Abstract
Adiponectin is reported to have both pro- and anti-inflammatory effects. Because adiponectin circulates in isoforms of various sizes, and some responses to adiponectin are isoform-dependent, it was postulated that the pro-inflammatory effects of adiponectin may isoform-specific. To test this, peripheral blood mononuclear cells (PBMC), microvascular endothelial cells (MVEC), and human glomerular mesangial cells (HMC) were treated with high or low molecular weight (HMW, LMW) recombinant human adiponectin, and chemokine production was measured. The PBMC were isolated from healthy volunteers by density gradient centrifugation of EDTA anticoagulated whole blood through endotoxin-free Ficoll. The MVEC were of dermal origin, and the HMC were isolated from kidneys not suitable for transplantation. Overnight (16 hours) incubation with HMW adiponectin (0.01–1μg/ml for PBMC; 5–20μg/ml for MVEC, HMC) induced a dose-dependent increase in production of monocyte chemoattractant protein-1 and interleukin-8 by PBMC and MVEC, but had no effect on HMC chemokine production (n=3–5). LMW adiponectin at the same concentrations did not induce chemokine production in any of the cell types tested, and did not block cytokine-induced chemokine production by PBMC or MVEC (n=3–5). These in vitro data suggested that the HMW adiponectin isoform is pro-inflammatory. To examine the possibility of a relationship between HMW adiponectin and inflammation in vivo, the urine of patients with systemic lupus erythematosus (SLE) and kidney involvement, previously shown to contain immunoreactive adiponectin, was examined for the presence of specific adiponectin isoforms by non-denaturing gel electrophoresis. HMW adiponectin was found in the urine of patients with active lupus nephritis. Therefore, HMW adiponectin may contribute to the renal inflammation of SLE.
Key Words: Adiponectin, Inflammation, Monocyte Chemotactic Protein-1, Interleukin-8, lupus
Introduction
Adiponectin is a 30 kDa adipocyte-derived cytokine (adipokine) that has been has been implicated in the regulation of metabolic disorders such as obesity and diabetes mellitus [1–7]. In plasma adiponectin circulates at concentrations of 3–20 μg/ml, as homo-trimers, hexamers, and high-molecular weight (HMW) structures [8, 9]. These isoforms are stable in vivo and do not interconvert [10]. The metabolic effects of adiponectin appear to be mediated by two specific receptors, AdipoR1 and R2 [11–14]. R1 signals through activation (i.e. phosphorylation) of AMP-activated protein kinase (AMPK) [15].
The physiologic effects of adiponectin may be isoform-dependant [9, 16]. For example hexamers and HMW isoforms can activate the transcription factor NF-κB, but trimers cannot [9, 16]. Conversely, in skeletal muscle trimers, but not higher order structures induce AMPK [16].
While doing an analysis of the urine proteome in patients with systemic lupus erythematosus (SLE) and glomerulonephritis, we identified adiponectin as one of the most abundant cytokines present in the urine of patients with active SLE nephritis [17]. Plasma adiponectin levels were also significantly higher in patients with SLE nephritis than healthy individuals, or patients with nonrenal SLE [17]. A preliminary longitudinal analysis of patient flare cycles showed that urine adiponectin increased significantly at renal but not nonrenal flare.
These puzzling lupus data were initially difficult to reconcile with the known metabolic effects of adiponectin. However, in addition to metabolic regulation, adiponectin was recently shown to modulate inflammation, an effect that could be relevant to an inflammatory disease such as SLE. Initial studies suggested adiponectin is anti-inflammatory because it blocked proinflammatory cytokine production, decreased leukocyte adherence, and increased production of anti-inflammatory cytokines [18–21]. Several of these studies used bacterially produced recombinant adiponectin that is not representative of the multimeric isoforms found in the human circulation [22]. In contrast, using human recombinant adiponectin prepared in mammalian cells, we and others have shown that adiponectin may actually be pro-inflammatory. It can induce the production of the pro-inflammatory chemokines and cytokines MCP-1, IL-8, IL-1β, TNFα, and IL-6 in vitro in various tissues [23–28]. Interestingly, adiponectin itself can be induced in several cell types by cytokines known to be expressed in SLE nephritis, such as interferon-γ and TNFα, suggesting production is not restricted to adipose tissue [29–32].
Because circulating adiponectin is comprised of different size isoforms, and there is evidence that at least some of its effects are isoform-specific [16, 33, 34], we postulated that the pro-inflammatory effects of adiponectin are size-dependent. Specifically, we postulated that the HMW isoforms, but not LMW adiponectin induce the expression of the pro-inflammatory chemokines MCP-1 and IL-8. This hypothesis seemed reasonable, given the fact that HMW, but not LMW adiponectin can active NF-κB, and both MCP-1 and IL-8 are NF-κB-dependent [35]. The present study was undertaken to test this hypothesis. Furthermore, because MCP-1 and IL-8 are involved in the pathogenesis of kidney injury during lupus nephritis [36], we sought in vivo evidence that pro-inflammatory, HMW adiponectin was present in the kidneys of patients with active SLE nephritis.
