Abstract
In this study, the authors' objective was to determine whether serum concentrations of polychlorinated biphenyls (PCBs), hexachlorobenzene, p,p′-dichlorodiphenyl trichloroethane (DDT), o,p′-DDT, and p,p′-dichlorodiphenyl dichloroethylene (DDE) are associated with thyroid function during pregnancy. These compounds, as well as thyroid-stimulating hormone, total thyroxine, and free thyroxine, were measured in serum samples collected between October 1999 and October 2000 from 334 pregnant women living in the Salinas Valley, California. Data were analyzed by multivariate linear regression. After adjustment for covariates, seven of the 19 PCB congeners detected in more than 75% of participants and the sum of those congeners were negatively associated with free thyroxine concentrations. PCBs 44, 52, and 183 remained significant after the exclusion of two outliers. Hexachlorobenzene concentrations were negatively associated with both free thyroxine and total thyroxine. PCB and hexachlorobenzene concentrations were strongly correlated, which hampered the authors' ability to identify their independent associations with thyroid function. None of the exposures under study were associated with thyroid-stimulating hormone. Results suggest that exposure to PCBs and/or hexachlorobenzene at background levels may affect thyroid function during pregnancy. These findings are of particular significance, since thyroid hormones of maternal origin may play an essential role in fetal neurodevelopment.
Keywords: DDT; dichlorodiphenyl dichloroethylene; hexachlorobenzene; hydrocarbons, chlorinated; polychlorinated biphenyls; pregnancy; thyroid hormones
Persistent organic pollutants are stable lipophilic chemicals that persist in the environment, bioaccumulate in the food chain, and are detected in most human populations (1). Because they are suspected to cause adverse effects on wildlife and human health, individual persistent organic pollutants were banned in the United States between 1978 and 2000. In 2001, more than 100 countries signed the Stockholm Convention on Persistent Organic Pollutants, committing to discontinue or restrict the use of 12 chemicals of concern (2). To date, as many as 154 countries have ratified the Convention. Despite this accord, several of these chemicals may still find their way into the environment. For instance, polychlorinated biphenyls (PCBs) are found in older US electrical transformers and capacitators (3), and the fungicide hexachlorobenzene is a byproduct of US-manufactured solvents (e.g., carbon tetrachloride, trichloroethylene, and tetrachloroethylene) and current-use pesticides (e.g., atrazine) and is an effluent from municipal incinerators (4). In addition, nearly 30 years after phasing out the use of the pesticide dichlorodiphenyl trichloroethane (DDT), the World Health Organization recently issued a position statement announcing that it would resume promoting its use in malaria-endemic areas (5).
Animal and epidemiologic studies suggest that prenatal exposure to PCBs (6–12), hexachlorobenzene (13, 14), and DDT (15, 16) and its breakdown product dichlorodiphenyl dichloroethylene (DDE) (12, 15, 17) may have a deleterious effect on neurodevelopment. Thyroid hormone disruption has been proposed as a potential mechanism of action for the neurodevelopmental effects of certain persistent organic pollutants, since thyroid hormone is essential for normal brain development in mammals (18). In humans, iodine deficiency-related fetal hypothyroidism can lead to cretinism, the leading preventable cause of mental retardation worldwide (19).
Recent findings underscore the importance of maternal thyroid hormone in fetal neurodevelopment. Rat pups of thyroidectomized dams (20) and of moderately and transiently hypothyroxinemic dams (21) have shown deficits in maze learning and altered neuronal migration, respectively. In humans, maternal hypothyroidism and low but normal free thyroxine concentrations (<10th percentile) during pregnancy have been found to be associated with poorer neurodevelopment in children between 10 months and 8 years of age (22–25).
PCBs and their hydroxylated metabolites, as well as hexachlorobenzene and its metabolite pentachlorophenol, have been shown to reduce circulating levels of total thyroxine and free thyroxine and in some cases to increase thyroid-stimulating hormone concentrations in both pregnant and nonpregnant animals (26–28). Although effects of DDT and DDE on thyroid hormone concentrations were not demonstrated in experimental studies conducted in mammals (29), a decrease in iodine uptake by the thyroid gland was observed after acute exposure to technical DDT in rats (30).
Only three human studies have investigated the effect of PCBs on thyroid hormone during pregnancy. Takser et al. (31) found inverse associations between concentrations of total triiodothyronine and PCBs 138, 153, and 180 and the sum of 14 PCB congeners in blood collected from 101 women during pregnancy. In a study conducted in the Netherlands, investigators observed significant negative correlations between PCB toxic equivalents (32) (weighing PCB congeners based on their potency to produce dioxin-like effects) measured in 78 breast-milk samples and total triiodothyronine during the last month of pregnancy and total triiodothyronine and total thyroxine, measured 9–14 days after delivery (33). In addition, the sum of 28 PCB congeners and thyroid-stimulating hormone concentrations were negatively correlated in 182 pregnant women from the Faeroe Islands, although the association did not quite reach statistical significance (p = 0.09) (34). In the only study that investigated associations between thyroid hormone in pregnant women and hexachlorobenzene, DDT, or DDE concentrations, Takser et al. (31) reported negative associations between total triiodothyronine and hexachlorobenzene and p,p′-DDE but not p,p′-DDT.
Several mechanisms of action have been proposed to explain the potential association between thyroid hormone and PCBs, DDT/DDE, and hexachlorobenzene, including the induction of uridinediphosphate glucuronosyltransferase (35–37), increased liver thyroxine uptake (38), pituitary resistance to thyroid-releasing hormone (38), thyroid gland resistance to thyroid-stimulating hormone (39), altered triiodothyronine and thyroxine synthesis (40), and binding to transport proteins and displacement of thyroxine (26, 41).
We have previously reported an association between prenatal exposure to PCBs, grouped according to their potential to induce thyroxine-metabolizing enzymes in animals, and neonatal thyroid-stimulating hormone (42). In the present investigation, we aimed to determine whether PCB, DDT/DDE, and/or hexachlorobenzene body burdens are associated with thyroid function in pregnant women.
MATERIALS AND METHODS
Participants
Women were enrolled between October 1999 and October 2000 in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS). The project was a birth cohort study investigating the health effects of environmental exposures to pregnant women and children in the Salinas Valley, California (43). Pregnant women were eligible to participate in the study if they were covered by Medi-Cal (subsidized health care), were ≥18 years of age, were at <20 weeks' gestation, spoke English or Spanish, and planned to deliver at Natividad Medical Center, the county hospital in Salinas, California. A total of 601 women were enrolled, 538 of whom were followed to delivery and had a livebirth. Participants with insufficient serum volume for analysis of PCBs/organochlorines (n = 118) or thyroid hormones (n = 6) or who were taking thyroid hormone supplements or anti-thyroid-hormone medications (n = 1) were excluded. Given that we ultimately wanted to examine the relation of maternal thyroid hormone to neurodevelopment (manuscript in preparation), we did not measure thyroid hormone concentrations in mothers whose children were not assessed at some point in the future (n = 79). Thus, the final sample included 334 participants.
The analyses described below were performed with weights equal to the inverse probability of inclusion in the final sample, as determined by multiple logistic regression, and without weights (44). Results were similar for both weighted and unweighted regressions, suggesting that selection bias due to participants' exclusion did not substantially affect our results. Unweighted analyses are presented.
This study was approved by the University of California, Berkeley, Committee for the Protection of Human Subjects. All participants gave written informed consent prior to inclusion.
Maternal interviews and medical records
Personal interviews with the women were conducted during pregnancy at the time of enrollment (13 weeks' gestation) and again at 26 weeks' gestation. Information was collected on the women's demographic characteristics, health, and health-related behaviors (see table 1). Medical records were also abstracted by a nurse to obtain information about medication use and general health status during pregnancy.
TABLE 1.
