Abstract
Kaposi's sarcoma occurs at high incidence among Zambian adults and children, but there is a paucity of data on human herpesvirus 8 (HHV-8) incidence and routes of infection, especially in children. Between 1998 and 2004, the authors conducted a prospective study of viral transmission in a cohort of 684 children in Lusaka, Zambia, to estimate the annual incidence of HHV-8 from birth through 48 months of age. Maternal and pediatric human immunodeficiency virus type 1 (HIV-1) infection status was also determined. The results, based on 1,532 child-years of follow-up, showed that HHV-8 seroconversion occurs early in life. The incidence rate of HHV-8 seroconversion was 13.8 infections per 100 child-years by 48 months of age. HIV-1-infected children were at substantially higher risk for HHV-8 seroconversion (adjusted hazard ratio = 4.60, 95% confidence interval: 2.93, 7.22). Maternal HIV-1 and HHV-8 infection status were not independently associated with risk of HHV-8 seroconversion in the child. HHV-8 antibody titers in children followed at all consecutive time points revealed seroreversion of HHV-8 antibodies, with undetectable titers in some children at one or more time points after seroconversion. These results demonstrate that cross-sectional serologic screening probably underestimates true HHV-8 seroprevalence in young Zambian children because of fluctuations in detectable antibody titers.
Keywords: herpesvirus 8, human; HIV-1; infection; sarcoma, Kaposi; Zambia
Human herpesvirus 8 (HHV-8) is the infectious etiologic agent of all forms of Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease (1–4). The global distributions of HHV-8 seroprevalence and Kaposi's sarcoma incidence are uneven (5). HHV-8 seroprevalence is generally low in the United States and Northern Europe, but it ranges from 20 percent to 80 percent in adult populations in Africa and the Mediterranean (6–11). In a previous study, He et al. (12) demonstrated that HHV-8 seroprevalence among adolescent and childbearing women in Zambia is approximately 50 percent.
The exact routes of HHV-8 transmission are still unclear and may differ by geographic region and risk group. Horizontal transmission via heterosexual and homosexual contact has been reported in adults (13–15). Vertical transmission to children seems to occur at a very low rate; a likely source of nonsexual transmission is saliva, and the possibility of transmission through breast milk is still controversial (16–18). A report from Uganda provided evidence for HHV-8 transmission through blood transfusion (19). In Kaposi's sarcoma-endemic regions, primary HHV-8 infection has been reported to occur during early childhood, suggesting that transmission occurs early in life (20, 21). Among children, HHV-8 seroprevalence generally increases with age, which suggests that horizontal transmission may play an important role (9, 21, 22). Epidemiologic studies from sub-Saharan Africa report a seroprevalence of 20–68 percent in adolescents (22–24). Socioeconomic factors such as low parental education, low household income, and use of a communal water source are associated with HHV-8 infection in African children; in addition, maternal coinfection with HHV-8 and human immunodeficiency virus type 1 (HIV-1) may be an important risk factor (20, 22, 25–27).
In Zambia, coincident with the emergence of the HIV-1 epidemic, there was a significant increase in the incidence of Kaposi's sarcoma in adults and children (28–30). By 1992, Kaposi's sarcoma accounted for approximately 25 percent of all childhood cancers diagnosed in Lusaka, the capital of Zambia (31). Previously, Mantina et al. (18) reported infrequent detection of HHV-8 DNA in Zambian infants born to HHV-8-infected mothers, suggesting a low level of in utero transmission. However, this fails to account for the high seroprevalence levels observed in early childhood. Zambian children appear to contract HHV-8 infection early in life, but the extent of HHV-8 infection, how children acquire the virus, and whether HIV-1 infection in the child is a risk factor remain unclear.
We evaluated early childhood incidence of HHV-8 seroconversion prospectively in a longitudinal cohort study of infants followed from birth through age 48 months. Furthermore, we assessed whether maternal and pediatric HIV-1 infections were associated with higher risk of HHV-8 acquisition during early childhood in Zambia.
MATERIALS AND METHODS
Setting
Between October 1998 and April 2004, pregnant women visiting the labor ward at University Teaching Hospital in Lusaka, Zambia, were screened for HHV-8 and HIV-1 (32). Women in early stages of labor were enrolled in a prospective cohort study after being counseled and educated about the study and giving written informed consent. The study was approved by the institutional review boards of the University of Zambia, the University of Nebraska, and the University of Miami.
Study population
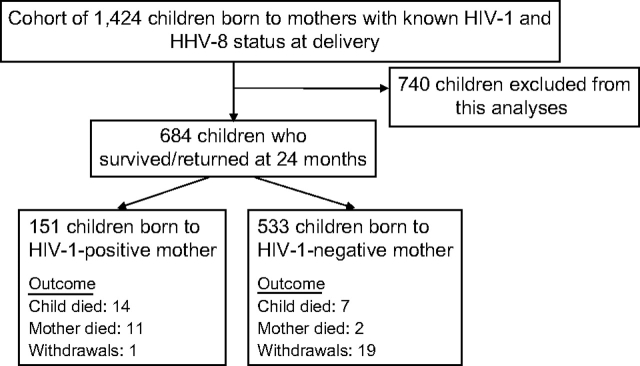
At delivery, mothers were divided into four groups based on single or dual seropositivity for HIV-1 and/or HHV-8. After delivery, mothers were encouraged to return with their children for follow-up visits. A total of 1,424 mother-infant pairs who returned for at least one postpartum visit constituted our longitudinal cohort (figure 1). This analysis included 684 children who survived and were followed for at least 24 months. Children who did not return at age 24 months (n = 740) were excluded from this analysis; reasons for exclusion included early mortality, early withdrawal, and loss to follow-up before HIV-1 serostatus could be reliably established. Children born to HIV-1-positive mothers were tested at 24 months or later to determine HIV-1 status. Here we report data collected from the 684 children who survived beyond 24 months of age and were followed prospectively for evaluation of both HHV-8 and HIV-1 seropositivity between 12 and 48 months of age. Of these 684 children, 54 percent (370/684) of the infants were born to HHV-8-seropositive mothers and 22 percent (151/684) were born to HIV-1-seropositive mothers. By 24 months of age, 6 percent (41/684) of the children tested positive for HIV-1.
FIGURE 1.