Methods
Cell culture and treatments
Human peripheral blood mononuclear cells (PBMC) were isolated from healthy volunteers by density gradient centrifugation of EDTA anti-coagulated whole blood through endotoxin-free Ficoll-Paque Plus (Amerhsam, Arlington Heights, IL). PBMCs (5 X 106) were plated in RPMI 1640 overnight to allow monocytes to adhere. After this incubation, the cultures were washed 3 times with fresh RPMI 1640 to remove non-adherent lymphocytes and non-viable cells. Monocytes account for almost all of the chemokine production by PBMC [37].
Immortalized human dermal microvascular endothelial cells (MVECs) were created at the Department of Dermatology, Emory University [38], and were a gift of Dr. X.L. Chen, Atherogenics Inc., Alpharetta, Georgia. The MVEC were cultured in EGM-2 basal medium (Lonza Walkersville Inc., Walkersville, MD) supplemented with EGM SingleQuot (Cambrex, Rockland, ME) containing 2% fetal bovine serum.
Human mesangial cells (HMC) were cultured from adult kidneys not suitable for transplantation, and were characterized as previously described [39]. Cells were used between passages 4 and 7, and were grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum (GIBCO, Grand Island, NY). All cells were maintained at 37°C under 5% CO2.
Cells were exposed to 0.01–20 μg/ml of recombinant human full-length high molecular weight (HMW) adiponectin, or trimeric, low molecular weight (LMW) adiponectin (BioVendor, Candler, NC) for 16 hours. The concentrations of adiponectin were originally chosen to reflect human plasma concentrations (5–20 μg/ml). However PBMC were very sensitive to adiponectin, and the concentrations were reduced for these cells to demonstrate a dose-response relationship. LMW and HMW adiponectin were produced in human embryonal kidney cells. The full-length adiponectin is enriched in HMW isoforms (78% HMW plus hexamer), but does contain about 22% of the LMW isoform. In the LMW form alanine is substituted for cysteine at position 39, allowing it to form trimers but not higher molecular weight isoforms [40]. The adiponectin preparations demonstrated the appropriate bands on gel electrophoresis and immunoblotting (data not shown). Adiponectin did not affect the viability of any of the cell types used, as assessed by the LDH release (Roche Diagnostics, Mannheim, Germany). Per the manufacturer, endotoxin was removed from both preparations with Detoxi-gel endotoxin removal reagent. Endotoxin levels of the HMW and LMW were similar and ≤ 0.5 EU/μg protein, measured with the Limulus Amebocyte Lysate Pyrochrome Kit (Associates of Cape Cod, East Falmouth, Massachusetts).
In some experiments cells were treated with 1.1 ng/ml human recombinant interleukin-1β (IL-1β, R and D Systems, Minneapolis, MN) to induce chemokine production.
Non-denaturing gel electrophoresis and immunoblotting for adiponectin
Urine and plasma samples from patients with SLE nephritis or healthy controls were obtained from our internal review board-approved Ohio SLE Study specimen bank [17]. Adiponectin isoforms in urine and plasma were identified by protein electrophoresis run under non-denaturing, non-reducing conditions. Briefly, 15μl of fresh urine or 2 μl plasma was mixed with 15μl or 38μl of Novex® Native Tris-Glycine Sample Buffer (Invitrogen, Carlsbad, CA) respectively, and 20μl of this mix was directly loaded on 3 –8% Tris-Acetate gels (Invitrogen) with Novex® Tris-Glycine native running buffer (Invitrogen). Proteins were transferred to membranes, and immunoblotting was done with goat antihuman adiponectin (AF1065, R&D Systems) at a concentration of 0.2μg/ml. To ensure specificity, nonimmune goat IgG was used in place of the primary antibody for some blots (not shown). Furthermore, to ensure that all the identified bands were adiponectin isoforms, 2 μg/ml recombinant human adiponectin was added to the incubation as a competitor in some experiments. Blots were developed with an enhanced chemiluminescence system (Amersham).
ELISAs
Human IL-8 was measured in the cell culture supernatants using the Hu IL-8 Cytoset kit from Biosource (Camarillo, CA). Human MCP-1 was determined with the BD OptEIA™ Human MCP-1 ELISA Set from BD Bioscience (San Diego, CA). In each experiment measurements were made in duplicate from three independent cultures. Chemokine levels were normalized to the amount of cell protein in the individual wells. Total cell protein was measured using the DC protein assay (Bio-Rad, Hercules, CA).
Plasma and urine adiponectin were measured by specific ELISA according to manufacturer’s directions (R&D Systems, Minneapolis, MN). The lower range of the assay was 3.9 ng/ml of adiponectin. Urine and plasma values below this were considered undetectable and assigned a value of zero. Urine adiponectin levels were standardized to urine creatinine measured in the same spot urine and expressed as ng/mg creatinine.
