irregularity and amenorrhea at each visit. Premenopause was defined as the presence of menses within the past 3 months, with no decrease in cycle predictability. Early perimenopause was defined as the presence of menses within the past 3 months that had become less predictable, and late perimenopause was defined as 3–11 months of amenorrhea (23–25). Twelve consecutive months of amenorrhea with no other cause were defined as postmenopause (25). On the basis of data indicating that reproductive hormones continue to fluctuate for 2 years after the final menstrual period (26), this category was divided into early (≤24 months of the final menstrual period) and late (>24 months after the final menstrual period). Menstrual patterns provide an easily measured marker of menopausal status in epidemiologic studies. We were able to confirm the results obtained using menstrual data by alternatively using levels of estradiol and follicle-stimulating hormone to represent menopausal status. Estradiol, the main premenopausal estrogen source, decreases during the menopause transition (26), while follicle-stimulating hormone increases and is a commonly used clinical marker of menopausal status (27, 28).
Age, smoking, medical history, and medication use were ascertained by self-report annually. Because few women changed smoking status, analyses used baseline smoking. Weight, height, and body mass index (weight (kg)/height (m)2) were ascertained annually.
At baseline and each follow-up, phlebotomy was performed in the morning after an overnight fast. Venipuncture was scheduled on days 2–5 (days 2–7 from January 1, 1996, through May 30, 1996) of a spontaneous menstrual cycle within 60 days of recruitment. Two attempts were made to obtain this sample from the early follicular phase. If this timed sample could not be obtained, or in noncycling women, a random fasting sample was taken within 90 days of recruitment (baseline) or within 90 days of the anniversary of recruitment (follow-up visits). Timed samples were obtained for 84% and 72% of pre- and perimenopausal observations, respectively.
Hormone assays were performed on an ACS-180 automated analyzer (Bayer Diagnostics Corporation, Tarrytown, New York) utilizing a double-antibody chemiluminescent immunoassay with a solid-phase anti-immunoglobulin G immunoglobulin conjugated to paramagnetic particles, antiligand antibody, and competitive ligand labeled with dimethylacridinium ester. The follicle-stimulating hormone assay is a modification of a manual assay kit (Bayer Diagnostics) utilizing 2 monoclonal antibodies directed to different regions on the beta subunit, with a lower limit of detection of 1.05 mIU/mL. The estradiol assay modifies the rabbit ACS-180 (anti-E2-6) immunoassay to increase sensitivity, with a lower limit of detection of 1.0 pg/mL. Duplicate estradiol assays were conducted with results reported as the arithmetic mean for each subject (coefficient of variation, 3%–12%). All other assays were single determinations.
Lipid analyses were performed at baseline and at the first and third through seventh follow-ups. Lipid assays at the second visit or beyond visit 7 were not funded in the current project. Lipid, lipoprotein, and apolipoprotein fractions were analyzed on ethylenediaminetetraacetic acid-treated plasma (29, 30). Plasma was frozen at −20°C and sent on dry ice to the Medical Research Laboratories (Highland Heights, Kentucky). Total cholesterol and triglycerides were analyzed by enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Indianapolis, Indiana) (29). High density lipoprotein cholesterol was isolated by using heparin-2m manganese chloride (30). Low density lipoprotein cholesterol was estimated (31) and set to missing when triglycerides exceeded 400 mg/dL. Low density lipoprotein cholesterol and triglycerides were set to missing when nonfasting samples were obtained (<2% of observations). The laboratory remained certified by the National Heart, Lung, and Blood Institute, Centers for Disease Control and Prevention, standardization program (32).
The percentages of calories from fat and alcohol consumption were measured at baseline and the fifth follow-up by an interviewer-administered modification of the 1995 Block Food Frequency Questionnaire (33). Physical activity was assessed at baseline and at the third, fifth, and sixth follow-ups by using 19 questions adapted from the Kaiser Physical Activity Survey based on the Baecke physical activity questionnaire (34, 35). This self-administered questionnaire ascertains physical activity in specific domains, including sports/exercise, household/care giving, and daily routine, by use of Likert responses. Responses in each domain are averaged, and an overall composite score is computed as the sum of domain-specific indices (21).
With regard to statistical analyses, associations of lipids with menopausal status were estimated by random effects linear regression (36). Analyses were repeated, substituting quartiles of estradiol or follicle-stimulating hormone to represent ovarian status. All analyses were adjusted for the following: ethnicity, study site, education, baseline values of age, smoking (never, past, current), height, and weight; change since baseline in age and weight; and concurrent diabetes, medications for hypertension or lipids, physical activity, alcohol consumption, and percentage of total calories from fat. Models including estradiol and follicle-stimulating hormone were also adjusted for day of cycle (days 2–5 vs. other), but not status, given its high correlation with these hormones. Analyses included height and weight, because changes in body mass index are due primarily to weight changes (21). Analyses using body mass index (not presented) were similar. Age and weight were each separated into the baseline value and change since baseline, to distinguish cross-sectional and longitudinal associations (36). Similarly, we included both baseline menopausal status or hormone level and concurrent menopausal status or hormone level.
To compare age-related lipid changes with menopausal status-related changes, we examined within-woman changes corresponding to 4.84 years of aging (mean within-woman age change during the pre- to late-perimenopause transition) along with changes during the transition from pre- to late perimenopause. Similar comparisons were done for the mean within-woman changes in estradiol or follicle-stimulating hormone corresponding to the transition from pre- to late perimenopause. The mean within-woman age and hormone changes were estimated from random effects linear regression of either age or hormones on menopausal status. This transition was selected because lipids peaked during late perimenopause.
