Abstract
Coloboma, an ocular birth defect seen in humans and other species, is caused by incomplete closure of the optic fissure. Here, we demonstrate that genetic deletion of Lrp6, a bottleneck coreceptor in the canonical Wnt signaling pathway, results in ocular coloboma and neuroretinal patterning defects in mice. The expression of ventral neuroretinal patterning gene Vax2 was conserved but with dorsally shifted expression domains; however, the dorsal neuroretinal patterning gene Tbx5 was lost in the Lrp6-mutant eyes at embryonic day 10.5. Both Bmp4 and phosphorylated Smad 1/5/8 were also significantly attenuated in the dorsal neuroretina. In addition, the retinoic acid synthesizing enzymes Raldh1 and Raldh3 were significantly changed in the mutant eyes. Our findings suggest that defective retinal patterning causes coloboma in the Lrp6-deficient mice, and that canonical Wnt signaling plays a primary role in dorsal neuroretinal patterning and related morphogenetic movements by regulation of both Bmp and retinoic acid signaling pathways.
Keywords: Lrp6, dorsoventral neuroretinal patterning, coloboma, Wnt, Bmp, retinoic acid, Raldh1, Raldh3, Tbx5, Vax2
Introduction
Coloboma, an ocular birth defect caused by incomplete closure of the optic fissure, has a prevalence in humans of 1 in 10,000 (Stoll et al., 1997). The processes that occur during vertebrate eye development are well documented and include formation of the optic vesicle with the subsequent invagination of the ventral surface of the optic vesicle and stalk, leading to the formation of a double-layered optic cup (Chow and Lang, 2001). This invagination gives rise to the optic fissure. Fusion of the edges of the optic fissure starts around embryonic day (E) 10.5–E11.5 in the mouse eye, and failure of optic fissure fusion results in coloboma (Gregory-Evans et al., 2004). Multiple signaling pathways (Shh, Bmp, Fgf, retinoic acid [RA]) and a variety of downstream transcription factors are involved in regulation of optic cup formation and optic fissure closure (Chow and Lang, 2001; Martinez-Morales et al., 2004; Yang, 2004; Matt et al., 2005; Molotkov et al., 2006). Mutations in several genes and signaling molecules have been associated with coloboma (Gregory-Evans et al., 2004; Molotkov et al., 2006). Coloboma may be accompanied by other congenital malformations. An example is the CHARGE syndrome, a multiple congenital disorders condition, that is characterized by ocular coloboma, microphthalmia, cleft palate and choanal atresia, heart defects, retarded growth and development, and ear defects (Sanlaville and Verloes, 2007).
Canonical Wnt/β-catenin signaling plays crucial roles in multiple developmental processes (Logan and Nusse, 2004; Nusse, 2005), particularly in the developing eyes (de Iongh et al., 2006). However, the role of Wnt signaling in early neuroretinal patterning and coloboma remains unknown. Three pathways are activated by Wnts: the β-catenin–dependent canonical Wnt pathway, the Wnt/Ca2+ pathway, and the planar cell polarity (PCP) pathway (Gordon and Nusse, 2006). The canonical Wnt pathway regulates the ability of β-catenin to activate the transcription of target genes. At the cell surface, Wnt ligands bind to the Fzd receptors and Lrp5/6 (low density lipoprotein receptor-related protein 5 or 6) coreceptors (He et al., 2004). Recruitment of Lrp5/6 appears to be restricted to the canonical Wnt pathway. Strong genetic evidence suggests that Lrp6 plays a critical role in canonical Wnt signaling (Pinson et al., 2000).
Microphthalmia, a reduced overall size of the eyes that may occur with coloboma, has been described in Lrp6-knockout (KO) mice (Pinson et al., 2000). Given that Lrp6 is ubiquitously expressed during development as a key coreceptor for the canonical Wnt signaling pathway, Lrp6-KO mice can be used to study the role of canonical Wnt signaling in diverse developmental processes. Recently, we have demonstrated that several severe forebrain developmental disorders occurred as a consequence of genetic inactivation of canonical Wnt signaling in Lrp6-KO mice (Zhou et al., 2004a,b, 2006). It has also been shown that Lrp6 plays a role in lens development (Stump et al., 2003; Smith et al., 2005). Here, we tested whether the Lrp6-mediated canonical Wnt pathway plays a role during early eye patterning and closure of the optic fissure. We demonstrate that genetic deletion of Lrp6 attenuates Bmp and retinoic acid signaling gene activation in the dorsal optic cup, thus altering the expression of dorsal and ventral neuroretina markers, which may lead to eye axis defects and ocular coloboma.