Demographic characteristics of pregnant women and their offspring according to serum concentrations of thyroid hormones, Salinas Valley, California, 1999–2000
| No. of subjects† | %† | Thyroid hormone |
|||
| Free thyroxine (mean) (ng/dl) | Total thyroxine (mean) (μg/dl) | Thyroid-stimulating hormone (geometric mean) (mIU/liter) | |||
| Child | |||||
| Sex | |||||
| Male | 164 | 49 | 0.83 (0.25)‡ | 10.6 (1.5)‡ | 1.14 (1.81)§ |
| Female | 170 | 51 | 0.82 (0.23) | 10.6 (1.6) | 1.19 (1.66) |
| Birth weight (g) | |||||
| <2,500 | 12 | 4 | 0.79 (0.22)* | 10.6 (1.8) | 1.34 (1.37) |
| 2,500–3,500 | 174 | 52 | 0.86 (0.25) | 10.8 (1.5) | 1.17 (1.84) |
| >3,500 | 148 | 44 | 0.79 (0.23) | 10.5 (1.6) | 1.14 (1.63) |
| Gestational age (weeks) at birth | |||||
| <37 | 24 | 7 | 0.71 (0.21)** | 10.4 (1.5) | 1.21 (1.67) |
| 37–42 | 310 | 93 | 0.83 (0.24) | 10.7 (1.6) | 1.16 (1.74) |
| >42 | 0 | 0 | |||
| Mother | |||||
| Age (years) | |||||
| 18–24 | 158 | 47 | 0.87 (0.25)*** | 10.8 (1.6)** | 1.09 (1.80)* |
| 25–29 | 108 | 32 | 0.81 (0.22) | 10.7 (1.6) | 1.30 (1.69) |
| 30–34 | 45 | 13 | 0.74 (0.22) | 10.4 (1.1) | 1.16 (1.59) |
| 35–45 | 23 | 7 | 0.71 (0.21) | 9.6 (1.5) | 1.07 (1.68) |
| Race/ethnicity | |||||
| White | 7 | 2 | 0.77 (0.21) | 10.9 (2.3) | 1.05 (1.50) |
| Latino | 320 | 96 | 0.83 (0.24) | 10.6 (1.6) | 1.17 (1.74) |
| Other | 7 | 2 | 0.89 (0.23) | 11.4 (1.5) | 0.91 (1.53) |
| Education | |||||
| ≤6th grade | 140 | 42 | 0.85 (0.24) | 10.4 (1.5) | 1.13 (1.90) |
| 7th–12th grade (no diploma) | 118 | 35 | 0.80 (0.25) | 10.7 (1.7) | 1.21 (1.63) |
| High school diploma or more | 76 | 23 | 0.83 (0.22) | 10.9 (1.5) | 1.13 (1.57) |
| Family income | |||||
| Poverty line or less | 188 | 60 | 0.82 (0.22) | 10.7 (1.6) | 1.11 (1.79) |
| Above poverty line–200% of poverty line | 111 | 36 | 0.81 (0.24) | 10.7 (1.5) | 1.25 (1.63) |
| >200% of poverty line | 12 | 4 | 0.91 (0.43) | 9.8 (1.4) | 1.30 (1.60) |
| Country of birth | |||||
| United States | 41 | 12 | 0.86 (0.28) | 11.2 (1.6)** | 1.12 (1.59)** |
| Mexico | 286 | 86 | 0.82 (0.24) | 10.5 (1.6) | 1.19 (1.71) |
| Other | 7 | 2 | 0.80 (0.13) | 11.1 (1.0) | 0.55 (2.99) |
| Parity | |||||
| 0 | 109 | 33 | 0.86 (0.26) | 10.6 (1.6) | 1.21 (1.57) |
| ≥1 | 225 | 67 | 0.81 (0.23) | 10.6 (1.6) | 1.14 (1.81) |
| Time (years) in the United States | |||||
| ≤5 | 182 | 54 | 0.84 (0.26) | 10.6 (1.5) | 1.22 (1.86) |
| 6–10 | 78 | 23 | 0.80 (0.19) | 10.4 (1.7) | 1.07 (1.62) |
| ≥11 | 74 | 22 | 0.82 (0.25) | 10.9 (1.6) | 1.11 (1.52) |
| Smoking during pregnancy | |||||
| Yes | 22 | 7 | 0.79 (0.21) | 10.9 (1.6) | 1.15 (1.50) |
| No | 312 | 93 | 0.83 (0.24) | 10.6 (1.6) | 1.16 (1.75) |
| Alcohol drinking during pregnancy | |||||
| Yes | 76 | 23 | 0.79 (0.21) | 10.5 (1.6) | 1.20 (1.55) |
| No | 254 | 77 | 0.83 (0.25) | 10.7 (1.6) | 1.15 (1.79) |
| Prepregnancy body mass index¶ | |||||
| <25 | 127 | 39 | 0.86 (0.24) | 10.6 (1.4) | 1.09 (1.90) |
| 25–30 | 131 | 41 | 0.82 (0.22) | 10.7 (1.6) | 1.24 (1.64) |
| >30 | 65 | 20 | 0.79 (0.23) | 10.6 (1.8) | 1.16 (1.64) |
* p < 0.10; **p < 0.05; ***p < 0.001 (two-sided p value calculated using analysis of variance).
Numbers do not always add to the total number of participants because of missing values. Percentages may not add to 100% because of rounding.
Numbers in parentheses, standard deviation.
Numbers in parentheses, geometric standard deviation.
Weight (kg)/height (m)2.
Laboratory analyses
Maternal concentrations of thyroid hormone and PCBs were measured in blood collected during the second interview (n = 320) or before delivery (n = 14). Samples were processed immediately at Natividad Medical Center and were stored at −80°C at the University of California, Berkeley, School of Public Health Biorepository until they were shipped to analytical laboratories. Thyroid hormone analyses were conducted by Quest Diagnostics' Nichols Institute (San Juan Capistrano, California). In pilot experiments, we determined that the number of freeze-thaw cycles was positively associated with measured free thyroxine concentrations (data not shown). Therefore, samples were thawed only once for aliquotting, sent refrigerated to the analytical laboratory, and analyzed within 48 hours. Thyroid-stimulating hormone was measured by ultrasensitive third-generation immunochemiluminometric assay (functional sensitivity, 0.01 mIU/liter; reference range, 0.8–5.2 mIU/liter or >2.5 mIU/liter (45); intraassay coefficients of variation, 2.3–6.0 percent); total thyroxine was determined by solid-phase immunochemiluminometric assay (functional sensitivity, 0.1 μg/dl; reference range, 5.6–13.7 μg/dl; intraassay coefficients of variation, 4.5–5.7 percent); and free thyroxine was measured by direct equilibrium dialysis followed by radioimmunoassay (functional sensitivity, 0.1 ng/dl; reference range, 0.5–2.7 ng/dl; intraassay coefficients of variation, 2.4–6.2 percent) (46). Previous studies have used immunoassays alone to measure free thyroxine, but these methods may be affected by the blood concentration of protein-bound thyroxine (47), which increases during pregnancy and varies among individuals (48). Equilibrium dialysis physically separates the bound hormone from the free portion before it is measured with a highly sensitive radioimmunoassay, thereby permitting accurate measurement of free thyroxine concentrations in samples with normal or high protein-bound thyroxine concentrations (49).
We measured 34 PCB congeners (International Union for Pure and Applied Chemistry numbers 18, 28, 44, 49, 52, 66, 74, 87, 99, 101, 118, 128, 138, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 199, 206, and 209) and eight organochlorines, including hexachlorobenzene, p,p′-DDT, o,p′-DDT, p,p′-DDE, γ-hexachlorocyclohexane, dieldrin, mirex, and trans-nonachlor, in maternal serum by high-resolution gas chromatography/high-resolution mass spectrometry with isotope dilution quantification (50). All exposures are expressed on a lipid basis. Blood lipid concentrations were calculated based on triglyceride and total cholesterol levels, as determined by standard enzymatic methods (Roche Chemicals, Indianapolis, Indiana) (51). Limits of detection ranged between 0.01 ng/g lipids and 1.09 ng/g lipids for PCBs and between <0.001 ng/g lipids and 9.54 ng/g lipids for organochlorines.