The interactions of all 11 baseline covariates with menopausal status and hormones were tested by using a significance level of 0.05/11 = 0.0045 and improvement in the goodness-of-fit Akaike Information Criterion statistic (37) to assess whether associations differed across subgroups. Backward elimination was used to eliminate nonpredictive interaction terms. Only interactions occurring consistently across lipid outcomes were reported.
To present lipid changes in relation to national screening guidelines (17), we estimated the relative odds of low density lipoprotein cholesterol (≥130 mg/dL) for each menopausal status relative to premenopause using random effects logistic regression (36), adjusting for the covariates indicated above and testing for interactions.
Weight, estradiol, follicle-stimulating hormone, and triglycerides were log transformed to handle right-skewness. For presentation, the adjusted means for log-triglycerides were back-transformed to the original scale; standard errors on the original scale were obtained by using bootstrapping (38). Analyses were repeated, excluding observations concurrent with diabetes and lipid-lowering medication and with adjustment for fasting glucose, with similar results (data not shown). Analyses used SAS, version 9.1, software (39).
RESULTS
Analyses included 10,387 observations from 2,659 women: 2,354 during premenopause, 5,367 during early perimenopause, 865 during late perimenopause, 818 during early postmenopause, and 983 during late postmenopause. At baseline, by design, the sample was approximately half pre- and half early perimenopausal (Table 1). Enrolled women had higher education, socioeconomic status, and less current smoking versus those screened and not enrolled (22).
Table 1.
Characteristics of the Analytical Sample at Baseline (N = 2,747), Study of Women's Health Across the Nation Study Population, 1995–1997
| % | No. | Median (Interquartile Range) | |
| Menopausal status | |||
| Premenopausal | 54.7 | 1,502 | |
| Early perimenopausal | 45.3 | 1,245 | |
| Estradiol, pg/mL | 55.6 (55.2) | ||
| Follicle-stimulating hormone, mIU/mL | 15.9 (15.5) | ||
| Age, years | 46.3 (4.2) | ||
| Ethnicity | |||
| African American | 26.0 | 714 | |
| Caucasian | 48.5 | 1,333 | |
| Chinese | 8.5 | 233 | |
| Hispanic | 7.6 | 209 | |
| Japanese | 9.4 | 258 | |
| Educational level | |||
| Less than or equal to high school diploma or equivalent | 24.5 | 673 | |
| Some college | 31.3 | 859 | |
| College degree | 20.2 | 555 | |
| Greater than college | 24.0 | 660 | |
| Smoking status | |||
| Never | 57.8 | 1,587 | |
| Past | 26.0 | 713 | |
| Current | 16.3 | 447 | |
| Weight, kg | 69.6 (24.8) | ||
| Body mass index, kg/m2 | 26.2 (9.0) | ||
| Underweight (<19) | 3.1 | 84 | |
| Normal (19–24.9) | 39.0 | 1,072 | |
| Overweight (25–29.9) | 27.0 | 741 | |
| Obese (≥30) | 30.9 | 850 | |
| Physical activity indices (range, 1–5) | |||
| Sports/exercise | 2.5 (1.8) | ||
| Household/care giving | 2.6 (1.2) | ||
| Daily routine | 2.5 (1.0) | ||
| Alcohol consumption | |||
| Any | 50.5 | 1,386 | |
| Nonzero amount, kcal | 37.2 (95.7) | ||
| Calories from fat, % | 32.9 (10.0) | ||
| Diabetic | 5.6 | 148 | |
| Antihypertensive medication use | 10.2 | 280 | |
| Lipid-lowering medication use | 0.3 | 8 |
Table 2 presents adjusted mean lipids by concurrent menopausal status, which was significantly associated with all lipids. Total cholesterol, low density lipoprotein cholesterol, lipoprotein(a), and triglycerides peaked during late peri- and early postmenopause, with little difference between pre- and early perimenopause. High density lipoprotein cholesterol also peaked during late perimenopause.
Table 2.
Adjusted Mean Lipids by Concurrent Menopausal Status, Study of Women's Health Across the Nation, 1995–2004
| Lipid | Adjusted Mean (SE) |
No. of Observations | No. of Women | Overall P Value for Differences by Status | ||||
| Premenopausal | Early Perimenopausal | Late Perimenopausal | Early Postmenopausal | Late Postmenopausal | ||||
| Total cholesterol, mg/dL | 196.7 (0.9) | 197.4 (0.7) | 205.5 (1.0) | 206.3 (1.0) | 205.2 (1.1) | 10,885 | 2,747 | <0.0001 |
| Low density lipoprotein cholesterol, mg/dL | 116.3 (0.8) | 115.5 (0.6) | 121.7 (0.8) | 123.4 (0.9) | 123.1 (1.0) | 10,741 | 2,734 | <0.0001 |
| High density lipoprotein cholesterol, mg/dL | 57.7 (0.3) | 58.4 (0.3) | 59.6 (0.3) | 58.7 (0.4) | 57.7 (0.4) | 10,882 | 2,747 | <0.0001 |
| Lipoprotein(a), mg/dL | 30.3 (0.7) | 30.1 (0.6) | 32.1 (0.8) | 30.9 (0.8) | 30.3 (0.8) | 10,587 | 2,747 | 0.0032 |
| Triglycerides, mg/dL | 100.2 (1.2) | 103.3 (1.0) | 105.8 (1.4) | 106.4 (1.6) | 106.4 (1.7) | 10,858 | 2,746 | 0.0014 |
Abbreviation: SE, standard error.