Measurement of adiponectin receptor expression and function
The partial cDNAs of adiponectin receptors R1 and R2 (GenBank accession numbers BC001594 and NM_024551, respectively) were amplified using SuperScript One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen) from total RNA extracted from cultured HMC, MVEC, or PBMC. Total RNA was treated with DNase I following the manufacture’s protocol before each RT-PCR reaction. The primers were designed to cross introns to indicate any contamination by DNA in the total RNA. The primer sequences were as follows:
| R1 924bp | Forward: 5′ CCTTTCCCCAAGCTGAAGCTGC 3′ |
| Reverse: 5′ CCTTGACAAAGCCCTCAGCGAT 3′ | |
| R2 446bp | Forward: 5′ AACGAGCCAACAGAAAACCGATTG 3′ |
| Reverse: 5′ CAAATGTTGCCTGTTTCTGTGTGTAT 3′ |
To test the function of R1 and R2, cultured HMC, MVEC or PBMC were treated with adiponectin, and the phosphorylation of AMP-kinase (AMPK) was measured. As a positive control, cells were also treated with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR, Sigma, St. Louis, MO), which directly activates AMPK. Total cell protein lysate (5 to 15μg) was loaded into each lane of 4–12% NuPage Bis-Tris gel (Invitrogen) and immunoblotted for phospho-AMPK and actin (I-19, Santa Cruz, Santa Cruz, CA). The primary antibody for phospho-AMPK-α was polyclonal antibody from Cell Signaling (Beverly, MA), used at a concentration of 1.0 μg/ml.
Statistical analysis
Data are presented as the mean ± standard error of the mean. Differences between 3 or more groups were verified by ANOVA, and specific groups compared by the Bonferroni post-hoc test. A P < 0.05 was considered significant. Statistical analysis was performed using GraphPad InStat version 3.06 from GraphPad Software, San Diego, CA.
Results
Adiponectin Isoforms and Chemokine Production In Vitro