Outline of a longitudinal study of human herpesvirus 8 (HHV-8) among children in Lusaka, Zambia, 1998–2004. Of the total cohort, 740 children were excluded from the analysis because of early mortality, early withdrawal, or loss to follow-up before human immunodeficiency virus type 1 (HIV-1) serostatus could be reliably established. “Outcome” indicates the reasons for attrition between 24 and 48 months of age.
Serologic testing for HHV-8 and HIV-1
HHV-8 serology.
Blood specimens were collected annually from children at birth and 12, 24, 36, and 48 months after birth. Specimens were coded by means of a unique identification number assigned to each mother-infant pair and were analyzed without knowledge of the personal identity of the study participants. Plasma was screened for evidence of HHV-8 seroconversion. Age at HHV-8 seroconversion was defined as the age at which the first HHV-8-positive test result was obtained using the assays described. To rule out detection of transplacental maternal HHV-8 antibodies, plasma from children younger than 12 months of age was not tested. In addition, the plasma of all HHV-8-seropositive children at 12 months who were born to HHV-8-seropositive mothers was titered at birth, at 6 months, and at 12 months to rule out detection of maternal antibodies.
BC-3 monoclonal antibody-enhanced immunofluorescence assay.
Antibodies against HHV-8 were detected by monoclonal antibody-enhanced immunofluorescence assay (mIFA) as described previously (33). BC-3 cells (American Type Culture Collection, Manassas, Virginia) stimulated by tetradecanoyl phorbol acetate were fixed and permeabilized, and mIFA was carried out as described (32). To reduce subjectivity in observing specific fluorescence, slides were read independently by two laboratory workers. All plasma determined to be positive by BC-3 mIFA was confirmed using Spodoptera frugiperda clone 9 (Sf9) mIFA as described below. For determination of HHV-8 antibody titers, serial twofold dilutions of plasma were performed, and each dilution was assayed using the BC-3 mIFA. The inverse of the last dilution that tested positive was taken as the endpoint titer.
Sf9 monoclonal antibody-enhanced immunofluorescence assay.
Recombinant baculoviruses expressing the glutathione S-transferase-tagged lytic proteins ORF65 and K8.1A and the latent protein ORF73 (provided by Dr. Bala Chandran, Rosalind Franklin University of Medicine and Science, Chicago, Illinois), were used to develop an Sf9 mIFA. Baculovirus-infected Sf9 cells expressing glutathione S-transferase alone were used as a negative control to detect background and nonspecific fluorescence. All infections were initiated separately, harvested at 72 hours postinfection, and fixed using the BC-3 cell method. The Sf9 mIFA procedure was similar to the BC-3 mIFA. A sample was considered HHV-8-seropositive only if it was positive at a standard serum dilution of 1:40 for both the BC-3 mIFA and the Sf9 mIFA (with at least one antigen). The quality of the slides was monitored for every batch, and appropriate positive and negative controls were used every time mIFAs were conducted.
HIV-1 serology.
Plasma from mothers (at delivery) and from children born to HIV-1-positive mothers (at 24 months or older) was screened for HIV-1 antibodies. Human immunodeficiency virus type 2 infection has not been reported in Zambia (34, 35). Children born to HIV-1-negative mothers were assumed to be HIV-1-negative. Children younger than 24 months were not screened for HIV-1 antibodies because of the risk of detecting persisting transplacental maternal antibodies. Plasma was screened by means of a standard rapid HIV-1 kit (Capillus HIV-1/2 agglutination test kit; Trinity Biotech PLC, Bray, Ireland) and confirmed by the Abbott Determine HIV-1/2 enzyme immunoassay test kit (Abbott Laboratories, Chicago, Illinois).
Statistical and analytic methods
The crude incidence rate per 100 child-years was calculated by dividing the number of new HHV-8 seroconverters by the total number of child-years at risk and multiplying by 100. Children contributed HHV-8-free child-years at risk until they tested positive for HHV-8. Because the actual date of seroconversion within the 1-year interval is unknown, a child was considered at risk for only half of the year in which he/she tested positive. All data were right-censored at 48 months of age. We present the crude incidence rate per 100 child-years according to covariates. We also compared stratum-specific incidence rates using the crude incidence rate ratio and its 95 percent confidence interval. To evaluate the risk of HHV-8 seroconversion over time, we estimated hazard rate ratios and 95 percent confidence intervals using Cox proportional hazards modeling in which we examined various characteristics individually and simultaneously to obtain adjusted hazard rate ratios and to generate hazard curves that represented the child's risk of seroconversion for HHV-8 over time (36). All comparisons were considered statistically significant at p ≤ 0.05. Data were analyzed using the statistical software packages SAS, version 9.1 (SAS Institute, Inc., Cary, North Carolina), and SPSS, version 15 (SPSS, Inc., Chicago, Illinois).
RESULTS
HHV-8 incidence and associated risk factors
Based on 1,532 total child-years of follow-up, the incidence rate of HHV-8 seroconversion in Zambian children was 13.8 infections per 100 child-years over 48 months (table 1). We observed a statistically significant increased risk of seroconversion among HIV-1-positive children after adjusting for multiple covariates (adjusted hazard rate ratio = 4.60, 95 percent confidence interval: 2.93, 7.22). No statistically significant difference in hazard rates was observed by sex of the child or mother's HHV-8 infection status at delivery. The association between HHV-8 seroconversion in children and maternal HIV-1 seropositivity was no longer statistically significant when results were adjusted for HIV-1 seropositivity of the child. Similar results were observed in children born to HIV-1-positive, HHV-8-negative mothers in comparison with children born to HHV-8- and HIV-1-negative mothers.
TABLE 1.