Corresponding results for concurrent estradiol categories are presented in Table 3. Consistent with results for menopausal status, observations in the highest estradiol quartile had the lowest levels of total cholesterol, low density lipoprotein cholesterol, and triglycerides, although the association with triglycerides was only marginally statistically significant. Contrary to findings for menopausal status, high density lipoprotein cholesterol was highest on average in the highest estradiol quartile. Estradiol was not significantly related to lipoprotein(a).
Table 3.
Adjusted Mean Lipids by Concurrent Estradiol Quartile, Study of Women's Health Across the Nation, 1995–2004
| Lipid | Adjusted Mean (SE) |
No. of Observations | No. of Women | P Value for Differences by Estradiol Quartile | |||
| First Quartile (<21.45 pg/mL) | Second Quartile (21.45–39.30 pg/mL) | Third Quartile (39.31–78.62 pg/mL) | Fourth Quartile (>78.62 pg/mL) | ||||
| Total cholesterol, mg/dL | 202.0 (0.7) | 198.9 (0.7) | 199.7 (0.7) | 196.2 (0.7) | 10,812 | 2,743 | <0.0001 |
| Low density lipoprotein cholesterol, mg/dL | 120.5 (0.7) | 117.5 (0.6) | 117.6 (0.7) | 113.9 (0.7) | 10,668 | 2,730 | <0.0001 |
| High density lipoprotein cholesterol, mg/dL | 57.9 (0.3) | 57.9 (0.3) | 58.2 (0.3) | 59.0 (0.3) | 10,809 | 2,743 | <0.0001 |
| Lipoprotein(a), mg/dL | 30.4 (0.7) | 30.5 (0.7) | 30.4 (0.7) | 30.3 (0.7) | 10,516 | 2,733 | 0.9495 |
| Triglycerides, mg/dL | 104.3 (1.1) | 103.1 (1.0) | 103.6 (1.2) | 102.0 (1.1) | 10,785 | 2,742 | 0.0550 |
Abbreviation: SE, standard error.
The adjusted associations of total cholesterol, low density lipoprotein cholesterol, and triglycerides with concurrent follicle-stimulating hormone (Table 4) were also in agreement with those for status. As would be expected, given the inverse relation of estradiol and follicle-stimulating hormone, the direction of associations reversed. In contrast, high density lipoprotein cholesterol was highest in the highest follicle-stimulating hormone quartile, and lipoprotein(a) was significantly positively associated with concurrent follicle-stimulating hormone.
Table 4.
Adjusted Mean Lipids by Concurrent Follicle-stimulating Hormone Quartile, Study of Women's Health Across the Nation, 1995–2004
| Lipid | Adjusted Mean (SE) |
No. of Observations | No. of Women | P Value for Differences by Follicle-stimulating Hormone Quartile | |||
| First Quartile (<13.2 mIU/mL) | Second Quartile (13.2–24.9 mIU/mL) | Third Quartile (25.0–62.2 mIU/mL) | Fourth Quartile (≥62.3 mIU/mL) | ||||
| Total cholesterol, mg/dL | 194.8 (0.8) | 196.2 (0.7) | 197.8 (0.7) | 207.7 (0.8) | 10,822 | 2,743 | <0.0001 |
| Low density lipoprotein cholesterol, mg/dL | 113.4 (0.7) | 115.0 (0.7) | 116.1 (0.7) | 124.7 (0.7) | 10,678 | 2,730 | <0.0001 |
| High density lipoprotein cholesterol, mg/dL | 58.0 (0.3) | 57.9 (0.3) | 58.2 (0.3) | 58.9 (0.3) | 10,819 | 2,743 | 0.0056 |
| Lipoprotein(a), mg/dL | 30.3 (0.7) | 30.2 (0.7) | 29.6 (0.7) | 31.5 (0.7) | 10,525 | 2,733 | 0.0003 |
| Triglycerides, mg/dL | 102.1 (1.2) | 102.3 (1.1) | 102.7 (1.1) | 106.0 (1.3) | 10,795 | 2,742 | 0.0041 |
Abbreviation: SE, standard error.