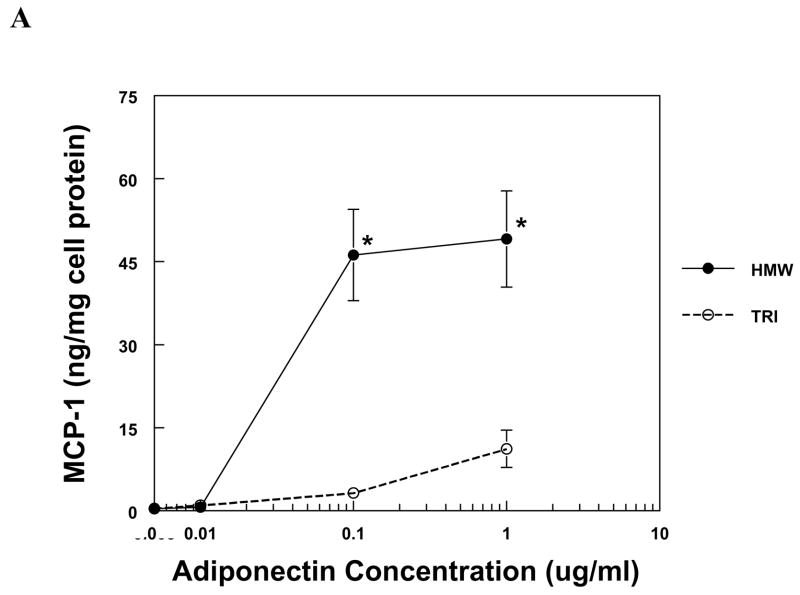
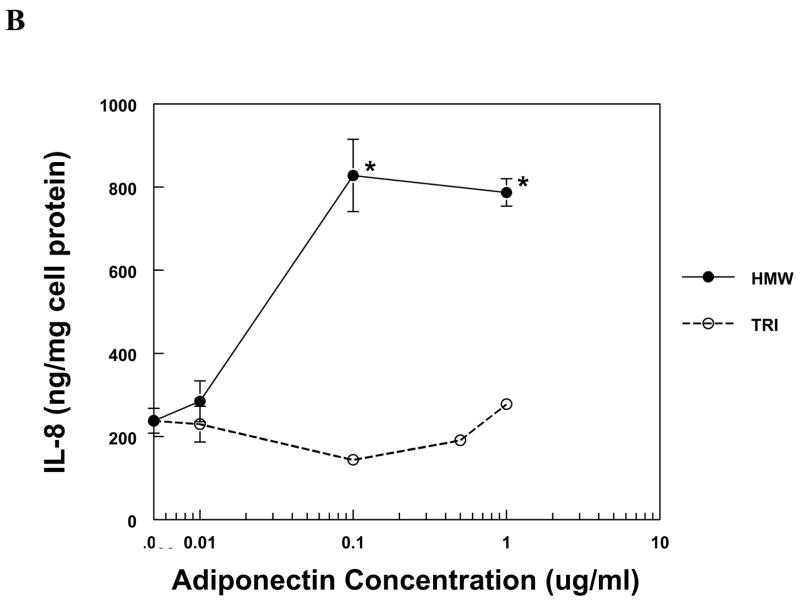
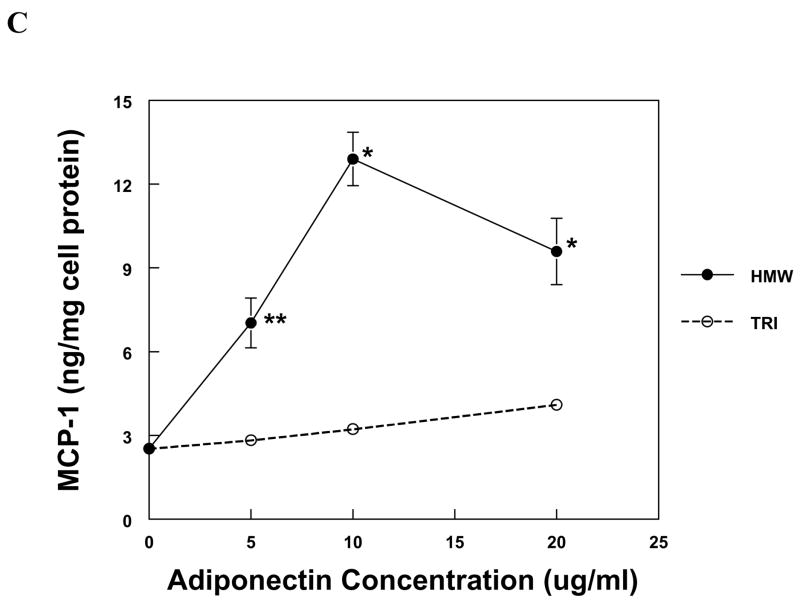
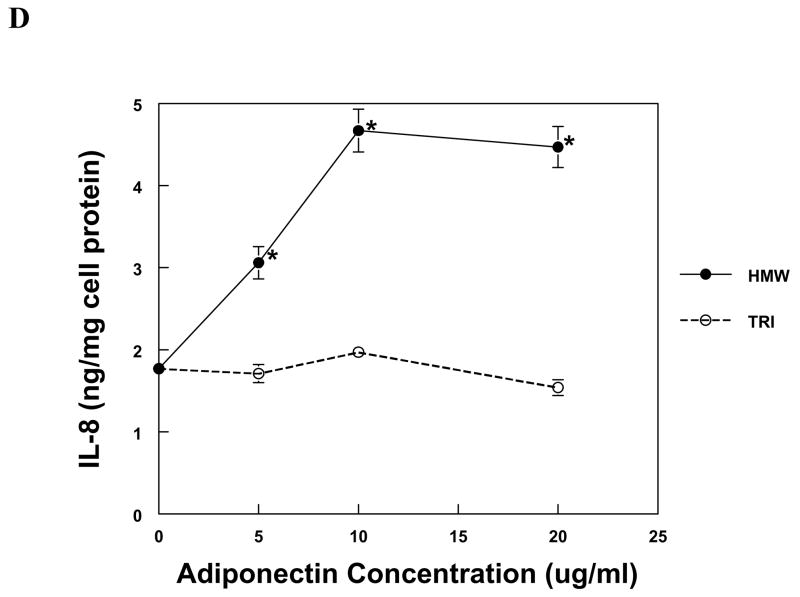
The production of IL-8 and MCP-1 by PBMC and MVEC was induced by HMW adiponectin in a dose-dependent fashion (Fig. 1). PBMC were more sensitive to adiponectin than MVEC, responding robustly to 50-fold lower concentrations of HMW adiponectin than MVEC. In contrast, trimeric LMW adiponectin was unable to increase the expression of IL-8 or MCP-1 in either cell type at the same concentrations as HMW adiponectin (Fig. 1). Neither HMW nor LMW adiponectin induced chemokine expression in human kidney mesangial cells, but the cells were functional because they responded to IL-1β with a significant increase in both IL-8 and MCP-1 production (not shown).
Figure 1. Chemokine production by PBMC and MVEC in response to different isoforms of adiponectin.
PBMC (a, b) or MVEC (c, d) were untreated (control), or treated with the indicated concentrations of HMW or LMW (tri) adiponectin for 16 hours. Supernatants were harvested and supernatant concentrations of MCP-1 or IL-8 were measured by specific ELISA. Each figure is representative of 3–5 independent experiments done in triplicate. Using PBMC, HMW adiponectin significantly increased MCP-1 and IL-8 compared to control at concentrations of 0.1 and 1μg/ml (*P < 0.001). LMW adiponectin did not increase MCP-1 or IL-8 production by PBMC at any concentration tested. Using MVEC, HMW adiponectin significantly increased MCP-1 compared to control at concentrations of 5 μg/ml (**P < 0.01), 10 and 20 μg/ml (*P < 0.001), and increased IL-8 compared to control at concentrations of 5, 10 and 20 μg/ml (*P < 0.001). LMW adiponectin did not increase MCP-1 or IL-8 production by MVEC at any concentration.