Results
Ocular Coloboma in Lrp6-KO Embryos
Our analysis of Lrp6-KO embryos from E11.5 to E18.5 revealed striking defects in eye morphogenesis (Figs. 1, 2). Compared with normal control embryos, Lrp6-KO embryos exhibited ocular defects with full penetrance (n > 100; both eyes or one eye) consisting of neuroretinal, retinal pigment epithelium (RPE), and optic nerve coloboma and reduced overall size of mutant eyes (microphthalmia; Fig. 1). Histological analyses of sagittal (Fig. 2A,B) and coronal (Fig. 2C–H) head sections of Lrp6-KO embryos during mid and late gestation demonstrated that the neuroretina and RPE in the ventral eye were completely absent in the coloboma region (Fig. 2B,D,F,H). Although the mutant retina was much smaller than in littermate controls (Fig. 2A,C,E,G), the cells of the mutant retina in the dorsal eye region were relatively well developed, as shown by the retinal precursors stained by hematoxylin & eosin (Fig. 2B shown at E11.5), the retinal ganglion cell axon projections immunolabeled for neurofilament protein (green in Fig. 2D,F shown at E13.5 and E16.5), and the compact proliferating neuroretinal cells which were all immunolabeled by bromodeoxyuridine (BrdU) incorporation (red in Fig. 2H shown at E18.5). The mutant lens was formed and labeled with Prox1 antibodies as expected (Fig. 2D,F), but its axis was rotated ventrally by approximately 45° may due to the absence of the retina in the ventral region (Fig. 2D,F,H). However, we did not detect obvious changes in proliferation by BrdU immunolabeling (Fig. 2H; not shown) or apoptosis by TUNEL assay in the mutant retina (data not shown).
Fig. 1.
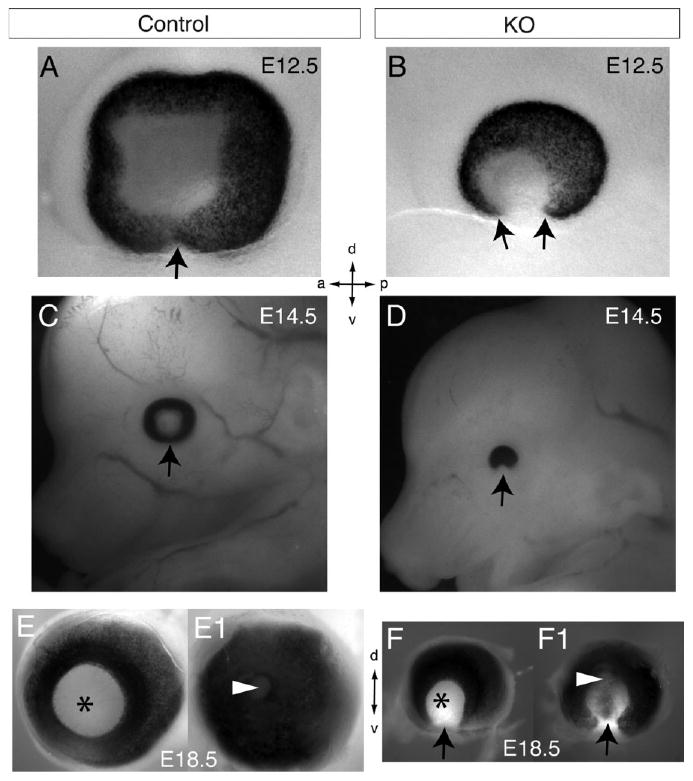
Whole-mount views of ocular coloboma in Lrp6-KO embryos. A,B: Whole eyes in normal control (A) and Lrp6-KO (B) embryos at embryonic day (E) 12.5. Arrow in A indicates the closed ventral eye region. Arrows in B indicate the defect in optic cup closure (coloboma) in the ventral eye region particularly evident by lacking the retinal pigment epithelium. C,D: Sagittal views of the embryo heads showing the eye of normal (C) and Lrp6-KO (D) at E14.5. Arrow in D indicates the eye coloboma. E–F1: Whole eyes dissected from E18.5 normal (E,E1) and Lrp6-KO (F,F1) embryos. Views from the front/lens (E,F) and back/optic nerve (E1,F1) sides of the eyes are shown. Arrows in F,F1 indicate the consistent coloboma in the mutant eyes. Arrowheads in E1,F1 indicate the optic nerve region, and asterisks in E,F indicate the lens. Axes: d, dorsal; v, ventral; a, anterior; p, posterior. Note that the mutant eyes are obviously smaller than the littermate control eyes at the respective ages.