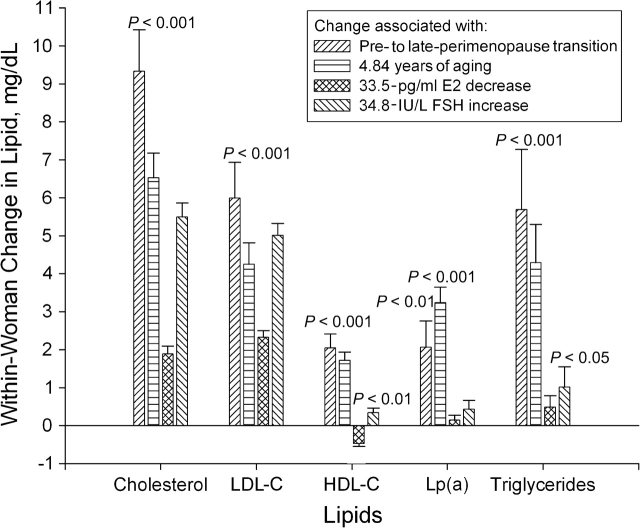
Figure 1 presents the adjusted mean within-woman changes in lipids associated with the transition from pre- to late perimenopause, as well as the changes in lipids attributable to 4.84 years of chronologic aging (the mean years elapsed during the pre- to late-perimenopause transition), the changes in lipids attributable to a 33.5-pg/mL decrease in estradiol (the mean decline during the pre- to late-perimenopause transition), and the changes in lipids attributable to a 34.8-IU/L increase in follicle-stimulating hormone (the mean increase during the pre- to late-perimenopause transition). With the exception of within-woman changes in lipoprotein(a) associated with changes in estradiol and follicle-stimulating hormone and in triglycerides associated with decline in estradiol, all within-woman changes in lipids were statistically significant.
Figure 1.
Adjusted within-woman change in lipids attributable to changes in menopausal status, age, estradiol, and follicle-stimulating hormone during the transition from pre- to late perimenopause, Study of Women's Health Across the Nation, 1995–2004. LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; Lp(a), lipoprotein(a); E2, estradiol; FSH, follicle-stimulating hormone. The statistical significance of within-woman changes is shown. Bonferroni correction for multiple comparisons was applied.
Comparing lipid changes associated with the pre- to late-perimenopause transition and changes associated with aging, status transition-related changes were not statistically significantly different from status-adjusted aging-related changes for any of the lipids. For example, after adjustment for other predictors, an average woman's cholesterol level rose by 9.33 mg/dL during the pre- to late-perimenopause transition, compared with a mean increase of 6.52 mg/dL with 4.84 years of aging (Pdifference = 0.07).
Estradiol-related changes were smaller in magnitude than aging-related changes (adjusted for estradiol) for all lipids (total cholesterol, 1.88 vs. 6.40 mg/dL, P < 0.0001; low density lipoprotein cholesterol, 2.32 vs. 3.91 mg/dL, P = 0.004; high density lipoprotein cholesterol, −0.47 vs. 1.69 mg/dL, P < 0.0001; lipoprotein(a), 0.14 vs. 2.85 mg/dL, P < 0.0001; triglycerides, 0.49 vs. 4.97 mg/dL, P < 0.0001). Follicle-stimulating hormone-related changes were larger than age-related changes (adjusted for follicle-stimulating hormone) for low density lipoprotein cholesterol (5.01 vs. 2.66 mg/dL, P = 0.0005) and triglycerides (1.01 vs. 4.67 mg/dL, P = 0.0008), smaller in magnitude for high density lipoprotein cholesterol (0.34 vs. 1.18 mg/dL, P = 0.001) and for lipoprotein(a) (0.43 vs. 2.70 mg/dL, P < 0.0001), and similar for total cholesterol (5.50 vs. 4.61 mg/dL, P = 0.25).
The most consistently significant interaction of a covariate with either menopausal status, estradiol, or follicle-stimulating hormone was for baseline weight. For total cholesterol, low density lipoprotein cholesterol, and triglycerides, the change in lipid during the transition from early to late perimenopause was blunted in the second weight tertile compared with the first; this effect was even more pronounced in the third weight tertile (Figure 2). Similar but smaller interactions with baseline weight were seen for estradiol in predicting total cholesterol and low density lipoprotein cholesterol, as well as for follicle-stimulating hormone in predicting total cholesterol, low density lipoprotein cholesterol, and triglycerides.
Figure 2.
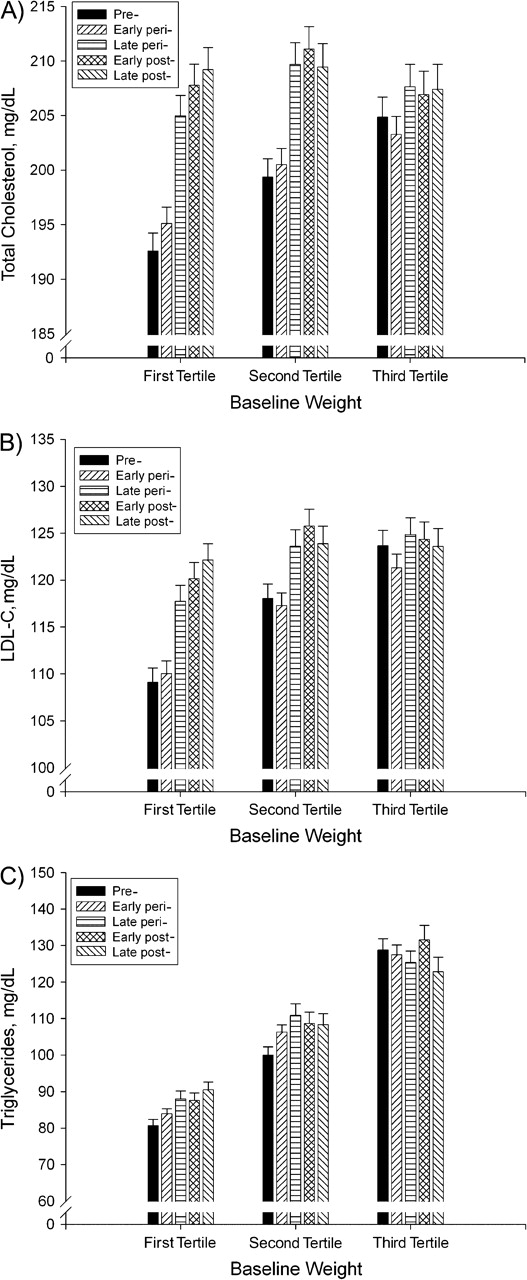
Adjusted mean (standard error) of total cholesterol (A), low density lipoprotein cholesterol (LDL-C) (B), and triglycerides (C), by menopausal status stratified on baseline weight tertile, Study of Women's Health Across the Nation, 1995–2004. Weight tertiles: first (37.6–62.4 kg); second (62.5–78.0 kg); third (78.1–175.4 kg). P values for differences by menopausal status within specific weight tertiles: in A, P < 0.0001 for tertiles 1 and 2 and P < 0.01 for tertile 3; in B, P < 0.0001 for tertiles 1 and 2 and P < 0.01 for tertile 3; in C, P < 0.0001 for tertiles 1 and 2 and P = not significant for tertile 3. For A, B, and C, the interaction of baseline weight tertile and menopausal status was P < 0.0001.