Because other exposures may also affect thyroid hormone concentrations, we also measured lead levels in maternal blood (n = 87) and umbilical cord blood (n = 185) using graphite furnace atomic absorption spectrophotometry. Lead levels measured at both time points were highly correlated (r = 0.8, p < 0.001), indicating that using cord blood levels to estimate maternal exposure was appropriate. Finally, we assessed maternal exposure to organophosphate pesticides by measuring dialkyl phosphate metabolites in urine collected at approximately 13 and 26 weeks' gestation. Dialkyl phosphate metabolites were analyzed by high-resolution gas chromatography-tandem mass spectrometry with isotope dilution quantification (52); the two measurements were creatinine-adjusted and averaged.
Statistical analyses
PCB congeners were examined individually and were summed as well as grouped as proposed in previous publications on the basis of the following factors: 1) dioxin-like properties determined using the toxic equivalent method (32); 2) structure-activity and occurrence frequency considerations (53); and 3) the potential induction of thyroxine-metabolizing enzymes (42). We used one-way analysis of variance to examine PCBs and organochlorines according to demographic characteristics. In order to determine the shape of relations between environmental exposures and thyroid hormone, we first plotted variables using LOWESS smoothers, which indicated log-linear relations for associations with free thyroxine and total thyroxine and log-log associations with thyroid-stimulating hormone. We used multivariate linear regression models to investigate associations between organochlorines/PCBs and thyroid-stimulating hormone, free thyroxine, and total thyroxine. Models were constructed and analyses carried out both with and without outliers as identified by the generalized extreme studentized deviate many-outlier procedure (54). Covariates considered for inclusion in the models were expressed as shown in table 1 or as indicated in parentheses and comprised age (years; continuous variable), race/ethnicity, education, income level, country of birth, number of years spent in the United States, parity, prepregnancy body mass index (weight (kg)/height (m)2; continuous variable), gestational age at the time of sample collection (weeks; continuous variable), and cigarette smoking, alcohol drinking, and caffeine consumption during pregnancy (yes vs. no). We also considered the potential confounding effect of environmental exposures such as blood lead (55), urine dialkyl phosphates (organophosphate metabolites) (56), and other organochlorine serum concentrations (57–59). Covariates kept in the final models comprised variables that changed the magnitude of one of the main effects by 10 percent or more (60).
Values below limits of detection and missing values for individual PCB congeners were randomly generated on the basis of a log-normal probability distribution whose parameters were determined by maximum likelihood estimation. This method generally produces unbiased parameter and variance estimates when detection frequencies are greater than 70 percent, whereas simple substitution methods, such as imputing values less than the limit of detection (LOD) by using the formula LOD/2 or LOD/√2, require detection frequencies greater than 90–95 percent (61). Therefore, only individual PCB congeners with a detection frequency greater than 75 percent, adding 5 percent as a safety margin (PCBs 18, 28, 44, 49, 52, 66, 74, 99, 101, 118, 138, 146, 153, 156, 180, 183, 187, 194, and 199), were considered in statistical analyses. Levels of PCBs, organochlorines, and thyroid-stimulating hormone were log10-transformed for analysis. The analyses were performed using Intercooled STATA, version 8.2 (Stata Corporation, College Station, Texas).
RESULTS
Population characteristics
The pregnant women participating in this study were mostly young (mean age = 25.5 years; standard deviation, 5), low-income, and Latina and from farmworker families (table 1). The majority of participants had been born in Mexico, had lived in the United States for less than 10 years, and did not have a high school diploma. The women were generally healthy; a small percentage had gestational diabetes (7 percent) or pregnancy-induced hypertension (4 percent).
Concentrations of PCBs, organochlorines, and thyroid hormone
Changes in body fat mass can modulate organochlorine/PCB serum concentrations by altering the volume of dilution (62). Therefore, prepregnancy concentrations in our population were probably higher than reported in table 2. Despite this, blood concentrations of p,p′-DDT, o,p′-DDT, p,p′-DDE, and hexachlorobenzene were substantially higher in our population than in the Third National Health and Nutrition Examination Survey (NHANES III), with the median p,p′-DDE concentration being about 3.9 times higher in CHAMACOS women than in nonpregnant women aged ≥20 years in NHANES III (1,003 ng/g lipids vs. 256 ng/g lipids) (63, 64). Less than 5 percent of NHANES III samples had detectable hexachlorobenzene serum concentrations, while 82 percent of the CHAMACOS samples were above the NHANES III limit of detection (31.4 ng/g lipids). PCB concentrations were low in comparison with those reported in NHANES III in both the general population and the Mexican-American subsample. All organochlorine/PCB concentrations increased with age (data not shown).
TABLE 2.
Serum concentrations* of polychlorinated biphenyls and organochlorine pesticides, detection frequencies, and ranges of limits of detection among pregnant women, Salinas Valley, California, 1999–2000
| No. of subjects | LOD† range | Detection frequency (%) | Geometric mean concentration | 95% confidence interval | Range of concentrations | |
| Organochlorines (ng/g) | ||||||
| p,p′-DDT† | 334 | 0.06–1.36 | 100.0 | 18.8 | 15.7, 22.5 | 1.6–33,174.0 |
| o,p′-DDT | 333 | 0.06–0.76 | 95.5 | 1.7 | 1.5, 2.0 | <LOD–1,257.3 |
| p,p′-DDE† | 334 | 0.06–1.36 | 100.0 | 1,302.1 | 1,140.2, 1,487.0 | 48.8–159,303.3 |
| Hexachlorobenzene | 334 | 0.02–1.00 | 100.0 | 65.8 | 60.2, 71.9 | 7.5–841.0 |
| PCB† groupings | ||||||
| ΣPCBs (ng/g)‡ | 334 | 0.03–1.09 | 100.0 | 65.3 | 61.9, 69.0 | 18.8–323.7 |
| Enzyme inducers (ng/g)§ | 334 | 0.03–1.09 | 100.0 | 17.6 | 16.7, 18.6 | 4.3–208.3 |
| Mono-ortho PCBs (ng/g)¶ | 334 | 0.05–0.79 | 100.0 | 30.9 | 29.0, 32.9 | 7.1–122.2 |
| Toxic equivalents (pg/g) | 334 | 0.05–0.63 | 100.0 | 1.07 | 1.00, 1.14 | 0.08–6.19 |
| Wolff's method group 3# | 334 | 0.05–0.76 | 100.0 | 8.4 | 7.8, 9.1 | 0.2–140.8 |
| Individual PCB congeners | ||||||
| PCB 18 | 325 | 0.03–0.88 | 100.0 | 6.6 | 6.0, 7.2 | 0.9–27.5 |
| PCB 28 | 334 | 0.03–0.79 | 100.0 | 17.9 | 16.5, 19.5 | 3.1–84.7 |
| PCB 44 | 272 | 0.06–0.84 | 98.9 | 2.5 | 2.2, 2.7 | <LOD–11.4 |
| PCB 49 | 290 | 0.05–0.80 | 99.3 | 1.6 | 1.4, 1.7 | <LOD–6.8 |
| PCB 52 | 304 | 0.05–0.80 | 99.7 | 3.2 | 2.9, 3.5 | <LOD–12.4 |
| PCB 66 | 328 | 0.04–0.63 | 100.0 | 2.9 | 2.7, 3.1 | 0.4–16.0 |
| PCB 74 | 320 | 0.04–0.65 | 100.0 | 4.3 | 4.0, 4.5 | 0.7–26.4 |
| PCB 99 | 305 | 0.06–1.09 | 100.0 | 1.9 | 1.7, 2.0 | 0.5–11.6 |
| PCB 101 | 280 | 0.06–0.87 | 95.7 | 1.0 | 0.9, 1.1 | <LOD–5.8 |
| PCB 118 | 313 | 0.05–0.62 | 99.7 | 3.5 | 3.2, 3.7 | <LOD–19.8 |
| PCB 138 | 308 | 0.03–0.59 | 100.0 | 2.6 | 2.4, 2.8 | 0.2–30.9 |
| PCB 146 | 293 | 0.05–0.60 | 86.7 | 0.45 | 0.40, 0.50 | <LOD–14.7 |
| PCB 153 | 314 | 0.04–0.69 | 100.0 | 5.7 | 5.3, 6.1 | 0.3–95.7 |
| PCB 156 | 322 | 0.06–0.55 | 83.9 | 0.40 | 0.36, 0.45 | <LOD–6.3 |
| PCB 180 | 258 | 0.06–0.59 | 100.0 | 1.5 | 1.4, 1.6 | 0.3–30.0 |
| PCB 183 | 304 | 0.06–0.55 | 77.6 | 0.32 | 0.28, 0.35 | <LOD–8.2 |
| PCB 187 | 266 | 0.04–0.71 | 97.0 | 0.93 | 0.84, 1.04 | <LOD–38.3 |
| PCB 194 | 310 | 0.03–0.50 | 95.2 | 0.52 | 0.48, 0.57 | <LOD–9.5 |
| PCB 199 | 321 | 0.03–0.62 | 86.3 | 0.36 | 0.33, 0.40 | <LOD–29.8 |
All polychlorinated biphenyl and organochlorine concentrations are expressed on a lipid basis (ng/g lipids). Toxic equivalents are expressed in pg/g lipids.