Incidence of human herpesvirus 8 infection per 100 child-years and associated hazard rate ratios in a longitudinal cohort study of 684 children, by maternal and child characteristics, Lusaka, Zambia, 1998–2004
| Characteristic | No. of children | % | No. of HHV-8*-positive children | No. of HHV-8-free child-years | Incidence rate per 100 child-years | Unadjusted HRR* | 95% CI* | Adjusted HRR | 95% CI |
| Total cohort | 684 | 212 | 1,532.1 | 13.8 | |||||
| Sex of child | |||||||||
| Male | 349 | 51 | 101 | 799.3 | 12.6 | 0.84 | 0.64, 1.10 | 0.84† | 0.64, 1.10 |
| Female | 335 | 49 | 111 | 732.8 | 15.2 | 1.00‡ | 1.00‡ | ||
| Mother's HIV-1* status at delivery | |||||||||
| Uninfected | 533 | 78 | 140 | 1,194.7 | 11.7 | 1.00‡ | 1.00‡ | ||
| Infected | 151 | 22 | 72 | 337.5 | 21.3 | 1.79 | 1.35, 2.38 | 1.16† | 0.82, 1.63 |
| Mother's HHV-8 status at delivery | |||||||||
| Negative | 314 | 46 | 104 | 672.5 | 15.5 | 1.00‡ | 1.00‡ | ||
| Positive | 370 | 54 | 108 | 859.7 | 12.6 | 0.82 | 0.62, 1.07 | 0.84† | 0.64, 1.10 |
| Mother's HIV-1 and HHV-8 status§ | |||||||||
| HIV-1− and HHV-8− | 240 | 35 | 63 | 511.8 | 12.3 | 1.00‡ | 1.00‡ | ||
| HIV-1− and HHV-8+ | 293 | 43 | 77 | 682.9 | 11.3 | 0.92 | 0.66, 1.29 | 0.91¶ | 0.65, 1.26 |
| HIV-1+ and HHV-8− | 74 | 11 | 41 | 160.7 | 25.5 | 2.05 | 1.38, 3.03 | 1.29¶ | 0.83, 2.02 |
| HIV-1+ and HHV-8+ | 77 | 11 | 31 | 176.8 | 17.5 | 1.40 | 0.91, 2.15 | 0.94¶ | 0.59, 1.49 |
| Child's HIV-1 status at age 24 months | |||||||||
| Uninfected | 643 | 94 | 178 | 1,478.1 | 12.0 | 1.00‡ | 1.00‡ | ||
| Infected | 41 | 6 | 34 | 54.0 | 63.0 | 5.17 | 3.55, 7.52 | 4.60† | 2.93, 7.22 |
HHV-8, human herpesvirus 8; HRR, hazard rate ratio; CI, confidence interval; HIV-1, human immunodeficiency virus type 1.
Adjusted for sex of the child, mother's HHV-8 status, mother's HIV-1 status, and child's HIV-1 status at age 24 months.
Reference category.
A positive sign (+) indicates seropositivity and a negative sign (−) indicates seronegativity.
Adjusted for sex of the child and child's HIV-1 status at age 24 months.
Table 2 shows a constant rate of annual HHV-8 infection occurring in children, reported as the crude incidence rate per 100 child-years. We also examined these crude incidence rates by maternal and child HIV-1 coinfection status and maternal HHV-8 serostatus and observed results similar to those presented in table 1. Note that none of the HIV-1-infected children in the cohort returned for follow-up after 36 months.
TABLE 2.
Crude incidence of human herpesvirus 8 per 100 child-years and associated incidence rate ratios in a longitudinal cohort study of 684 children, by maternal and pediatric human immunodeficiency virus type 1 status and maternal human herpesvirus 8 status, Lusaka, Zambia, 1998–2004
| Characteristic and study period | No. of HHV-8*-positive children | No. of HHV-8-free child-years | Crude incidence rate per 100 child-years | No. of HHV-8-positive children | No. of HHV-8-free child-years | Crude incidence rate per 100 child-years | IRR* | 95% confidence interval |
| Total cohort | All children | |||||||
| Birth to 12 months | 92 | 638.4 | 14.4 | |||||
| Birth to 24 months | 152 | 1,086.6 | 14.0 | |||||
| Birth to 36 months | 195 | 1,393.2 | 14.0 | |||||
| Birth to 48 months | 212 | 1,532.1 | 13.8 | |||||
| Sex of child | Female | Male | IRRfemale/male | |||||
| Birth to 12 months | 51 | 309.5 | 16.5 | 41 | 329.0 | 12.5 | 1.32 | 0.88, 1.99 |
| Birth to 24 months | 78 | 521.2 | 15.0 | 74 | 565.4 | 13.1 | 1.14 | 0.83, 1.57 |
| Birth to 36 months | 101 | 668.3 | 15.1 | 94 | 724.9 | 13.0 | 1.17 | 0.88, 1.54 |
| Birth to 48 months | 111 | 732.8 | 15.2 | 101 | 799.3 | 12.6 | 1.20 | 0.92, 1.57 |
| Mother's HIV-1* status at delivery | Negative | Positive | IRRpositive/negative | |||||
| Birth to 12 months | 62 | 501.5 | 12.4 | 30 | 136.9 | 21.9 | 1.77 | 1.15, 2.74 |
| Birth to 24 months | 98 | 844.7 | 11.6 | 54 | 241.9 | 22.3 | 1.92 | 1.38, 2.68 |
| Birth to 36 months | 131 | 1,081.3 | 12.1 | 64 | 311.9 | 20.5 | 1.69 | 1.26, 2.28 |
| Birth to 48 months | 140 | 1,194.7 | 11.7 | 72 | 337.5 | 21.3 | 1.82 | 1.37, 2.42 |
| Mother's HHV-8 serostatus at delivery | Negative | Positive | IRRpositive/negative | |||||
| Birth to 12 months | 48 | 290.6 | 16.5 | 44 | 347.9 | 12.6 | 0.77 | 0.51, 1.15 |
| Birth to 24 months | 79 | 485.3 | 16.3 | 73 | 601.3 | 12.1 | 0.75 | 0.54, 1.02 |
| Birth to 36 months | 94 | 614.9 | 15.3 | 101 | 778.4 | 13.0 | 0.85 | 0.64, 1.12 |
| Birth to 48 months | 104 | 672.5 | 15.5 | 108 | 859.7 | 12.6 | 0.81 | 0.62, 1.06 |
| Child's HIV-1 status at age 24 months | Negative | Positive | IRRpositive/negative | |||||
| Birth to 12 months | 76 | 604.9 | 12.6 | 16 | 33.6 | 47.7 | 3.79 | 2.21, 6.50 |
| Birth to 24 months | 124 | 1,909.9 | 6.5 | 28 | 50.0 | 56.0 | 8.62 | 5.72, 13.00 |
| Birth to 36 months | 161 | 2,212.5 | 7.3 | 34 | 54.0 | 63.0 | 8.65 | 5.98, 12.53 |
| Birth to 48 months | 178 | 2,351.4 | 7.6 | 34 | 54.0 | 63.0 | 8.32 | 5.76, 12.00 |
HHV-8, human herpesvirus 8; IRR, incidence rate ratio; HIV-1, human immunodeficiency virus type 1.