Significant interactions were also seen for ethnicity with lipoprotein(a), both with menopausal status and with follicle-stimulating hormone. Menopause transition-related and follicle-stimulating hormone-related within-woman changes were largest for African Americans and were not statistically significant for Chinese, Hispanics, or Japanese. The interaction between menopausal status and ethnicity also was statistically significant for high density lipoprotein cholesterol, with the largest menopause transition-related changes for Caucasians. None of the other factors tested exhibited consistent interactions that contributed to the fit of the models.
The relative odds of elevated low density lipoprotein cholesterol (≥130 mg/dL) for early peri-, late peri-, early post-, and late postmenopause relative to premenopause were 0.98 (95% confidence interval (CI): 0.81, 1.19), 1.50 (95% CI: 1.12, 2.01), 2.07 (95% CI: 1.51, 2.85), and 1.80 (95% CI: 1.28, 2.54), respectively (Figure 3). The peak in the relative odds for elevated low density lipoprotein cholesterol in postmenopause mirrors the peak in continuous low density lipoprotein cholesterol. The figure also displays the relative odds stratified on baseline weight tertile. Status-related increases in risk of elevated low density lipoprotein cholesterol are greatest for women in the lowest weight tertile and decline with increasing weight tertile (Pinteraction = 0.0009).
Figure 3.
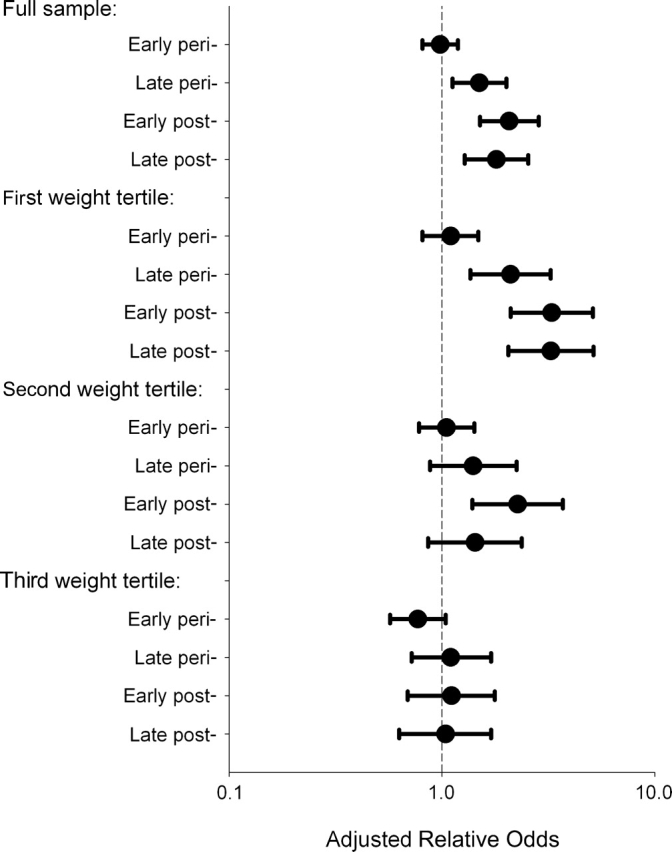
Estimated relative odds of low density lipoprotein cholesterol (≥130 mg/dL) for peri- and postmenopausal compared with premenopausal women, full sample and stratified by baseline weight tertile, Study of Women's Health Across the Nation, 1995–2004. Weight tertiles: first (37.6–62.4 kg); second (62.5–78.0 kg); third (78.1–175.4 kg).
DISCUSSION
In SWAN, serum lipids increased modestly during the menopause transition, peaking during late peri- and early postmenopause. A unique strength of SWAN is that we were able to confirm the results obtained for status based on prospective bleeding patterns, with analyses using estradiol and follicle-stimulating hormone as markers of status. The similarity of results validates the use of prospectively collected self-report of amenorrhea and menstrual irregularity to define menopausal status. In addition, we were able to examine the impact of natural menopause relative to aging effects. The impact of menopause versus age among women with early oophorectomy would also be of interest. Because such women were not included in SWAN, this question could not be addressed here.