LOD, limit of detection; DDT, dichlorodiphenyl trichloroethane; DDE, dichlorodiphenyl dichloroethylene; PCB, polychlorinated biphenyl.
Sum for the 19 PCB congeners with a detection frequency greater than 75%.
Enzyme inducers include PCBs 99, 101, 118, 153, 156, 157, 167, 180, 183, 187, 194, 189, and 199.
Mono-ortho PCBs include PCBs 28, 66, 74, 118, 156, 157, 167, and 189.
Group 3 includes PCBs 99, 153, 180, 183, and 196.
PCB congeners tended to correlate according to their chlorine content (data not shown). Concentrations of p,p′-DDT, o,p′-DDT, and p,p′-DDE were strongly correlated (r = 0.81–0.92, p < 0.001) but only weakly associated with PCB congeners and hexachlorobenzene concentrations (r = −0.09 to 0.19, p < 0.001–0.92). Hexachlorobenzene was more strongly correlated with less chlorinated PCBs (PCBs 18–99: r = 0.29–0.42; PCBs 101–199: r = 0.14–0.31, p < 0.05) and with the sum of the 19 PCB congeners detected in more than 75 percent of participants (ΣPCBs) (r = 0.46, p < 0.001).
Mean concentrations of total thyroxine and free thyroxine were 10.6 μg/dl (standard deviation, 1.6) and 0.82 ng/dl (standard deviation, 0.24), respectively; the geometric mean for thyroid-stimulating hormone was 1.16 mIU/liter (geometric standard deviation, 1.74). Free thyroxine was low (<0.5 ng/dl) in nine participants; 17 had a high thyroid-stimulating hormone value (>2.5 mIU/liter), and 13 had low total thyroxine (<8.0 μg/dl) concentrations. Thyroid-stimulating hormone was suppressed (<0.01 mIU/liter) in two women. As is shown in table 1, free thyroxine and total thyroxine concentrations decreased with age, and free thyroxine was lower in women who delivered preterm. Total thyroxine was lower and thyroid-stimulating hormone was higher in women born in Mexico as compared with those born in the United States.
Associations between PCBs and thyroid hormone concentrations
As table 3 and table 4 show, of the 19 PCB congeners detected in more than 75 percent of participants, 15 were negatively associated with free thyroxine and four were negatively associated with total thyroxine (no significant positive association). After adjustment for confounders, negative associations with free thyroxine remained significant for the less chlorinated PCB congeners (PCBs 18, 28, 44, 49, and 52; β = −0.11 to −0.08, p < 0.05) and for PCBs 101 (β = −0.07, 95 percent confidence interval (CI): −0.14, −0.00) and 183 (β = −0.11, 95 percent CI: –0.18, −0.03). PCBs 44, 52, and 183 remained significant after the exclusion of the two outliers whose free thyroxine values were more than four standard deviations above the mean but within the reference range provided by the analytic laboratory for women in their second and third trimester of pregnancy. Associations between free thyroxine and PCBs 99, 146, and 199 almost reached statistical significance (β = −0.11 to −0.07, p = 0.052–0.065). Only PCB 44 remained significantly negatively associated with total thyroxine in full models (β = −0.57, 95 percent CI: −1.07, −0.07). No PCBs were associated with thyroid-stimulating hormone concentrations.
TABLE 3.
Unadjusted and adjusted associations between serum concentrations* of polychlorinated biphenyls and organochlorine pesticides and free thyroxine in pregnant women† (n = 333), Salinas Valley, California, 1999–2000
| Change in free thyroxine concentration (ng/dl) per 10-fold increase in exposure |
||||
| Unadjusted |
Adjusted‡ |
|||
| β | 95% CI§ | β | 95% CI | |
| Organochlorines (ng/g) | ||||
| p,p′-DDT§ | 0.01 | −0.02, 0.05 | 0.02 | −0.01, 0.06 |
| o,p′-DDT | 0.01 | −0.03, 0.06 | 0.03 | −0.01, 0.07 |
| p,p′-DDE§ | −0.01 | −0.06, 0.04 | 0.01 | −0.03, 0.06 |
| Hexachlorobenzene | −0.10 | −0.17, −0.03 | −0.08 | −0.15, −0.01 |
| PCB§ groupings | ||||
| ΣPCBs (ng/g)¶ | −0.16 | −0.28, −0.05 | −0.12 | −0.24, −0.01 |
| Toxic equivalents (pg/g) | −0.12 | −0.22, −0.01 | −0.05 | −0.16, 0.06 |
| Enzyme inducers (ng/g)# | −0.17 | −0.28, −0.05 | −0.09 | −0.20, 0.03 |
| Mono-ortho PCBs (ng/g)** | −0.11 | −0.21, 0.00 | −0.09 | −0.19, 0.01 |
| Wolff's method group 3†† | −0.14 | −0.24, −0.04 | −0.07 | −0.18, 0.04 |
| Individual PCB congeners (ng/g) | ||||
| PCB 18 | −0.07 | −0.15, 0.00 | −0.08 | −0.16, −0.01 |
| PCB 28 | −0.07 | −0.15, 0.01 | −0.08 | −0.16, −0.01 |
| PCB 44 | −0.11 | −0.19, −0.04 | −0.11 | −0.19, −0.03 |
| PCB 49 | −0.08 | −0.16, −0.01 | −0.09 | −0.16, −0.01 |
| PCB 52 | −0.10 | −0.18, −0.02 | −0.10 | −0.18, −0.02 |
| PCB 66 | −0.07 | −0.16, 0.02 | −0.07 | −0.16, 0.02 |
| PCB 74 | −0.05 | −0.16, 0.05 | −0.01 | −0.11, 0.10 |
| PCB 99 | −0.17 | −0.27, −0.07 | −0.11 | −0.21, 0.00 |
| PCB 101 | −0.07 | −0.14, −0.00 | −0.07 | −0.14, −0.00 |
| PCB 118 | −0.11 | −0.21, −0.01 | −0.05 | −0.15, 0.06 |
| PCB 138 | −0.10 | −0.19, −0.01 | −0.04 | −0.13, 0.05 |
| PCB 146 | −0.11 | −0.18, −0.04 | −0.07 | −0.15, 0.00 |
| PCB 153 | −0.12 | −0.21, −0.03 | −0.06 | −0.15, 0.04 |
| PCB 156 | −0.11 | −0.17, −0.04 | −0.06 | −0.13, 0.01 |
| PCB 180 | −0.04 | −0.12, 0.05 | 0.02 | −0.07, 0.11 |
| PCB 183 | −0.14 | −0.21, −0.07 | −0.11 | −0.18, −0.03 |
| PCB 187 | −0.08 | −0.16, −0.01 | −0.04 | −0.12, 0.03 |
| PCB 194 | −0.11 | −0.19, −0.03 | −0.06 | −0.14, 0.02 |
| PCB 199 | −0.11 | −0.18, −0.04 | −0.07 | −0.14, 0.00 |
All polychlorinated biphenyl and organochlorine concentrations are expressed on a lipid basis (ng/g lipids) and were log10-transformed. Toxic equivalents are expressed in pg/g lipids. Free thyroxine is expressed in ng/dl.