Because of the difference in the molecular masses of HMW and LMW adiponectin, the molar concentration of LMW adiponectin was higher than that of HMW adiponectin in the experiments described in Figure 1. To exclude the possibility that a lower dose of the LMW isoform is necessary for chemokine induction, MVECs were incubated with the trimer at concentrations between 0.5 and 4 μg/ml and supernatant MCP-1 and IL-8 were measured. There was no effect of LMW adiponectin on chemokine expression at any tested concentration (data not shown). In these experiments HMW adiponectin (10 μg/ml) was used as a positive control, and significantly increased MCP-1 and IL-8, as expected.
LMW Adiponectin and Cytokine-Induced Chemokine Expression
LMW adiponectin was tested to determine if this isoform could block IL-1β-induced chemokine expression by PBMC and MVEC. In PBMC, IL-1β (1.1ng/ml) increased MCP-1 production 6-fold, but this was unaffected by LMW adiponectin (0.5 μg/ml) added one hour before IL-1β, or at the same time as IL-1β. Similarly, IL-1β (1.1 ng/ml) caused an 8–15-fold increase in IL-8 production by MVEC that was unaffected by preincubation or simultaneous addition of LMW adiponectin at concentrations of 5 and 10 μg/ml.
Adiponectin Receptors as Mediators of the Pro-Inflammatory Effects of Adiponectin
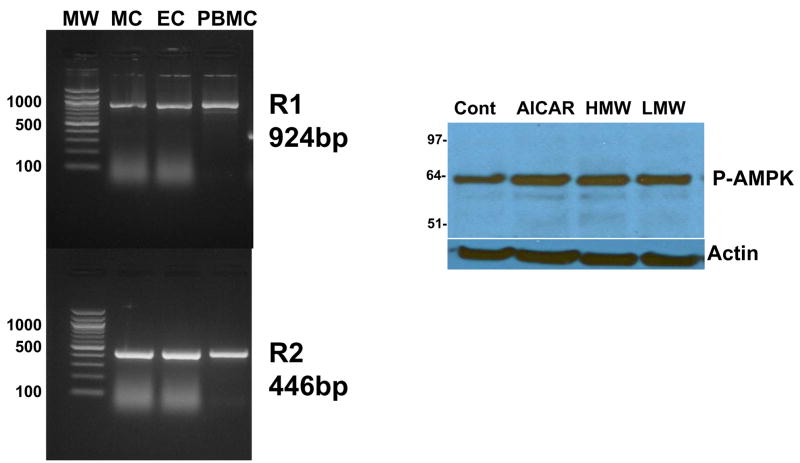
To determine if the pro-inflammatory effect of HMW adiponectin could be mediated by Adipo R1 or R2, we first investigated whether PBMC, MVEC, or HMC express Adipo R1 or R2. The mRNAs for Adipo R1 and R2 were found in each cell type under unstimulated conditions (Fig. 2), but Adipo R1 and R2 protein could not be demonstrated by immunoblotting (not shown). Thus indirect evidence for functional receptors was sought by examining whether adiponectin could activate AMPK. As shown in Figure 2, both HMW adiponectin and LMW increased phosphorylation of AMPK in MVEC to a small extent. In 4 experiments the fold-increase in incubation with HMW adiponectin increased phospho-AMPK 1.20±0.12 fold, and LMW adiponectin increased phospho-AMPK by 1.22±0.12 fold, neither of which were significantly different than control. The positive control AICAR increased phospho-AMPK 1.72±0.17 fold (p < 0.05 compared to control). In PBMC AMPK was expressed at very low to undetectable levels at baseline, and treatment with AICAR, HMW, or LMW adiponectin did not induce phospho-AMPK expression. In HMC phospho-AMPK was robustly expressed at baseline, but also was not increased by HMW or LMW adiponectin.
Figure 2. Adiponectin receptor expression in PBMC, MVEC, and HMC.
The left panel demonstrates the presence of adiponectin receptor R1 and R2 mRNA in resting PBMC, HMC (MC), and MVECs (EC) at the expected sizes of 924 and 446 bases respectively, by RT-PCR. MW indicates molecular weight markers in base pairs. The right panel shows the phosphorylation of AMPK after treatment with AICAR (1 mM, positive control), or HMW and LMW adiponectin both at 10 μg/ml for 10 minutes. The blot was reprobed for actin to demonstrate equivalent loading. One of 4 experiments.