Both low density lipoprotein cholesterol and total cholesterol peaked in late peri- and early postmenopause. This is consistent with results from studies suggesting that increases in total cholesterol and low density lipoprotein cholesterol are gradual, occurring late in the menopause transition. Matthews et al. (16) reported that the transition from pre- to early perimenopause was not associated with significant changes in low density lipoprotein cholesterol, with changes occurring later in the transition. A small prospective study has shown a gradual increase in low density lipoprotein cholesterol during the menopause transition that continues during the year following menopause (40).
High density lipoprotein cholesterol increased gradually between pre- and late perimenopause, with a subsequent gradual decline to the premenopausal level. Although this is in contrast to prior studies demonstrating a gradual postmenopausal decrease in high density lipoprotein cholesterol (4, 9, 10), the observed trend is consistent with others showing a gradual increase between pre- and late perimenopause (10, 41). One small prospective study observed that high density lipoprotein cholesterol peaked and then declined between pre- and postmenopause (41). Similarly, the Melbourne Women's Midlife Health Project (10) observed a slight increase in high density lipoprotein cholesterol in the year prior to menopause, followed by a similar decrease in the year following the final menstrual period. Additional follow-up is required to determine whether, in SWAN, late postmenopausal high density lipoprotein cholesterol will return to premenopausal levels.
We observed small increases in triglycerides across the menopause transition independent of age. Although some prior studies have shown associations between menopause and increasing triglyceride levels (3, 6), others have reported either no association or have shown that increases were explained by age effects (2, 7, 10).
The influence of menopause on lipoprotein(a) has not been established (13, 14). Baseline SWAN analyses showed no association between either follicle-stimulating hormone or estradiol concentrations and lipoprotein(a) (15). The present analysis showed that, similar to total cholesterol and low density lipoprotein cholesterol, lipoprotein(a) levels peaked during late peri- and postmenopause. Lipoprotein(a) was highest in the highest quartile of follicle-stimulating hormone but was not significantly associated with estradiol. This is consistent with prior SWAN analyses showing that follicle-stimulating hormone is more highly correlated with health outcomes than is estradiol (42–44).
Few studies have compared the relative magnitude of aging effects with menopause effects on lipids. The ability of prior studies to disentangle age and menopause effects has been limited, because few have followed women through the entire menopause transition. This analysis suggests that the impact of menopause is similar in magnitude to the impact of aging. Status-related changes were not statistically different from age effects, and changes attributable to estradiol or follicle-stimulating hormone were smaller than aging effects, with the exception that follicle-stimulating hormone effects were greater than age effects for low density lipoprotein cholesterol and triglycerides.
To date, not all women in the SWAN cohort have transitioned to postmenopause. Those who transitioned were slightly older and more likely to be perimenopausal at baseline. However, they were similar to other women in terms of smoking, vasomotor symptoms, socioeconomic status, education, and waist circumference. This was true at visit 7 (the last for which lipid measurements are available), when 51.4% were naturally postmenopausal, and at the most recent (ninth) evaluation, when 68.3% were postmenopausal. Thus, this analysis should not be biased toward women who transitioned early.
To our knowledge, the observed interaction of weight with menopausal status with respect to lipids has not been previously reported. Increases in total cholesterol and low density lipoprotein cholesterol were smallest in women in the highest baseline weight tertiles. This may be due to higher circulating estrogen levels among late peri- and postmenopausal women with higher weight, consistent with prior observations in the SWAN cohort that, while body mass index was inversely associated with estradiol levels in pre- and early perimenopause, among late perimenopausal and postmenopausal women, higher body mass index was associated with increased concentrations of estradiol (18). Addition of either estradiol or follicle-stimulating hormone to our regression models reduced the magnitude of the effect modification by weight on the relation of menopausal status to change in total cholesterol or low density lipoprotein cholesterol (data not shown). Thus, although overweight and obese women are at increased risk of elevated lipid levels (17), thinner women may experience the greatest hormone-related increases in low density lipoprotein cholesterol during the menopause transition. Within the lowest weight tertile, late perimenopausal and postmenopausal women had a greater than 2-fold risk of having low density lipoprotein cholesterol classified as high by national screening guidelines relative to premenopausal women.
Inferences regarding ethnicity interactions are limited by the small sample within status-specific ethnic groups. Menopause-related changes in total cholesterol and low density lipoprotein cholesterol were similar across ethnic groups. There was an interaction with lipoprotein(a) such that African Americans experienced the greatest menopause-related increases. African-American women in SWAN also have the highest mean lipoprotein(a) prior to menopause (45), suggesting that postmenopausal levels of this risk factor may be particularly elevated. An interaction with high density lipoprotein cholesterol was also suggested, with the greatest increase among Caucasians. These observations require confirmation in other samples.
Further follow-up is required to determine whether the observed peaks in late peri- and early postmenopause will continue into late postmenopause. Results suggest the importance of continued lipid screening throughout menopause, as increases may not occur until late in the transition. Although absolute lipid changes were modest, the continuous relation between lipoproteins and cardiovascular risk has been established, and even small reductions may lead to a reduction in risk. Compared with premenopausal women, early postmenopausal women had a 2-fold risk of low density lipoprotein cholesterol above the level recommended by national guidelines.