One participant with an invalid free thyroxine measurement was dropped.
Results were adjusted for age and prepregnancy body mass index.
CI, confidence interval; DDT, dichlorodiphenyl trichloroethane; DDE, dichlorodiphenyl dichloroethylene; PCB, polychlorinated biphenyl.
Sum for the 19 PCB congeners with a detection frequency greater than 75%.
Enzyme inducers include PCBs 99, 101, 118, 153, 156, 157, 167, 180, 183, 187, 194, 189, and 199.
Mono-ortho PCBs include PCBs 28, 66, 74, 118, 156, 157, 167, and 189.
Group 3 includes PCBs 99, 153, 180, 183, and 196.
TABLE 4.
Unadjusted and adjusted associations between serum concentrations* of polychlorinated biphenyls and organochlorine pesticides and total thyroxine in pregnant women† (n = 333), Salinas Valley, California, 1999–2000
| Change in total thyroxine concentration (μg/dl) per 10-fold increase in exposure |
||||
| Unadjusted |
Adjusted‡ |
|||
| β | 95% CI§ | β | 95% CI | |
| Organochlorines (ng/g) | ||||
| p,p′-DDT§ | 0.08 | −0.16, 0.32 | 0.13 | −0.11, 0.36 |
| o,p′-DDT | 0.12 | −0.16, 0.39 | 0.17 | −0.11, 0.44 |
| p,p′-DDE§ | 0.04 | −0.29, 0.36 | 0.17 | −0.15, 0.49 |
| Hexachlorobenzene | −0.67 | −1.13, −0.20 | −0.51 | −0.97, −0.04 |
| PCB§ groupings | ||||
| ΣPCBs (ng/g)¶ | −0.53 | −1.30, 0.24 | −0.29 | −1.06, 0.47 |
| Toxic equivalents (pg/g) | −0.18 | −0.87, 0.51 | 0.26 | −0.45, 0.96 |
| Enzyme inducers (ng/g)# | −0.86 | −1.60, −0.11 | −0.31 | −1.10, 0.48 |
| Mono-ortho PCBs (ng/g)** | −0.19 | −0.86, 0.48 | −0.13 | −0.78, 0.53 |
| Wolff's method group 3†† | −0.97 | −1.66, −0.29 | −0.51 | −1.23, 0.22 |
| Individual PCB congeners (ng/g) | ||||
| PCB 18 | −0.23 | −0.73, 0.28 | −0.29 | −0.79, 0.20 |
| PCB 28 | −0.20 | −0.71, 0.32 | −0.27 | −0.78, 0.23 |
| PCB 44 | −0.58 | −1.09, −0.06 | −0.57 | −1.07, −0.07 |
| PCB 49 | −0.25 | −0.74, 0.24 | −0.31 | −0.79, 0.18 |
| PCB 52 | −0.38 | −0.90, 0.15 | −0.43 | −0.94, 0.08 |
| PCB 66 | −0.14 | −0.73, 0.45 | −0.17 | −0.75, 0.40 |
| PCB 74 | −0.25 | −0.95, 0.45 | 0.02 | −0.68, 0.71 |
| PCB 99 | −0.67 | −1.36, 0.02 | −0.28 | −0.99, 0.44 |
| PCB 101 | −0.40 | −0.86, 0.07 | −0.43 | −0.88, 0.03 |
| PCB 118 | −0.16 | −0.84, 0.52 | 0.26 | −0.43, 0.95 |
| PCB 138 | −0.42 | −1.01, 0.17 | −0.04 | −0.65, 0.57 |
| PCB 146 | −0.61 | −1.09, −0.14 | −0.34 | −0.83, 0.15 |
| PCB 153 | −0.77 | −1.37, −0.17 | −0.37 | −1.00, 0.26 |
| PCB 156 | −0.42 | −0.86, 0.03 | −0.05 | −0.52, 0.42 |
| PCB 180 | −0.77 | −1.33, −0.20 | −0.42 | −1.01, 0.17 |
| PCB 183 | −0.14 | −0.62, 0.35 | 0.15 | −0.34, 0.64 |
| PCB 187 | −0.41 | −0.90, 0.08 | −0.11 | −0.61, 0.39 |
| PCB 194 | −0.34 | −0.86, 0.18 | 0.07 | −0.48, 0.62 |
| PCB 199 | −0.14 | −0.60, 0.33 | 0.22 | −0.26, 0.70 |
All polychlorinated biphenyl and organochlorine concentrations are expressed on a lipid basis (ng/g lipids) and were log10-transformed. Toxic equivalents are expressed in pg/g lipids. Total thyroxine is expressed in μg/dl.
One participant with an invalid total thyroxine measurement was dropped.
Results were adjusted for age and prepregnancy body mass index.
CI, confidence interval; DDT, dichlorodiphenyl trichloroethane; DDE, dichlorodiphenyl dichloroethylene; PCB, polychlorinated biphenyl.
Sum for the 19 PCB congeners with a detection frequency greater than 75%.
Enzyme inducers include PCBs 99, 101, 118, 153, 156, 157, 167, 180, 183, 187, 194, 189, and 199.
Mono-ortho PCBs include PCBs 28, 66, 74, 118, 156, 157, 167, and 189.
Group 3 includes PCBs 99, 153, 180, 183, and 196.
Although all PCB groupings were significantly associated with free thyroxine in unadjusted models, only ΣPCBs remained significant after inclusion of covariates (β = −0.12, 95 percent CI: −0.24, −0.01). Therefore, a 10-fold increase in ΣPCBs was associated with a 0.12-ng/dl decrease in free thyroxine, corresponding to a 0.15-ng/dl decrement (equivalent to about 0.6 standard deviation units) over the full range of ΣPCBs. This result was no longer significant after exclusion of two outliers whose free thyroxine values were more than four standard deviations above the mean. None of the PCB groupings were associated with total thyroxine or thyroid-stimulating hormone concentrations. Results were similar when we summed the concentrations of all measured PCBs.
Associations of organochlorine pesticides with thyroid hormone concentrations
None of the DDT/DDE isomers were associated with any of the thyroid hormone measurements. Hexachlorobenzene was negatively associated with both total thyroxine and free thyroxine in adjusted models (figure 1; tables 3 and 4) but not with thyroid-stimulating hormone concentrations (β = 0.02, 95 percent CI: −0.05, 0.10). A 10-fold increase in hexachlorobenzene concentration was associated with 0.08-ng/dl (95 percent CI: −0.15, −0.01) and 0.51-μg/dl (95 percent CI: −0.98, −0.04) decreases in free thyroxine and total thyroxine, corresponding to decrements of 0.16 ng/dl and 1.04 μg/dl, respectively (equivalent to approximately 0.7 standard deviation units each) over the full range of hexachlorobenzene values. Excluding outliers did not materially alter these results.
FIGURE 1.
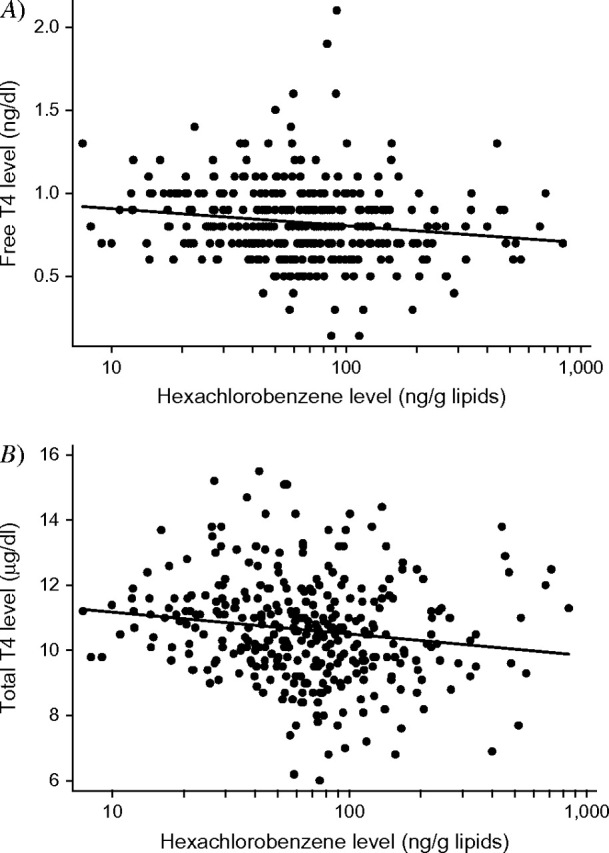
Association between hexachlorobenzene and A) free and B) total thyroxine (T4) concentrations in pregnant women (n = 333), Salinas Valley, California, 1999–2000.