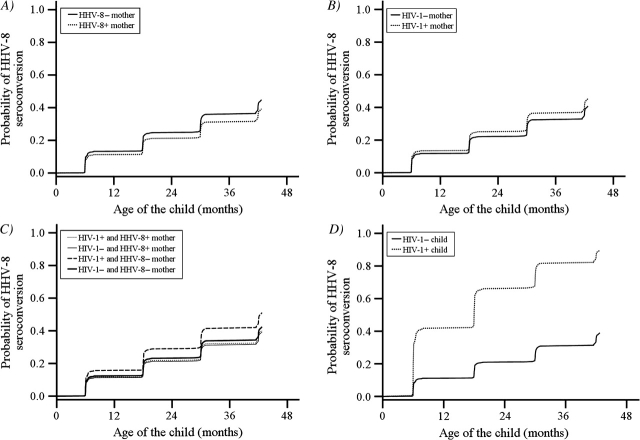
Figure 2 presents the probabilities of HHV-8 seroconversion as hazard curves, stratified by covariates and adjusted for confounders. These graphs show that there is little difference in the probability of HHV-8 seroconversion according to maternal HHV-8 status (p = 0.2), maternal HIV-1 infection (p = 0.41), or maternal HIV-1 and HHV-8 coinfection status (p = 0.38) (panels A, B, and C). The most important independent risk factor was HIV-1 infection in children, where the probability of HHV-8 seroconversion was significantly lower in the HIV-1-uninfected children than in the infected children (p < 0.001) (panel D).
FIGURE 2.
Results from survival analysis estimating the probability of becoming seropositive for human herpesvirus 8 (HHV-8) in a longitudinal cohort of 684 children followed from birth to 48 months of age, Lusaka, Zambia, 1998–2004. Results are presented by the HHV-8 serostatus of the mother at delivery (panel A); the human immunodeficiency virus type 1 (HIV-1) infection status of the mother at delivery (panel B); maternal coinfection with HIV-1 and HHV-8 at delivery (panel C); and the HIV-1 infection status of the child at age 24 months (panel D).
Figure 3 (panel A) shows the annual (12-month) percentage of children newly acquiring HHV-8 infection within each period. Because HIV-1 infection of the child was the most prominent risk factor observed for HHV-8 seroconversion, we also present annual percentages of seroconversion according to the child's HIV-1 status at 24 months (panel B). This figure shows that HHV-8 seroconversion in HIV-1-uninfected children was essentially constant during each annual period, while HHV-8 seroconversion in HIV-1-infected children was significantly higher and increased during each annual period.
FIGURE 3.
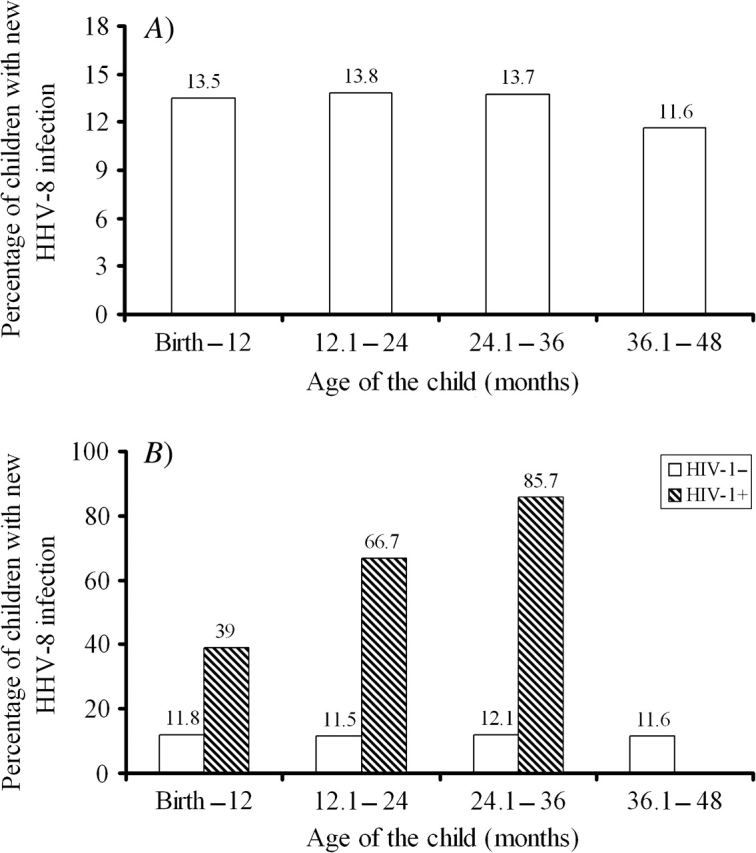
Proportions (%) of 684 children seroconverting for human herpesvirus 8 (HHV-8) during each annual follow-up period, Lusaka, Zambia, 1998–2004. Results are presented for all children followed to 48 months of age (panel A) and stratified by the human immunodeficiency virus type 1 (HIV-1) infection status of the child at age 24 months (panel B). None of the HIV-1-infected children survived or returned beyond 36 months of age.
Variations in antibody titers in HHV-8-positive children over time
The different HHV-8 seroreactivity patterns are summarized in table 3. We monitored the HHV-8 antibody responses of 171 children who returned for all four follow-up visits to study the temporal persistence of HHV-8 antibodies. Sixty percent (103/171) of these children were persistently HHV-8-seronegative and 68 of the 171 children (40 percent) seroconverted by 48 months, but only three of the 68 children remained persistently HHV-8-seropositive at all time points. We frequently observed fluctuations in antibody titers, which dropped below the detection limit at one or more time points, leading to seroreversion.
TABLE 3.
Human herpesvirus 8 seroreactivity patterns among 171 children with completion of all planned study visits at 12, 24, 36, and 48 months of age, Lusaka, Zambia, 1998–2004
| HHV-8* seroreactivity pattern† |
No. of children | % | |||
| 12 months | 24 months | 36 months | 48 months | ||
| − | − | − | − | 103 | 60.2 |
| − | − | − | + | 11 | 6.4 |
| − | − | + | + | 7 | 4.1 |
| − | + | + | + | 4 | 2.3 |
| + | + | + | + | 3 | 1.8 |
| + | + | + | − | 1 | 0.6 |
| + | + | − | − | 2 | 1.2 |
| + | − | − | − | 10 | 5.8 |
| + | − | − | + | 4 | 2.3 |
| − | + | − | − | 9 | 5.3 |
| − | + | + | − | 4 | 2.3 |
| − | + | − | + | 7 | 4.1 |
| − | − | + | − | 6 | 3.5 |
| Total for HHV-8-negative children | 103 | 60.2 | |||
| Total for HHV-8-positive children | 68 | 39.8 | |||
| Total for all children | 171 | 100 | |||
HHV-8, human herpesvirus 8.