Acknowledgments
Author affiliations: Albert Einstein College of Medicine, Bronx, New York (Carol A. Derby); University of Massachusetts Medical School, Worcester, Massachusetts (Sybil L. Crawford); Merck Research Laboratories, Rahway, New Jersey (Richard C. Pasternak); University of Michigan School of Public Health, Ann Arbor, Michigan (MaryFran Sowers); University of California at Davis and Kaiser Permanente, Oakland, California (Barbara Sternfeld); and University of Pittsburgh, Pittsburgh, Pennsylvania (Karen A. Matthews).
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the Office of Research on Women's Health (grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495).
The authors thank the study staff at each site.
Clinical Centers: University of Michigan, Ann Arbor, Michigan—MaryFran Sowers, Principal Investigator; Massachusetts General Hospital, Boston, Massachusetts—Robert Neer, Principal Investigator, 1994–1999; Joel Finkelstein, Principal Investigator, 1999–present; Rush University Medical Center, Chicago, Illinois—Lynda Powell, Principal Investigator; University of California, Davis/Kaiser, California—Ellen Gold, Principal Investigator; University of California, Los Angeles, California—Gail Greendale, Principal Investigator; University of Medicine and Dentistry of New Jersey Medical School, Newark, New Jersey—Gerson Weiss, Principal Investigator, 1994–2004; Nanette Santoro, Principal Investigator, 2004–present; and the University of Pittsburgh, Pittsburgh, Pennsylvania—Karen Matthews, Principal Investigator. NIH Program Office: National Institute on Aging, Bethesda, Maryland—Marcia Ory, 1994–2001; Sherry Sherman, 1994–present; National Institute of Nursing Research, Bethesda, Maryland—Program Officers. Central Laboratory: University of Michigan, Ann Arbor, Michigan—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: New England Research Institutes, Watertown, Massachusetts—Sonja McKinlay, Principal Investigator, 1995–2001; University of Pittsburgh, Pittsburgh, Pennsylvania—Kim Sutton-Tyrrell, Principal Investigator, 2001–present. Steering Committee: Chris Gallagher, Chair; Susan Johnson, Chair.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Nursing Research, Office of Research on Women's Health, or the National Institutes of Health.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- SWAN
Study of Women's Health Across the Nation
References
- 1.Kannel W, Hjortland MC, McNamara PM, et al. Menopause and risk of cardiovascular disease: the Framingham Study. Ann Intern Med. 1976;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 2.Davis C, Pajak A, Rywik S, et al. Natural menopause and cardiovascular disease risk factors: the Poland and US Collaborative Study on Cardiovascular Disease Epidemiology. Ann Epidemiol. 1994;4(6):445–448. doi: 10.1016/1047-2797(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 3.Lindquist O, Bengtsson C, Lapidus L. Relationships between the menopause and risk factors for ischaemic heart disease. Acta Obstet Gynecol Scand Suppl. 1985;130:43–47. doi: 10.3109/00016348509157146. [DOI] [PubMed] [Google Scholar]
- 4.Jensen J, Nilas L, Christiansen C. Influence of menopause on serum lipids and lipoproteins. Maturitas. 1990;12(4):321–331. doi: 10.1016/0378-5122(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 5.Akahoshi M, Soda M, Nakashima E, et al. Effects of menopause on trends of serum cholesterol, blood pressure, and body mass index. Circulation. 1996;94(1):61–66. doi: 10.1161/01.cir.94.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Bonithon-Kopp C, Scarabin PY, Darne B, et al. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. Int J Epidemiol. 1990;19(1):42–48. doi: 10.1093/ije/19.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Campos H, McNamara JR, Wilson PW, et al. Differences in low density lipoprotein subfractions and apolipoproteins in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 1988;67(1):30–35. doi: 10.1210/jcem-67-1-30. [DOI] [PubMed] [Google Scholar]
- 8.Bush T, Cowan L, Heiss G, et al. Ovarian function and lipid/lipoprotein levels. Results from the Lipid Research Clinics Program [abstract] Am J Epidemiol. 1984;120(3):489. [Google Scholar]
- 9.Matthews KA, Meilahn EN, Kuller LH, et al. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 10.Do KA, Green A, Guthrie JR, et al. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151(6):584–593. doi: 10.1093/oxfordjournals.aje.a010246. [DOI] [PubMed] [Google Scholar]
- 11.Dahlen GH, Guyton JR, Attar M, et al. Associations of levels of lipoprotein Lp(a), plasma lipids and other lipoproteins with coronary artery disease documented by angiography. Circulation. 1986;74(4):758–765. doi: 10.1161/01.cir.74.4.758. [DOI] [PubMed] [Google Scholar]
- 12.Hoefler G, Harnoncourt F, Paschke E, et al. Lipoprotein Lp(a). A risk factor for myocardial infarction. Arteriosclerosis. 1988;8(4):398–401. doi: 10.1161/01.atv.8.4.398. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich J, Sandkamp M, Kokott R, et al. Relationship of lipoprotein(a) to variables of coagulation and fibrinolysis in a healthy population. Clin Chem. 1991;37(11):1950–1954. [PubMed] [Google Scholar]
- 14.Jenner JL, Ordovas JM, Lamon-Fava S, et al. Effects of age, sex, and menopausal status on plasma lipoprotein(a) levels: the Framingham Offspring Study. Circulation. 1993;87(4):1135–1141. doi: 10.1161/01.cir.87.4.1135. [DOI] [PubMed] [Google Scholar]