Associations controlling for environmental exposures
Concentrations of lead, dialkyl phosphate metabolites, and organochlorine pesticides other than hexachlorobenzene did not confound any of the above associations. Out of the seven PCB congeners negatively associated with free thyroxine, only associations with PCB 44 (β = −0.09, 95 percent CI: −0.17, −0.01) remained significant after inclusion of hexachlorobenzene in the models. Adjustment for hexachlorobenzene also altered the statistical significance of the association between ΣPCBs and free thyroxine (β = −0.08, 95 percent CI: −0.21, 0.05). Similarly, inclusion of ΣPCBs nullified the significant result for the association of hexachlorobenzene with free thyroxine (β = −0.06, 95 percent CI: −0.13, 0.02) but not for the association with total thyroxine (β = −0.53, 95 percent CI: −1.05, −0.01).
DISCUSSION
We report significant inverse associations between exposure to ΣPCBs (the 19 PCB congeners detected in more than 75 percent of participants) and PCBs 18, 28, 44, 49, 52, 101, and 183 and free thyroxine, as well as between concentrations of the fungicide hexachlorobenzene and both free thyroxine and total thyroxine, in pregnant women. Concentrations of hexachlorobenzene and the less chlorinated PCBs were strongly correlated, and except for associations between PCB 44 and free thyroxine and between hexachlorobenzene and total thyroxine, adjustment for PCBs or hexachlorobenzene caused results to lose statistical significance, making it impossible to tease out their independent effects. None of the exposure measurements were associated with thyroid-stimulating hormone. Furthermore, despite the high concentrations of p,p′-DDT, o,p′-DDT, and p,p′-DDE in this population, we observed no associations between these exposures and thyroid function.
None of the previous studies investigating associations between PCBs or organochlorines and thyroid function in pregnant women controlled for exposure to other environmental chemicals, except for smoking. Our results on PCBs and hexachlorobenzene differ from those obtained in Canadian (31) and Dutch (33) studies, which found no association in pregnant women between free thyroxine concentrations and hexachlorobenzene, 14 individual PCB congeners, ΣPCBs, or the PCB toxic equivalents. Our results may differ from previous findings, because we used the direct equilibrium dialysis method to measure free thyroxine, whereas most previous studies used immunoassays. However, in one smaller study (n = 128) that used equilibrium dialysis, Steuerwald et al. (34) found no association with the sum of 28 PCB congeners. These disparities may also be partly explained by differences in exposure levels: Langer et al. (65) reported negative correlations between the sum of 15 PCB congeners and free thyroxine and total triiodothyronine at lower exposure levels (<530 ng/g lipids) but positive correlations at higher exposure levels. Hexachlorobenzene concentrations in our population were approximately an order of magnitude higher than in the Canadian study; PCB concentrations were similar to those from the Canadian population but approximately 20 and 85 times lower than those in the Dutch and Faroese studies, respectively (66). However, we probably underestimated exposure to dioxin-like compounds, since we did not measure two key PCB congeners (PCBs 126 and 169). We also had no data on polychlorinated dibenzo-p-dioxins or polychlorinated dibenzofurans. Confounding by iodine intake, which was not measured in previous studies, may also have affected our results. It is noteworthy, however, that investigators who reported significant results consistently found negative associations between PCBs, hexachlorobenzene, and thyroid hormone concentrations, suggesting, as our results do, that these exposures may exert a hypothyroidic effect. Negative associations were reported between the PCB toxic equivalents and total thyroxine (33), as well as between hexachlorobenzene, ΣPCBs, and PCBs 138, 153, and 180 and total triiodothyronine concentrations (31).
Studies carried out in animals have lent support to the hypothesis that adverse associations between prenatal exposure to PCBs and neurodevelopment arise at least partly from thyroxine suppression. For example, in rats, injection with thyroxine normalized PCB-induced depression of choline acetyltransferase after prenatal and lactational exposures (67), and hearing loss caused by pre- and perinatal PCB exposure was ameliorated by the administration of thyroxine (68). Similarly, several animal studies found that exposure to hexachlorobenzene decreased thyroxine concentrations and that prenatal exposure to hexachlorobenzene resulted in adverse neurodevelopmental effects (hypoactivity, hyperactivity, and reduced operant behavior efficiency) (13, 69). However, we are aware of no studies that specifically investigated whether adverse neurodevelopmental effects associated with PCB or organochlorine pesticide exposure may be mediated by thyroid hormone disruption.
This study had a number of strengths. First, we used direct equilibrium dialysis to measure free thyroxine in pregnant women, a method which, contrary to widely used immunoassays (47), is not affected by the increase in thyroxine-bound proteins that occurs in pregnancy (48, 49). We were also able to consider the potential confounding effect of a large number of demographic characteristics and environmental exposures. In addition, our population was homogenous in terms of socioeconomic status (low income level and primarily agricultural), country of origin, and race/ethnicity, thereby reducing the likelihood of uncontrolled confounding.
However, similarly to previous studies, we did not have data on maternal autoimmune thyroid disease, which is the most common cause of acquired hypothyroidism (70). Results from NHANES III showed that 17.0 percent (standard deviation, 0.5) of American women were positive for antithyroperoxidase antibodies and that a strong association existed between antithyroperoxidase antibodies and clinical hypothyroidism (odds ratio = 39.7, 95 percent CI: 11.6, 136.1) (71). Given the strength of this association, even a weak association between exposures examined in the present study and antithyroperoxidase antibodies could have substantially confounded, in an undetermined direction, the results of our study, as well as those of previous studies (72). In addition, because of the intercorrelation among PCB congeners and between PCB congeners and hexachlorobenzene, identifying the chemical or combination of chemicals that may affect thyroid hormone concentrations remains a challenge.
In summary, this study was the first to observe a negative association between the sum of PCB congeners and free thyroxine as well as between hexachlorobenzene and either free thyroxine or total thyroxine concentrations in pregnant women. We found the association with ΣPCBs despite a low level of exposure. Hexachlorobenzene concentrations were within the range of non-occupationally exposed populations previously studied but substantially higher than concentrations measured in the general US population (63). The strong correlation between ΣPCBs and hexachlorobenzene, however, hampered our ability to determine their independent associations with thyroid function. Maternal thyroxine crosses the placenta, is associated with fetal thyroxine concentrations before the onset of fetal thyroid function, and continues to reach the fetus throughout pregnancy (73). Both animal and human studies demonstrate that thyroid hormone is essential for normal brain development, and evidence suggests that maternal free thyroxine may be of particular importance in humans (23, 24). Our results therefore suggest that the neurodevelopmental effects of PCBs and/or hexachlorobenzene reported in previous studies may be partly mediated through thyroid hormone disruption, even at background levels of exposure. We plan to examine this hypothesis in an upcoming analysis of neurodevelopment in this birth cohort.
Acknowledgments
This study was made possible by grants from the National Institute for Occupational Safety and Health (RO1 OH007400), the National Institute of Environmental Health Sciences (PO1 ES009605), and the Environmental Protection Agency (RD 83171001). Additional funding was provided by the University of California Institute for Mexico and the United States, the Fonds de la Recherche en Santé du Québec (J. C.), and the Canadian Institutes for Health Research (J. C.).
The authors acknowledge the contributions of Dr. Ira B. Tager (manuscript review and statistical consultation), Alan Ho (manager of the University of California, Berkeley, School of Public Health Biorepository), and Drs. Jerald C. Nelson, R. Bruce Wilcox, and Jon M. Nakamoto (consultations regarding thyroid hormone analyses). They are particularly grateful to the CHAMACOS staff.