A positive sign (+) indicates HHV-8 seropositivity at that age, and a negative sign (−) indicates HHV-8 seronegativity.
The titers of four representative patients are shown in figure 4 to demonstrate the fluctuations in anti-HHV-8 antibody titers over time. Patients A and B were born to HHV-8-seropositive mothers, and patients C and D were born to HHV-8-seronegative mothers.
FIGURE 4.
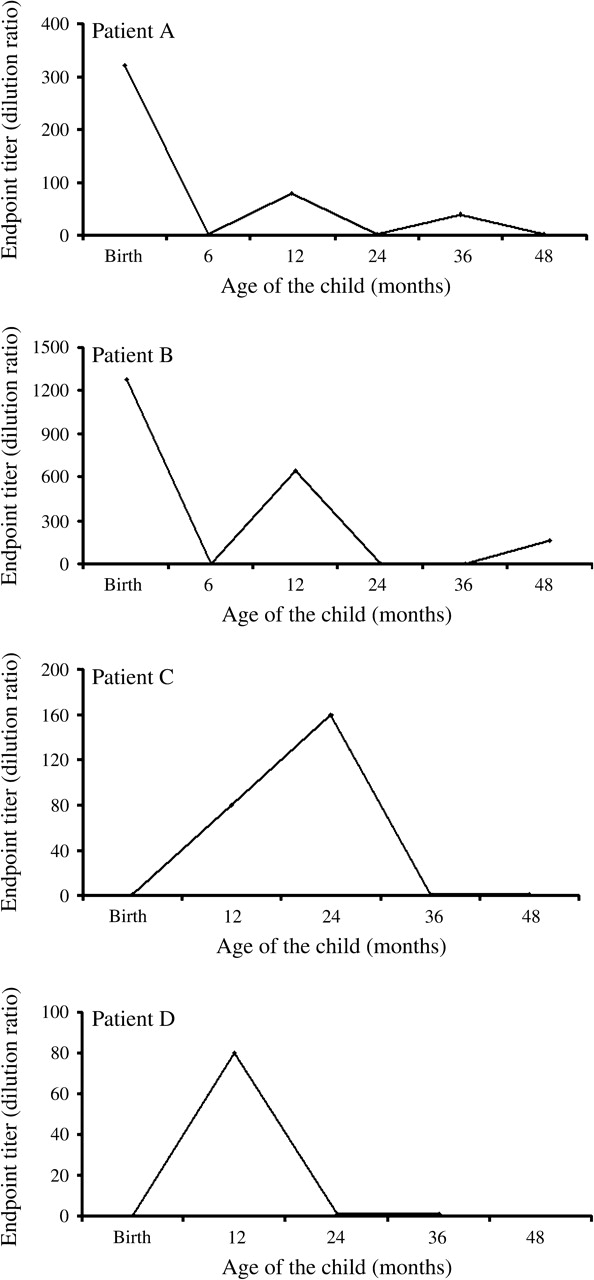
Observed fluctuations in titers of antibodies to human herpesvirus 8 (HHV-8) during early childhood, Lusaka, Zambia, 1998–2004. Children who had detectable antibody responses were titered by serial twofold dilutions of each test serum sample, beginning with 1:40, as described in Materials and Methods. The inverse of the last positive dilution was considered to be the endpoint titer. Patients A and B were born to HHV-8-seropositive mothers, and patients C and D were born to HHV-8-seronegative mothers.
DISCUSSION
The major strengths of the present study were its size, its prospective nature, and the availability of HHV-8 and HIV-1 coinfection data from mother-infant pairs collected at multiple time points from birth to 48 months of follow-up. These strengths enabled us to provide the first documentation of annual HHV-8 incidence rates in early childhood in an African endemic area. Our results indicate that the HIV-1 status of the child is a strong predictor of HHV-8 seroconversion. Incidence rates were generally high among these children between birth and 48 months of age. In addition, fluctuations in detectable HHV-8 titers leading to seroreversions among HHV-8-seroconverted children may produce frequent underestimation of childhood HHV-8 seroprevalence in cross-sectional studies.
The observed HHV-8 seroprevalence in Zambian children is generally comparable to prevalences reported in cross-sectional studies from other parts of sub-Saharan Africa (17, 23, 26). While the prospective, longitudinal design of this study makes it difficult to compare its results directly with those of published cross-sectional studies conducted in the region, HHV-8 seroprevalence of 20–60 percent has been reported among young children (22–24). Kaplan-Meier analysis (data not shown) estimating the probability of HHV-8 seroconversion in this longitudinally followed cohort revealed that more than 40 percent of the children seroconverted for HHV-8 by age 48 months, with a clear increase in HHV-8 seroprevalence with age. These results show that children become infected at a young age in Zambia and that adult seroprevalence levels may be reached relatively early in life. These results indicate that HIV-1-infected children are more likely to become HHV-8-infected, but it remains unclear whether increased risk is due to HIV-1-infected children's having 1) a higher likelihood of being exposed to HHV-8, 2) a higher likelihood of becoming infected when exposed, or 3) a higher likelihood of antibody detection when infected. In addition, the specific risk factors associated with each of these possibilities have yet to be determined.
Potential routes of horizontal HHV-8 transmission are poorly understood, but salivary contact may be the major route of transmission in early childhood. Our laboratory and others have previously found that HHV-8 DNA can be readily detected in the saliva of infected persons and is detected more frequently in persons with higher antibody titers (16, 37–39). While Zambian children are usually breast-fed up to the age of 18–24 months, HHV-8 cannot be easily detected in breast milk, suggesting that it is an unlikely source of infection (16). We frequently observed HHV-8 seroconversion in children born to mothers who were HHV-8-seronegative at delivery, indicating that horizontal transmission is possible in early childhood. These results are consistent with Mantina et al.'s finding that in utero infection of infants is infrequent (18). However, we cannot rule out the possibility that some children who are HHV-8-seropositive at 12 months have HHV-8 that is due to perinatal transmission. Incidence in this cohort could still be underestimated if the perinatally infected children seroreverted before age 12 months. Determining serostatus in children below age 12 months is difficult because of the presence of maternal antibodies.