- 15.Sowers M, Crawford SL, Cauley JA, et al. Association of lipoprotein(a), insulin resistance, and reproductive hormones in a multiethnic cohort of pre- and perimenopausal women (the SWAN Study) Am J Cardiol. 2003;92(5):533–537. doi: 10.1016/s0002-9149(03)00720-3. [DOI] [PubMed] [Google Scholar]
- 16.Matthews KA, Wing RR, Kuller LH, et al. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994;154(20):2349–2355. [PubMed] [Google Scholar]
- 17.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Randolph JF, Jr, Sowers M, Bondarenko IV, et al. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89(4):1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 19.Heiss G, Johnson NJ, Reiland S, et al. The epidemiology of plasma high-density lipoprotein cholesterol levels: the Lipid Research Clinics Program prevalence study summary. Circulation. 1980;62(suppl IV) IV116–IV136. [PubMed] [Google Scholar]
- 20.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 21.Sternfeld B, Wang H, Quesenberry CP, Jr, et al. Physical activity and changes in weight and waist circumference in midlife women: findings from the Study of Women's Health Across the Nation. Am J Epidemiol. 2004;160(9):912–922. doi: 10.1093/aje/kwh299. [DOI] [PubMed] [Google Scholar]
- 22.Sowers MF, Crawford S, Sternfeld B, et al. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. New York, NY: Academic Press; 2000. [Google Scholar]
- 23.Brambilla DJ, McKinlay SM, Johannes CB. Defining the perimenopause for application in epidemiologic investigations. Am J Epidemiol. 1994;140(12):1091–1095. doi: 10.1093/oxfordjournals.aje.a117209. [DOI] [PubMed] [Google Scholar]
- 24.Dudley EC, Hopper JL, Taffe J, et al. Using longitudinal data to define the perimenopause by menstrual cycle characteristics. Climacteric. 1998;1(1):18–25. doi: 10.3109/13697139809080677. [DOI] [PubMed] [Google Scholar]
- 25.Research on the menopause in the 1990s. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1996;866:1–107. [PubMed] [Google Scholar]
- 26.Burger HG, Dudley EC, Hopper JL, et al. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J Clin Endocrinol Metab. 1999;84(11):4025–4030. doi: 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- 27.Burger HG. Diagnostic role of follicle-stimulating hormone (FSH) measurements during the menopausal transition—an analysis of FSH, oestradiol and inhibin. Eur J Endocrinol. 1994;130(1):38–42. doi: 10.1530/eje.0.1300038. [DOI] [PubMed] [Google Scholar]
- 28.Cooper GS, Baird DD. The use of questionnaire data to classify peri- and premenopausal status. Epidemiology. 1995;6(6):625–628. doi: 10.1097/00001648-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Steiner P, Freidel J, Bremner W, et al. Standardization of micromethods for plasma cholesterol, triglyceride and HDL-cholesterol with the Lipid Clinics’ methodology [abstract] J Clin Chem Clin Biochem. 1981;19(8):850. [Google Scholar]
- 30.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin manganese precipitation procedure for estimating high-density lipoprotein cholesterol. J Lipid Res. 1978;19(1):65–76. [PubMed] [Google Scholar]
- 31.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 32.Myers GL, Cooper GR, Winn CL, et al. The Centers for Disease Control-National Heart, Lung, and Blood Institute Lipid Standardization Program: an approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9(1):105–135. [PubMed] [Google Scholar]
- 33.Huang MH, Schocken M, Block G, et al. Variation in nutrient intakes by ethnicity: results from the Study of Women's Health Across the Nation (SWAN) Menopause. 2002;9(1):309–319. doi: 10.1097/00042192-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Sternfeld B, Ainsworth BA, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 35.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 36.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, United Kingdom: Clarendon Press, Publishers; 1994. [Google Scholar]
- 37.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Contr. 1974;19(6):716–723. [Google Scholar]
- 38.Efron B. The Jackknife, the Bootstrap, and Other Resampling Plans. Philadelphia, PA: SIAM, Publishers; 1982. [Google Scholar]
- 39.SAS OnlineDoc®, Version 9.1. Cary, NC: SAS Institute, Inc, Publishers; 1999. SAS Institute, Inc. [Google Scholar]
- 40.Fukami K, Koike K, Hirota K, et al. Perimenopausal changes in serum lipids and lipoproteins: a 7-year longitudinal study. Maturitas. 1995;22(3):193–197. doi: 10.1016/0378-5122(95)00927-d. [DOI] [PubMed] [Google Scholar]
- 41.Hall G, Collins A, Csemiczky G, et al. Lipoproteins and BMI: a comparison between women during transition to menopause and regularly menstruating healthy women. Maturitas. 2002;41(3):177–185. doi: 10.1016/s0378-5122(01)00258-4. [DOI] [PubMed] [Google Scholar]
- 42.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopause transition. J Clin Endocrinol Metab. 2006;91(4):1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 43.Sowers MR, Greendale GA, Bondarenko I, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14(3):191–197. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 44.Randolph JF, Jr, Sowers M, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90(11):6106–6112. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 45.Matthews KA, Sowers MF, Derby CA, et al. Ethnic differences in cardiovascular risk factor burden among middle-aged women: Study of Women's Health Across the Nation (SWAN) Am Heart J. 2005;149(6):1066–1073. doi: 10.1016/j.ahj.2004.08.027. [DOI] [PubMed] [Google Scholar]