Adiponectin Isoforms in Urine
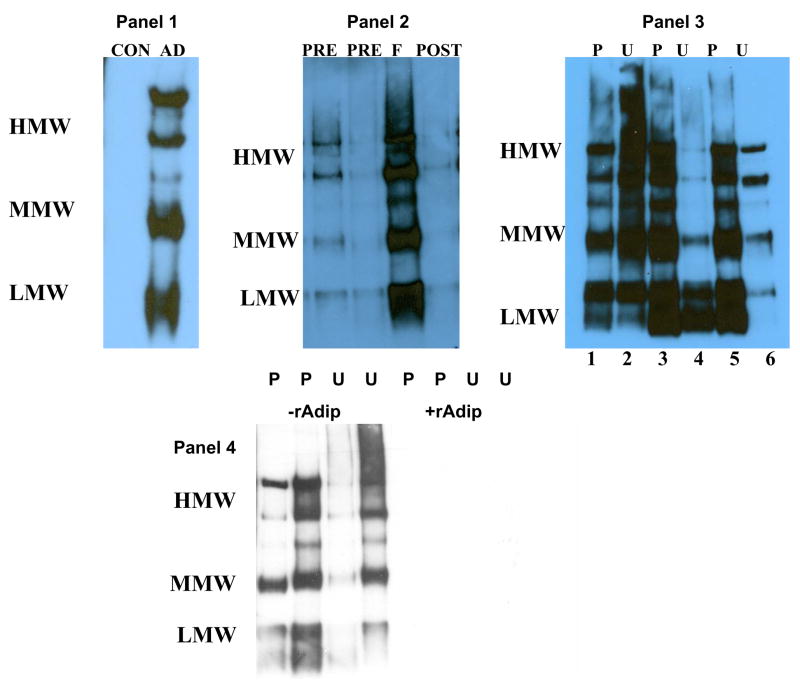
We previously found that adiponectin, which is not normally present in the urine of healthy individuals, appears in the urine of patients with SLE who develop nephritis flares [17]. This is of particular interest given our in vitro data that HMW adiponectin may be pro-inflammatory, and suggests a potential relationship between adiponectin and kidney inflammation in lupus. In our previous work immunoreactive adiponectin was identified in urine after samples had been reduced and denatured, which destroys the multimeric structure and reduces adiponectin to its 30 kDa monomeric form [17]. Therefore the urine of patients with SLE nephritis was examined to determine whether kidney parenchyma is exposed to intact HMW and MMW multimers in vivo during flares. This question is relevant because HMW and MMW adiponectin are large, and may not be filtered even by the inflamed glomeruli of lupus nephritis. As shown in Figure 3, the urine adiponectin isoform profile was found to be similar to that of plasma in several (n=6) flaring lupus patients, and contained intact HMW, MMW and LMW adiponectin. In 4 patients urine and plasma adiponectin levels were found to be 390 ± 126 ng/mg creatinine and 32.8 ± 12.6 μg/ml. In contrast, adiponectin was not detected in the urine of healthy individuals, and higher molecular weight isoforms were less prominent when lupus nephritis was not flaring, even if patients were still significantly proteinuric (Fig. 3). All of the plasma and urine adiponectin bands were completely abolished in the presence of recombinant human adiponectin, demonstrating that these bands were all specific for isoforms of adiponectin (Fig. 3).
Figure 3. Adiponectin isoforms found in the urine and plasma of patients with SLE nephritis.
Urine or plasma proteins from 3 healthy individuals and 6 patients with lupus nephritis were separated on non-reducing, non-denaturing gels to preserve native protein structure, and were immunoblotted for adiponectin. The high, middle (hexamer), and low molecular weight adiponectin isoforms are labeled HMW, MMW, LMW, respectively. In panel 1, CON indicates a urine sample from a healthy individual (representative of 3), and AD represents the adiponectin isoforms produced by human fat as a reference. Panel 2 shows urine adiponectin isoforms during a lupus nephritis flare cycle. The samples were from four and two months before flare (PRE), at renal flare (F), and 4 months after flare (POST). Panel 3 shows plasma (P) and urine (U) adiponectin isoforms from two lupus patients during renal flare (patient 1 lanes 1, 2; patient 2 lanes 5, 6). Lanes 3 and 4 are from a third SLE patient 10 months after a renal flare was diagnosed. The patient was improving but still had over 5 grams of urine protein/day. Panel 4 shows the plasma (P) and urine (U) of two patients blotted for adiponectin in the absence and presence of human recombinant adiponectin (rAdip).
Discussion
Adiponectin has been reported to have both pro- and anti-inflammatory effects. To determine if these opposing findings could be explained by differential effects of adiponectin isoforms, this study examined chemokine induction by HMW and LMW adiponectin isoforms. Using primary human PBMC and a microvascular endothelial cell line, HMW but not LMW adiponectin was found to dose-dependently increase the production of IL-8 and MCP-1, two chemokines critical for tissue inflammation. Thus the HMW isoform of adiponectin appears to be pro-inflammatory, at least in vitro.
Adiponectin’s pro-inflammatory effect could not be generalized to all cell types. Primary human mesangial cells did not respond to either isoform with a change in chemokine production. The reason for this is not clear, but could be explained if the pro-inflammatory effects are mediated by a specific receptor that is not present on HMC.
The receptors that do transduce the pro-inflammatory effects of HMW adiponectin are not known. Because the mRNA for adiponectin receptors R1 and R2 is expressed in both responsive (MVEC, PBMC) and non-responsive cell types (HMC), it is less likely that they mediate inflammatory cytokine production. Furthermore, the putative R1 signal transduction pathway through AMPK was not activated by HMW or LMW adiponectin in PBMC, despite the responsiveness of PBMC to HMW adiponectin. Additionally, although there was a trend, albeit not-significant, toward increased phosphorylation of AMPK in MVEC, the lack of differential activation of R1 by the HMW and LMW isoforms further suggests R1 is not the pro-inflammatory receptor. R2 is less likely to be involved in mediating the pro-inflammatory effects of HMW adiponectin because it signals through PPARα, a pathway felt in general to be anti-inflammatory [41]. T-cadherin, which binds only HMW adiponectin [42], is a potential candidate for the pro-inflammatory receptor, but because it is GPI-linked it would likely need a partner for pro-inflammatory signal transduction.