In this cohort, maternal HHV-8 infection was not an independent risk factor associated with transmission of HHV-8 to children—a finding which was somewhat unexpected, because in the early phase of life the mother is usually the primary caregiver and has close contact with the child. This suggests that mothers may not be the only source of transmission to children and that other members of the household or nonfamilial contacts could be responsible for horizontal transmission to young children. Some reports from sub-Saharan Africa have shown a positive correlation between the HHV-8 status of the mother and that of the child (24, 26). However, other studies reported only a marginal-to-weak correlation (25, 40), and a recent study demonstrated that young infants' risk of acquiring HHV-8 infection in South Africa was not dependent on maternal serostatus (41). Molecular evidence for this has come from Uganda, where a mother and child were demonstrated to have different HHV-8 subtypes (42). The strength of this association could depend on locally common child-care practices, such as kissing, premastication of food, or sharing of food and utensils (43). The impact of such practices has not been explored fully and may be different in Zambia than in other HHV-8-endemic countries.
Brayfield et al. (16) previously reported risk factors associated with HHV-8 infection in a much smaller group of infants at 12 months while active follow-up of mother-infant pairs was still ongoing. A commercially available enzyme-linked immunosorbent assay was used to validate BC-3 mIFA results, and we observed that it underestimated HHV-8 seroprevalence by missing patients with distinct punctate nuclear staining, which led us to develop the Sf9 mIFA. We believe that patients with specific punctate staining could have low-titer antibodies that were missed by enzyme-linked immunosorbent assay because of the higher optical density cutoff values. Employing two assays as part of an algorithm provided us with a reliable, highly specific and conservative method. Sf9 mIFAs have matched negative controls that are lacking for BC-3 mIFAs, thus contributing to a low number of false-positive results. Analysis of a panel of positive and negative control serum samples revealed a high concordance between the two mIFAs (κ = 0.75; unpublished data). Zhu et al. (44) have suggested that confirmation of HHV-8 serostatus should not be based on a single antigen, since infected persons may demonstrate variable reactivity against different antigens. This variability may explain the fluctuations in the anti-HHV-8 antibody titers seen in this study and may explain why certain children have undetectable antibody titers during follow-up. Such variation has been observed in adults, and seroreversion has been reported for other herpesviruses and for hepatitis C virus (45–49). HHV-8 DNA has been detected in certain HHV-8-seronegative patients, and biopsy-proven Kaposi's sarcoma patients in full remission have been reported to undergo HHV-8 seroreversion (50, 51).
Seroreversion may be partial or complete, resulting from a loss of detectable titer to one or more antigens or a total loss of all specific antibodies. We believe that the observed seroreversions in our cohort could be both partial and complete seroreversions. It is unlikely that the HHV-8 seropositivity of children who tested positive at 12 months and subsequently tested negative was due to residual maternal HHV-8 antibodies, because all children born to HHV-8-seropositive mothers were titered at birth and at age 6 months. Eight of 10 children who were seropositive at 12 months and were seronegative at all later time points were born to HHV-8-seronegative mothers and were themselves seronegative at birth. It is possible that the observed seroreversions in our cohort were due to antibody titers that were below the limit of detection of our assays or due to the stringent detection criteria used. However, seroreversion has also been reported in studies using enzyme-linked immunosorbent assay (45). The lack of a gold-standard assay with established 100 percent accuracy makes it difficult to confirm these results. Performance studies of type-specific commercial assays designed to distinguish between herpes simplex virus types 1 and 2 have also reported that seroreversions were introduced because of poor assay sensitivity (52). An absence of antigenic stimulation after establishment of latency or viral clearance could lead to seroreversion. In addition, immunocompetent children may be able to efficiently control further reactivation, and thus the antibody titers drop below detection levels. It has been proposed that HHV-8-specific antibodies might be more readily detectable due to broadening of epitope recognition over time or due to subsequent reactivation after primary HHV-8 infection (45). HIV-1-related immunosuppression has also been proposed to be a factor responsible for HHV-8 seroreversion, especially in the Zambian population, which is experiencing a generalized HIV-1 epidemic. We observed that only four out of 41 HIV-1-infected children underwent seroreversion at one or more time points, but this number was too small for us to draw any conclusion, and a much larger cohort of HIV-1-infected children will be needed in order to understand this phenomenon.
Our study did have some important limitations. Although the number of HIV-1-infected children followed was limited, we found significantly higher HHV-8 incidence among HIV-1-infected children than among uninfected children. The high rate of attrition in HIV-1-infected children probably led to an underestimate of the true HHV-8 incidence and of the impact of child HIV-1 infection status on the risk of HHV-8 acquisition during early childhood. The number of children born to HIV-1-positive mothers was higher in the group that was not included in the analysis. To be eligible for inclusion in the analysis, a child had to survive and return at 24 months of age for reliable detection of HIV-1 antibodies. In addition, neither degree of clinical immunosuppression nor HIV-1 viral load could be assessed, because methods for determining CD4 cell count and viral load were not yet available in Zambia. Transmission from siblings could not be examined because siblings were not recruited. Attrition due to mortality was high in children, especially those who were HIV-1-infected, because of lack of availability of antiretroviral therapy at the time of the study (53). None of the HIV-1-infected children returned after 36 months, probably because of high mortality in this group, which was without access to antiretroviral agents during the time period of this study. Pediatric antiretroviral therapy has since been implemented at primary health-care clinics throughout Lusaka (54). Common reasons for withdrawal from the study were religious beliefs, disapproval of a spouse or family, or lack of interest. Some families were untraceable because both the mother and the child died, relocated, or provided a wrong address. Most members of the study population were of lower socioeconomic status, which contributed to their being highly mobile.
In conclusion, horizontal transmission appears to be the major route of HHV-8 transmission in early childhood, and HIV-1 infection of the child is an important risk factor for HHV-8 acquisition in an area that is highly endemic for both viruses. The frequent seroreversions observed demonstrate that cross-sectional serologic screening for HHV-8 underestimates true rates of HHV-8 infection and may not provide a true representation of HHV-8 prevalence and incidence in a population.
Acknowledgments
This work was supported by several grants to C. W.: US Public Health Service grants RO1 CA75903 and T32 AI060547 from the National Institutes of Health, Fogarty International training grant D43 TW01492, and National Center for Research Resources Centers of Biomedical Research Excellence grant P20 RR15635. T. M. was a Fogarty fellow. K. L. C. was supported by a Ruth L. Kirschstein National Research Service Award from the National Institute of Allergy and Infectious Diseases and by the INBRE (IDeA Networks of Biomedical Research Excellence) program (grant P20 RR016469) of the National Center for Research Resources.