This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders or the Centers for Disease Control and Prevention.
Conflict of interest: none declared.
Glossary
Abbreviations
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
- CI
confidence interval
- DDE
dichlorodiphenyl dichloroethylene
- DDT
dichlorodiphenyl trichloroethane
- NHANES III
Third National Health and Nutritional Examination Survey
- PCB(s)
polychlorinated biphenyl(s)
References
- 1.Needham LL, Ozkaynak H, Whyatt RM, et al. Exposure assessment in the National Children's Study: introduction. Environ Health Perspect. 2005;113:1076–82. doi: 10.1289/ehp.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Environment Programme. New York, NY: United Nations Environment Programme; 2001. Final act of the Conference of Plenipotentiaries on the Stockholm Convention on Persistent Organic Pollutants. [Google Scholar]
- 3.Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention. Toxicological profile for polychlorinated biphenyls (PCBs). Atlanta, GA: Centers for Disease Control and Prevention; 2000. p. 948. [PubMed] [Google Scholar]
- 4.Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention. Toxicological profile for hexachlorobenzene. Atlanta, GA: Centers for Disease Control and Prevention; 2002. p. 352. [Google Scholar]
- 5.World Health Organization. WHO Position Statement. Geneva, Switzerland: World Health Organization; 2006. Indoor residual spraying: use of indoor residual spraying for scaling up global malaria control and elimination. [Google Scholar]
- 6.Grandjean P, Weihe P, Burse VW, et al. Neurobehavioral deficits associated with PCB in 7-year-old children prenatally exposed to seafood neurotoxicants. Neurotoxicol Teratol. 2001;23:305–17. doi: 10.1016/s0892-0362(01)00155-6. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996;335:783–9. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson JL, Jacobson SW, Humphrey HE. Effects of in utero exposure to polychlorinated biphenyls and related contaminants on cognitive functioning in young children. J Pediatr. 1990;116:38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson SW, Fein GG, Jacobson JL, et al. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 1985;56:853–60. [PubMed] [Google Scholar]
- 10.Koopman-Esseboom C, Weisglas-Kuperus N, de Ridder MA, et al. Effects of polychlorinated biphenyl/dioxin exposure and feeding type on infants' mental and psychomotor development. Pediatrics. 1996;97:700–6. [PubMed] [Google Scholar]
- 11.Rogan WJ, Gladen BC. PCBs, DDE, and child development at 18 and 24 months. Ann Epidemiol. 1991;1:407–13. doi: 10.1016/1047-2797(91)90010-a. [DOI] [PubMed] [Google Scholar]
- 12.Rogan WJ, Gladen BC, McKinney JD, et al. Neonatal effects of transplacental exposure to PCBs and DDE. J Pediatr. 1986;109:335–41. doi: 10.1016/s0022-3476(86)80397-3. [DOI] [PubMed] [Google Scholar]
- 13.Lilienthal H, Benthe C, Heinzow B, et al. Impairment of schedule-controlled behavior by pre- and postnatal exposure to hexachlorobenzene in rats. Arch Toxicol. 1996;70:174–81. doi: 10.1007/s002040050257. [DOI] [PubMed] [Google Scholar]
- 14.Sala M, Ribas-Fitó N, de Muga LE. Hexachlorobenzene and other organochlorine compounds incorporation to the new-borns and its effects on neonatal neurological development at 6–8 weeks of life. Organohalogen Compd. 1999;44:241–2. [Google Scholar]
- 15.Eskenazi B, Marks AR, Bradman A, et al. In utero exposure to dichlorodiphenyltrichloroethane (DDT) and dichlorodiphenyldichloroethylene (DDE) and neurodevelopment among young Mexican American children. Pediatrics. 2006;118:233–41. doi: 10.1542/peds.2005-3117. [DOI] [PubMed] [Google Scholar]
- 16.Ribas-Fitó N, Torrent M, Carrizo D, et al. In utero exposure to background concentrations of DDT and cognitive functioning among preschoolers. Am J Epidemiol. 2006;164:955–62. doi: 10.1093/aje/kwj299. [DOI] [PubMed] [Google Scholar]
- 17.Ribas-Fitó N, Cardo E, Sala M, et al. Breastfeeding, exposure to organochlorine compounds, and neurodevelopment in infants. Pediatrics. 2003;111:e580–5. doi: 10.1542/peds.111.5.e580. (Electronic article) [DOI] [PubMed] [Google Scholar]
- 18.Porterfield SP. Thyroidal dysfunction and environmental chemicals—potential impact on brain development. Environ Health Perspect. 2000;108(suppl 3):433–8. doi: 10.1289/ehp.00108s3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunn JT. Iodine supplementation and the prevention of cretinism. Ann N Y Acad Sci. 1993;678:158–68. doi: 10.1111/j.1749-6632.1993.tb26119.x. [DOI] [PubMed] [Google Scholar]
- 20.Friedhoff AJ, Miller JC, Armour M, et al. Role of maternal biochemistry in fetal brain development: effect of maternal thyroidectomy on behaviour and biogenic amine metabolism in rat progeny. Int J Neuropsychopharmacol. 2000;3:89–97. doi: 10.1017/S1461145700001863. [DOI] [PubMed] [Google Scholar]
- 21.Auso E, Lavado-Autric R, Cuevas E, et al. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology. 2004;145:4037–47. doi: 10.1210/en.2004-0274. [DOI] [PubMed] [Google Scholar]
- 22.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 23.Pop VJ, Brouwers EP, Vader HL, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 24.Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 25.Smit BJ, Kok JH, Vulsma T, et al. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr. 2000;89:291–5. [PubMed] [Google Scholar]
- 26.van Raaij JA, Frijters CM, van den Berg KJ. Hexachlorobenzene-induced hypothyroidism. Involvement of different mechanisms by parent compound and metabolite. Biochem Pharmacol. 1993;46:1385–91. doi: 10.1016/0006-2952(93)90103-4. [DOI] [PubMed] [Google Scholar]
- 27.Sinjari T, Darnerud PO. Hydroxylated polychlorinated biphenyls: placental transfer and effects on thyroxine in the foetal mouse. Xenobiotica. 1998;28:21–30. doi: 10.1080/004982598239722. [DOI] [PubMed] [Google Scholar]
- 28.Lilienthal H, Weinand-Harer A, Winterhoff H, et al. Effects of maternal exposure to 3,3′,4,4′-tetrachlorobiphenyl or propylthiouracil in rats trained to discriminate apomorphine from saline. Toxicol Appl Pharmacol. 1997;146:162–9. doi: 10.1006/taap.1997.8245. [DOI] [PubMed] [Google Scholar]
- 29.Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention. Toxicological profile for DDT, DDE and DDD. Atlanta, GA: Centers for Disease Control and Prevention; 2002. p. 497. [PubMed] [Google Scholar]
- 30.Goldman M. The effect of a single dose of DDT on thyroid function in male rats. Arch Int Pharmacodyn Ther. 1981;252:327–34. [PubMed] [Google Scholar]
- 31.Takser L, Mergler D, Baldwin M, et al. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ Health Perspect. 2005;113:1039–45. doi: 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koopman-Esseboom C, Morse DC, Weisglas-Kuperus N, et al. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994;36:468–73. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Steuerwald U, Weihe P, Jorgensen PJ, et al. Maternal seafood diet, methylmercury exposure, and neonatal neurologic function. J Pediatr. 2000;136:599–605. doi: 10.1067/mpd.2000.102774. [DOI] [PubMed] [Google Scholar]
- 35.Visser TJ, Kaptein E, van Toor H, et al. Glucuronidation of thyroid hormone in rat liver: effects of in vivo treatment with microsomal enzyme inducers and in vitro assay conditions. Endocrinology. 1993;133:2177–86. doi: 10.1210/endo.133.5.8404669. [DOI] [PubMed] [Google Scholar]
- 36.van Raaij JA, Kaptein E, Visser TJ, et al. Increased glucuronidation of thyroid hormone in hexachlorobenzene-treated rats. Biochem Pharmacol. 1993;45:627–31. doi: 10.1016/0006-2952(93)90136-k. [DOI] [PubMed] [Google Scholar]