Our data are consistent with recent studies showing that HMW adiponectin induces the pro-inflammatory cytokines IL-8 and IL-6 in human macrophages, monocytes, and the THP-1 monocytic cell line [24, 28, 43]. Conversely, LMW (trimeric) adiponectin has been shown to block endotoxin-induced IL-6 secretion from THP-1 cells, and to stimulate the secretion of the anti-inflammatory cytokine IL-10 [28]. Endotoxin-mediated IL-6 production was also attenuated by LMW adiponectin in primary human monocytes, but only by about 20–30% [43]. Interestingly, in our hands LMW adiponectin did not block IL-1β-induced expression of MCP-1 or IL-8 by PBMC or MVEC. The reason for this discrepancy is not clear, however the recombinant LMW form that demonstrated anti-inflammatory activity was produced in insect cells in contrast to the mammalian-derived LMW trimer used in the current study.
The potential relevance of these data to inflammation in vivo is currently speculative, but examination of adiponectin isoforms in the urine of patients with SLE nephritis suggests an interesting possibility. As shown, in patients with SLE and a history of kidney involvement, all of the isoforms of adiponectin found in plasma can also be found in urine specifically during nephritis flares. Although the renal vasculature is routinely exposed to HMW, MMW, and LMW adiponectin, the renal parenchyma is not. It is conceivable that during flare, HMW adiponectin present in the renal parenchyma could interact with infiltrating monocytes and augment the production of pro-inflammatory cytokines and chemokines, thus worsening kidney inflammation. Pro-inflammatory adiponectin may also be relevant in other kidney diseases. For example, although isoforms were not measured, adiponectin was shown to be excreted in the urine of patients with type 2 diabetes mellitus and overt nephropathy [44]. Importantly, the chemokine MCP-1 has been implicated in the pathogenesis of diabetic nephropathy [45, 46].
In summary, this work is novel because it demonstrates that the pro-inflammatory effects of adiponectin are specifically due to its HMW isoform. Furthermore, HMW adiponectin is found in the urine of patients during active lupus nephritis. Normally, HMW adiponectin is sequestered from interacting with the renal interstitium. Thus, in SLE nephritis HMW adiponectin may be involved in the pathogenesis and/or maintenance of renal inflammation. Finally, LMW adiponectin of human origin does not seem to affect inflammatory cytokine production, at least in the cell types tested. These findings may be relevant to the regulation of inflammation in vivo, particularly in organs or tissues normally protected from interaction with HMW adiponectin.
Acknowledgments
This work was supported in part through the NIH-NIDDK grant DK074661
Abbreviations
- SLE
systemic lupus erythematosus
- PBMC
peripheral blood mononuclear cells
- MVEC
microvascular endothelial cells
- HMC
human mesangial cells
- HMW
high molecular weight
- LMW
low molecular weight
- IL-1β
interleukin-1β
- MCP-1
monocyte chemoattractant protein-1
- IL-8
interleukin-8, AMPK, AMP-kinase
- AICAR
5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside
Footnotes
Disclosure information: The authors have nothing to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specifice collagen-like factor, apM1. Biochem Biophys Res Comm. 1996;221:286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 2.Diez JJ, Iglesias P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endo. 2003;148:293–300. doi: 10.1530/eje.0.1480293. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Fruebis J, Tsao T-S, Javorschi S, Ebbetts-Reed D, Erickerson MRS, Yen FT, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005–10. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schalkwijk CG, Chaturvedi N, Schram MT, Fuller JH, Stehouwer CDA. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endo Metab. 2006;91:129–35. doi: 10.1210/jc.2005-1117. [DOI] [PubMed] [Google Scholar]
- 7.Gueere-Millo M. Adiponectin: An Update. Diabetes Metab. 2008;34:12–8. doi: 10.1016/j.diabet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Comm. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Tsao T-S, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kB signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–62. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 10.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endo. 2005;153:409–17. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate anitdiabetic metabolic effects. Nature. 2003;423:762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Zhang H, Jia Y, Zhang Z, Craig R, Wang X, et al. Adiponectin receptor 1 gene as a candidate for type 2 diabetes and insulin resistance. Diabetes. 2004;53:2132–6. doi: 10.2337/diabetes.53.8.2132. [DOI] [PubMed] [Google Scholar]
- 13.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endo Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett. 2008;582:74–80. doi: 10.1016/j.febslet.2007.11.070. [DOI] [PubMed] [Google Scholar]