The authors thank the late Dr. Ganapati Bhat for his contribution to the study design. They thank Chafye Siuluta, Darius Simbeye, and the laboratory and clinical staff of the Department of Paediatrics and Child Health at University Teaching Hospital, Lusaka, for their contributions to recruitment, data collection, and management. The authors thank Dr. Bala Chandran for providing them with the recombinant baculoviruses and Dr. Clinton Jones for supplying Sf9 cells. They thank Drs. Harold Jaffe and Sheila Dollard for helpful comments on an earlier draft of the manuscript. In addition, they thank Danielle Shea for technical assistance.
This paper was presented in part at the 13th Conference on Retroviruses and Opportunistic Infections, Denver, Colorado, February 5–8, 2006 (abstract 819).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Conflict of interest: none declared.
Glossary
Abbreviations
- HHV-8
human herpesvirus 8
- HIV-1
human immunodeficiency virus type 1
- mIFA
monoclonal antibody-enhanced immunofluorescence assay
- Sf9
Spodoptera frugiperda clone 9
References
- 1.Cesarman E, Chang Y, Moore PS, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–91. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 3.Schulz TF. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) J Gen Virol. 1998;79:1573–91. doi: 10.1099/0022-1317-79-7-1573. [DOI] [PubMed] [Google Scholar]
- 4.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–80. [PubMed] [Google Scholar]
- 5.Cook-Mozaffari P, Newton R, Beral V, et al. The geographical distribution of Kaposi's sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–8. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blauvelt A, Sei S, Cook PM, et al. Human herpesvirus 8 infection occurs following adolescence in the United States. J Infect Dis. 1997;176:771–4. doi: 10.1086/517298. [DOI] [PubMed] [Google Scholar]
- 7.Dedicoat M, Newton R. Review of the distribution of Kaposi's sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi's sarcoma. Br J Cancer. 2003;88:1–3. doi: 10.1038/sj.bjc.6600745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med. 1996;2:925–8. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 9.Martro E, Bulterys M, Stewart JA, et al. Comparison of human herpesvirus 8 and Epstein-Barr virus seropositivity among children in areas endemic and non-endemic for Kaposi's sarcoma. J Med Virol. 2004;72:126–31. doi: 10.1002/jmv.10548. [DOI] [PubMed] [Google Scholar]
- 10.Pellett PE, Wright DJ, Engels EA, et al. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion. 2003;43:1260–8. doi: 10.1046/j.1537-2995.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiss RA, Whitby D, Talbot S, et al. Human herpesvirus type 8 and Kaposi's sarcoma. J Natl Cancer Inst Monogr. 1998;23:51–4. doi: 10.1093/oxfordjournals.jncimonographs.a024173. [DOI] [PubMed] [Google Scholar]
- 12.He J, Bhat G, Kankasa C, et al. Seroprevalence of human herpesvirus 8 among Zambian women of childbearing age without Kaposi's sarcoma (KS) and mother-child pairs with KS. J Infect Dis. 1998;178:1787–90. doi: 10.1086/314512. [DOI] [PubMed] [Google Scholar]
- 13.Cannon MJ, Dollard SC, Smith DK, et al. Blood-borne and sexual transmission of human herpesvirus 8 in women with or at risk for human immunodeficiency virus infection. N Engl J Med. 2001;344:637–43. doi: 10.1056/NEJM200103013440904. [DOI] [PubMed] [Google Scholar]
- 14.Martin JN, Ganem DE, Osmond DH, et al. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 15.Melbye M, Cook PM, Hjalgrim H, et al. Risk factors for Kaposi's-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981 –1996. Int J Cancer. 1998;77:543–8. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Brayfield BP, Kankasa C, West JT, et al. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J Infect Dis. 2004;189:2260–70. doi: 10.1086/421119. [DOI] [PubMed] [Google Scholar]
- 17.Dedicoat M, Newton R, Alkharsah KR, et al. Mother-to-child transmission of human herpesvirus 8 in South Africa. J Infect Dis. 2004;190:1068–75. doi: 10.1086/423326. [DOI] [PubMed] [Google Scholar]
- 18.Mantina H, Kankasa C, Klaskala W, et al. Vertical transmission of Kaposi's sarcoma-associated herpesvirus. Int J Cancer. 2001;94:749–52. doi: 10.1002/ijc.1529. [DOI] [PubMed] [Google Scholar]
- 19.Hladik W, Dollard SC, Mermin J, et al. Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med. 2006;355:1331–8. doi: 10.1056/NEJMoa055009. [DOI] [PubMed] [Google Scholar]
- 20.Plancoulaine S, Abel L, van Beveren M, et al. Human herpesvirus 8 transmission from mother to child and between siblings in an endemic population. Lancet. 2000;356:1062–5. doi: 10.1016/S0140-6736(00)02729-X. [DOI] [PubMed] [Google Scholar]
- 21.Whitby D, Luppi M, Sabin C, et al. Detection of antibodies to human herpesvirus 8 in Italian children: evidence for horizontal transmission. Br J Cancer. 2000;82:702–4. doi: 10.1054/bjoc.1999.0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817–20. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 23.Gessain A, Mauclere P, van Beveren M, et al. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int J Cancer. 1999;81:189–92. doi: 10.1002/(sici)1097-0215(19990412)81:2<189::aid-ijc4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Mbulaiteye SM, Pfeiffer RM, Whitby D, et al. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–5. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 25.Mbulaiteye SM, Biggar RJ, Pfeiffer RM, et al. Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr. 2005;38:474–9. doi: 10.1097/01.qai.0000132495.89162.c0. [DOI] [PubMed] [Google Scholar]
- 26.Plancoulaine S, Abel L, Trégouët D, et al. Respective roles of serological status and blood specific antihuman herpesvirus 8 antibody levels in human herpesvirus 8 intrafamilial transmission in a highly endemic area. Cancer Res. 2004;64:8782–7. doi: 10.1158/0008-5472.CAN-04-2000. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler JL, Katongole-Mbidde E. Kaposi's sarcoma in childhood: an analysis of 100 cases from Uganda and relationship to HIV infection. Int J Cancer. 1996;65:200–3. doi: 10.1002/(SICI)1097-0215(19960117)65:2<200::AID-IJC12>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 28.Bayley AC. Occurrence, clinical behaviour and management of Kaposi's sarcoma in Zambia. Cancer Surv. 1991;10:53–71. [PubMed] [Google Scholar]