- 37.Lubet RA, Dragnev KH, Chauhan DP, et al. A pleiotropic response to phenobarbital-type enzyme inducers in the F344/NCr rat. Effects of chemicals of varied structure. Biochem Pharmacol. 1992;43:1067–78. doi: 10.1016/0006-2952(92)90614-o. [DOI] [PubMed] [Google Scholar]
- 38.Khan MA, Hansen LG. Ortho-substituted polychlorinated biphenyl (PCB) congeners (95 or 101) decrease pituitary response to thyrotropin releasing hormone. Toxicol Lett. 2003;144:173–82. doi: 10.1016/s0378-4274(03)00203-0. [DOI] [PubMed] [Google Scholar]
- 39.Byrne JJ, Carbone JP, Hanson EA. Hypothyroidism and abnormalities in the kinetics of thyroid hormone metabolism in rats treated chronically with polychlorinated biphenyl and polybrominated biphenyl. Endocrinology. 1987;121:520–7. doi: 10.1210/endo-121-2-520. [DOI] [PubMed] [Google Scholar]
- 40.Collins WT, Jr, Capen CC. Fine structural lesions and hormonal alterations in thyroid glands of perinatal rats exposed in utero and by the milk to polychlorinated biphenyls. Am J Pathol. 1980;99:125–42. [PMC free article] [PubMed] [Google Scholar]
- 41.Chauhan KR, Kodavanti PR, McKinney JD. Assessing the role of ortho-substitution on polychlorinated biphenyl binding to transthyretin, a thyroxine transport protein. Toxicol Appl Pharmacol. 2000;162:10–21. doi: 10.1006/taap.1999.8826. [DOI] [PubMed] [Google Scholar]
- 42.Chevrier J, Eskenazi B, Bradman A, et al. Associations between prenatal exposure to polychlorinated biphenyls and neonatal thyroid-stimulating hormone levels in a Mexican-American population, Salinas Valley, California. Environ Health Perspect. 2007;115:1490–6. doi: 10.1289/ehp.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eskenazi B, Bradman A, Gladstone EA, et al. CHAMACOS, a longitudinal birth cohort study: lessons from the fields. J Child Health. 2003;1:3–27. [Google Scholar]
- 44.Hogan JW, Roy J, Korkontzelou C. Handling drop-out in longitudinal studies. Stat Med. 2004;23:1455–97. doi: 10.1002/sim.1728. [DOI] [PubMed] [Google Scholar]
- 45.Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid. 2005;15:44–53. doi: 10.1089/thy.2005.15.44. [DOI] [PubMed] [Google Scholar]
- 46.Nelson JC, Tomei RT. Direct determination of free thyroxin in undiluted serum by equilibrium dialysis/radioimmunoassay. Clin Chem. 1988;34:1737–44. [PubMed] [Google Scholar]
- 47.Wang R, Nelson JC, Weiss RM, et al. Accuracy of free thyroxine measurements across natural ranges of thyroxine binding to serum proteins. Thyroid. 2000;10:31–9. doi: 10.1089/thy.2000.10.31. [DOI] [PubMed] [Google Scholar]
- 48.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 49.Nelson JC, Weiss RM, Wilcox RB. Underestimates of serum free thyroxine (T4) concentrations by free T4 immunoassays. J Clin Endocrinol Metab. 1994;79:76–9. doi: 10.1210/jcem.79.1.8027258. [DOI] [PubMed] [Google Scholar]
- 50.Barr JR, Maggio VL, Barr DB, et al. New high-resolution mass spectrometric approach for the measurement of polychlorinated biphenyls and organochlorine pesticides in human serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:137–48. doi: 10.1016/s1570-0232(03)00451-3. [DOI] [PubMed] [Google Scholar]
- 51.Phillips DL, Pirkle JL, Burse VW, et al. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- 52.Bravo R, Caltabiano LM, Weerasekera G, et al. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. J Expo Anal Environ Epidemiol. 2004;14:249–59. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- 53.Wolff MS, Camann D, Gammon M, et al. Proposed PCB congener groupings for epidemiological studies. Environ Health Perspect. 1997;105:13–14. doi: 10.1289/ehp.9710513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosner B. Percentage points for a generalized ESD many-outlier procedure. Technometrics. 1983;25:165–72. [Google Scholar]
- 55.Singh B, Chandran V, Bandhu HK, et al. Impact of lead exposure on pituitary-thyroid axis in humans. Biometals. 2000;13:187–92. doi: 10.1023/a:1009201426184. [DOI] [PubMed] [Google Scholar]
- 56.Satar S, Satar D, Kirim S, et al. Effects of acute organophosphate poisoning on thyroid hormones in rats. Am J Ther. 2005;12:238–42. [PubMed] [Google Scholar]
- 57.Bondy G, Curran I, Doucet J, et al. Toxicity of trans-nonachlor to Sprague-Dawley rats in a 90-day feeding study. Food Chem Toxicol. 2004;42:1015–27. doi: 10.1016/j.fct.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Matsuura I, Saitoh T, Tani E, et al. Evaluation of a two-generation reproduction toxicity study adding endpoints to detect endocrine disrupting activity using lindane. J Toxicol Sci. 2005 doi: 10.2131/jts.30.s135. 30(spec no.):135–61. [DOI] [PubMed] [Google Scholar]
- 59.Rathore M, Bhatnagar P, Mathur D, et al. Burden of organochlorine pesticides in blood and its effect on thyroid hormones in women. Sci Total Environ. 2002;295:207–15. doi: 10.1016/s0048-9697(02)00094-3. [DOI] [PubMed] [Google Scholar]
- 60.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 61.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–6. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chevrier J, Dewailly E, Ayotte P, et al. Body weight loss increases plasma and adipose tissue concentrations of potentially toxic pollutants in obese individuals. Int J Obes Relat Metab Disord. 2000;24:1272–8. doi: 10.1038/sj.ijo.0801380. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention. Third national report on human exposure to environmental chemicals. Atlanta, GA: Centers for Disease Control and Prevention; 2005. p. 475. [Google Scholar]
- 64.Bradman AS, Schwartz JM, Fenster L, et al. Factors predicting organochlorine pesticide levels in pregnant Latina women living in a United States agricultural area. J Expo Sci Environ Epidemiol. 2007;17:388–99. doi: 10.1038/sj.jes.7500525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langer P, Kocan A, Tajtakova M, et al. Possible effects of persistent organochlorinated pollutants cocktail on thyroid hormone levels and pituitary-thyroid interrelations. Chemosphere. 2007;70:110–18. doi: 10.1016/j.chemosphere.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 66.Longnecker MP, Wolff MS, Gladen BC, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect. 2003;111:65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Juarez de Ku LM, Sharma-Stokkermans M, Meserve LA. Thyroxine normalizes polychlorinated biphenyl (PCB) dose-related depression of choline acetyltransferase (ChAT) activity in hippocampus and basal forebrain of 15-day-old rats. Toxicology. 1994;94:19–30. doi: 10.1016/0300-483x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 68.Goldey ES, Crofton KM. Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats. Toxicol Sci. 1998;45:94–105. doi: 10.1006/toxs.1998.2495. [DOI] [PubMed] [Google Scholar]
- 69.Goldey ES, Taylor DH. Developmental neurotoxicity following premating maternal exposure to hexachlorobenzene in rats. Neurotoxicol Teratol. 1992;14:15–21. doi: 10.1016/0892-0362(92)90024-5. [DOI] [PubMed] [Google Scholar]
- 70.Roberts CGP, Ladenson PW. Hypothyroidism. Lancet. 2004;363:793–803. doi: 10.1016/S0140-6736(04)15696-1. [DOI] [PubMed] [Google Scholar]
- 71.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 72.Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. Am J Epidemiol. 2007;166:646–55. doi: 10.1093/aje/kwm165. [DOI] [PubMed] [Google Scholar]
- 73.de Escobar GM, Obregon MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–48. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]