- 15.Viollet B, Mounier R, Leclerc J, Yazigi A, Foretz M, Andreelii F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33:395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Tsao T-S, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. J Biol Chem. 2003;278:50810–7. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 17.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, et al. Plasma, urine, and renal expresssion of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–33. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 18.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in procine macrophages. Biochem Biophys Res Comm. 2004;316:924–9. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 19.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules. Circ. 1999;100:2473–6. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 20.Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochem Biophys Res Comm. 2004;314:415–9. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 21.Kumada M, Kihara S, Noriyuki O, Kobayashi H, Okamoto Y, Ohashi K, et al. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circ. 2004;109:2046–9. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 22.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–49. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 23.Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006;120:99–105. doi: 10.1016/j.clim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Saijo S, Nagata K, Nakano Y, Tobe T, Kobayashi Y. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Comm. 2005;334:1180–3. doi: 10.1016/j.bbrc.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Lappas M, Permezel M, Rice GE. Leptin and adiponectin stimulate the release of proinflammatory cytokines and prostaglandins from human placenta and maternal adipose tissue via nuclear factor-kB, peroxisomal proliferator-activated receptor-gamma, and extracellularly regulated kinase 1/2. Endocrinology. 2005;146:3334–43. doi: 10.1210/en.2005-0406. [DOI] [PubMed] [Google Scholar]
- 26.Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochimica et Biophysica Acta. 2006 doi: 10.1016/j.bbadis.2006.06.008. in press. [DOI] [PubMed] [Google Scholar]
- 27.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, et al. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–78. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 28.Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, Scholmerich J, et al. Different effects of adiponectin isoforms in human moncytic cells. J Leuk Biol. 2006;79:803–8. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 29.Delaigle AM, Jonas J-C, Bauche IB, Cornu O, Brichard SM. Induction of adipokines in skeletal muscle by inflammatory cytokines: in Vivo and in Vitro studies. Endocrinology. 2004;145:5589–97. doi: 10.1210/en.2004-0503. [DOI] [PubMed] [Google Scholar]
- 30.Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: A new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006;54:2295–9. doi: 10.1002/art.21944. [DOI] [PubMed] [Google Scholar]
- 31.Takemura Y, Osuga Y, Yamauchi T, Kobayashi M, Harada M, Hirata T, et al. Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology. 2006;147:3203–10. doi: 10.1210/en.2005-1510. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Yu F, Saegusa S, Sukmino H, Nakahashi T, Kunimitsu I, et al. Impaired expression of cardiac adiponectin in leptin-deficient mice with viral myocarditis. Int Heart J. 2006;47:107–23. doi: 10.1536/ihj.47.107. [DOI] [PubMed] [Google Scholar]
- 33.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/Adiponectin. J Biol Chem. 2003;278:9073–85. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 34.Haugen F, Drevon CA. Activation of nuclear factor-kB by high molecular weight and globular adiponectin. Endocrinology. 2007;148:5478–86. doi: 10.1210/en.2007-0370. [DOI] [PubMed] [Google Scholar]
- 35.Rovin BH. Chemokines as therapeutic targets in renal inflammation. Am J Kid Dis. 1999;34:761–4. doi: 10.1016/S0272-6386(99)70406-3. [DOI] [PubMed] [Google Scholar]
- 36.Rovin BH. The chemokine network in systemic lupus erythematosis nephritis. Frontiers in Bioscience. 2007;13:904–22. doi: 10.2741/2731. [DOI] [PubMed] [Google Scholar]
- 37.Marsh CB, Wewers MD, Tan LC, Rovin BH. FcgammaR cross-linking induces peripheral blood mononuclear cell MCP-1 expression: Role of lymphocyte FcgammaR III. J Immunol. 1997;158:1078–84. [PubMed] [Google Scholar]
- 38.Xu Y, Swerlick RA, Sepp N, Boss D, Ades EW, Lawley TJ. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1) J Invest Dermatol. 1994;102:833–7. doi: 10.1111/1523-1747.ep12382086. [DOI] [PubMed] [Google Scholar]
- 39.Rovin BH, Yoshiumura T, Tan L. Cytokine induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J Immunol. 1992;148:2148–53. [PubMed] [Google Scholar]
- 40.Wang Y, Lam KSL, Xu JY, Lu G, Xu LY, Cooper GJS, et al. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341–7. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 41.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 42.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao T-S, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA. 2004;101:10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schober F, Neumeier M, Weigert J, Wurm S, Wanninger J, Schaffler A, et al. Low molecular weight adiponectin negatively correlates with the waist circumference and monocytic IL-6 release. Biochem Biophys Res Comm. 2007;361:968–73. doi: 10.1016/j.bbrc.2007.07.106. [DOI] [PubMed] [Google Scholar]
- 44.Koshimura J, Fujita H, Narita T, Shimotomai T, Hosoba M, Yoshioka N, et al. Urinary adiponectin excretion is increased in patients with overt diabetic nephropathy. Biochem Biophys Res Comm. 2004;316:165–9. doi: 10.1016/j.bbrc.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 45.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Frontiers in Bioscience. 2008;13:944–55. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 46.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol. 2008;294:F697–F701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]