- 29.Patil P, Elem B, Zumla A. Pattern of adult malignancies in Zambia (1980 –1989) in light of the human immunodeficiency virus type 1 epidemic. J Trop Med Hyg. 1995;98:281–4. [PubMed] [Google Scholar]
- 30.Patil PS, Elem B, Gwavava NJ, et al. The pattern of paediatric malignancy in Zambia (1980 –1989): a hospital-based histopathological study. J Trop Med Hyg. 1992;95:124–7. [PubMed] [Google Scholar]
- 31.Chintu C, Athale UH, Patil PS. Childhood cancers in Zambia before and after the HIV epidemic. Arch Dis Child. 1995;73:100–4. doi: 10.1136/adc.73.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brayfield BP, Phiri S, Kankasa C, et al. Postnatal human herpesvirus 8 and human immunodeficiency virus type 1 infection in mothers and infants from Zambia. J Infect Dis. 2003;187:559–68. doi: 10.1086/367985. [DOI] [PubMed] [Google Scholar]
- 33.Lennette ET, Blackbourn DJ, Levy JA. Antibodies to human herpesvirus type 8 in the general population and in Kaposi's sarcoma patients. Lancet. 1996;348:858–61. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- 34.Kanki PJ, Allan J, Barin F, et al. Absence of antibodies to HIV-2/HTLV-4 in six central African nations. AIDS Res Hum Retroviruses. 1987;3:317–22. doi: 10.1089/aid.1987.3.317. [DOI] [PubMed] [Google Scholar]
- 35.National AIDS. Lusaka, Zambia: National AIDS Council; 2006. Council. Zambia counseling and testing guidelines. [Google Scholar]
- 36.Allison PD. Cary, NC: SAS Publishing; 1999. Logistic regression using the SAS system: theory and application. [Google Scholar]
- 37.Marcelin AG, Gorin I, Morand P, et al. Quantification of Kaposi's sarcoma-associated herpesvirus in blood, oral mucosa, and saliva in patients with Kaposi's sarcoma. AIDS Res Hum Retroviruses. 2004;20:704–8. doi: 10.1089/0889222041524689. [DOI] [PubMed] [Google Scholar]
- 38.Mbulaiteye SM, Pfeiffer RM, Engels EA, et al. Detection of Kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. J Infect Dis. 2004;190:1382–6. doi: 10.1086/424489. [DOI] [PubMed] [Google Scholar]
- 39.Taylor MM, Chohan B, Lavreys L, et al. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and -seronegative Kenyan women. J Infect Dis. 2004;190:484–8. doi: 10.1086/421466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serraino D, Locatelli M, Songini M, et al. Human herpes virus 8 infection among pregnant women and their children: results from the Sardinia-IDDM Study 2. Int J Cancer. 2001;91:740–1. [PubMed] [Google Scholar]
- 41.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–5. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]
- 42.Mbulaiteye S, Marshall V, Bagni RK, et al. Molecular evidence for mother-to-child transmission of Kaposi sarcoma-associated herpesvirus in Uganda and K1 gene evolution within the host. J Infect Dis. 2006;193:1250–7. doi: 10.1086/503052. [DOI] [PubMed] [Google Scholar]
- 43.Gaur A, Dominguez K, Kalish M, et al. Practice of offering a child pre-masticated food: an unrecognized possible risk factor for HIV transmission. (Abstract) Presented at the 15th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, February 3–6, 2008. ( http://www.retroconference.org/2008/) [Google Scholar]
- 44.Zhu L, Wang R, Sweat A, et al. Comparison of human sera reactivities in immunoblots with recombinant human herpesvirus (HHV)-8 proteins associated with the latent (ORF73) and lytic (ORFs 65, K8.1A, and K8.1B) replicative cycles and in immunofluorescence assays with HHV-8-infected BCBL-1 cells. Virology. 1999;256:381–92. doi: 10.1006/viro.1999.9674. [DOI] [PubMed] [Google Scholar]
- 45.Biggar RJ, Engels EA, Whitby D, et al. Antibody reactivity to latent and lytic antigens to human herpesvirus 8 in longitudinally followed homosexual men. J Infect Dis. 2003;187:12–18. doi: 10.1086/345866. [DOI] [PubMed] [Google Scholar]
- 46.Cherpes TL, Ashley RL, Meyn LA, et al. Longitudinal reliability of focus glycoprotein G-based type-specific enzyme immunoassays for detection of herpes simplex virus types 1 and 2 in women. J Clin Microbiol. 2003;41:671–4. doi: 10.1128/JCM.41.2.671-674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinlivan EB, Wang RX, Stewart PW, et al. Longitudinal sero-reactivity to human herpesvirus 8 (KSHV) in the Swiss HIV Cohort 4.7 years before KS. J Med Virol. 2001;64:157–66. doi: 10.1002/jmv.1031. [DOI] [PubMed] [Google Scholar]
- 48.Rerksuppaphol S, Hardikar W, Dore GJ. Long-term outcome of vertically acquired and post-transfusion hepatitis C infection in children. J Gastroenterol Hepatol. 2004;19:1357–62. doi: 10.1111/j.1440-1746.2004.03463.x. [DOI] [PubMed] [Google Scholar]
- 49.Zavitsanou A, Sypsa V, Petrodaskalaki M, et al. Human herpesvirus 8 infection in hemodialysis patients. Am J Kidney Dis. 2006;47:167–70. doi: 10.1053/j.ajkd.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Beyari MM, Hodgson TA, Cook RD, et al. Multiple human herpesvirus 8 infection. J Infect Dis. 2003;188:678–89. doi: 10.1086/377504. [DOI] [PubMed] [Google Scholar]
- 51.Albrecht D, Meyer T, Lorenzen T, et al. Epidemiology of HHV-8 infection in HIV-positive patients with and without Kaposi sarcoma: diagnostic relevance of serology and PCR. J Clin Virol. 2004;30:145–9. doi: 10.1016/j.jcv.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Ashley RL. Sorting out the new HSV type specific antibody tests. Sex Transm Infect. 2001;77:232–7. doi: 10.1136/sti.77.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Baets AJ, Bulterys M, Abrams EJ, et al. Care and treatment of HIV-infected children in Africa: issues and challenges at the district hospital level. Pediatr Infect Dis J. 2007;26:163–73. doi: 10.1097/01.inf.0000253040.82669.22. [DOI] [PubMed] [Google Scholar]
- 54.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]