Fig. 2.
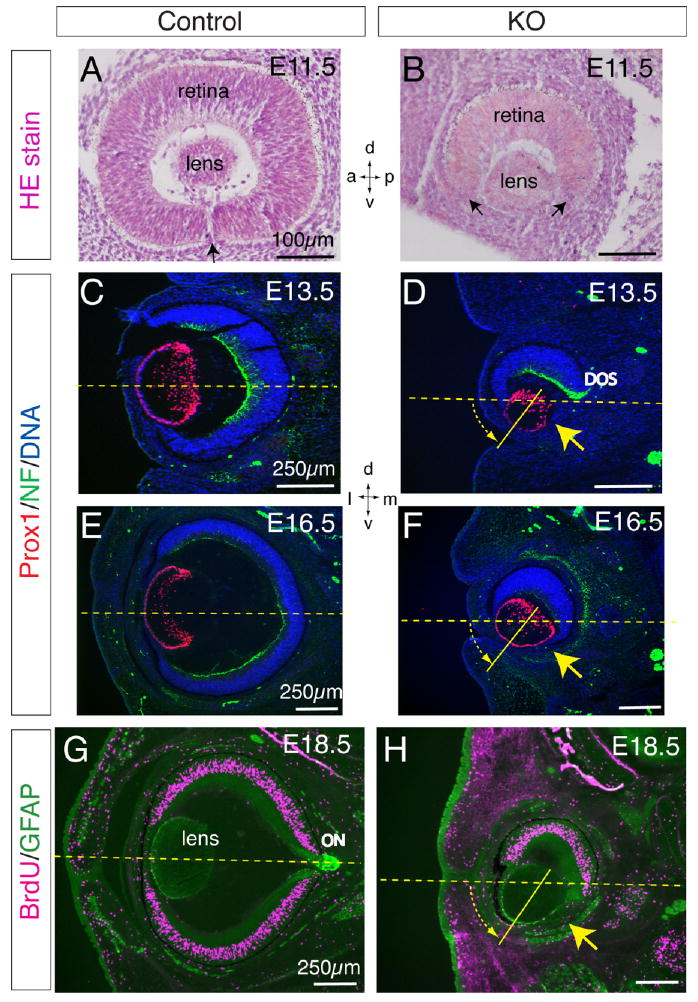
Histological analyses of retinal defects and lens rotation in Lrp6-knockout (KO) embryos. A,B: Hematoxylin & eosin staining of the frontal eye sections of the normal control and Lrp6-KO embryos at embryonic day (E) 11.5. Arrow in A indicates the closing site of the ventral retina. Arrows in B indicate the retinal coloboma, a big gap between the ventral ends of the retina (refer to arrows in Fig. 1B). C–H: Coronal head sections through mid eye regions of the control (C,E,G) and Lrp6-KO (D,F,H) embryos demonstrate the complete absence of the neuroretina and retinal pigment epithelium (RPE) in the ventral-middle eye of Lrp6-KO (yellow arrows; refer to arrows in Fig. 1D,F). C–F: Double immunolabeling for Prox1 (red, lens cells) and neurofilament protein (NF, green, retinal ganglion axons) at E13.5 (C,D) and E16.5 (E,F). Only the dorsal optic stalk (DOS; green in D) immunolabeled by neurofilament is observed in the mutant eye. Nuclei were counterstained by 4′,6-diamidine-2-phenylidole-dihydrochloride (DAPI). G,H: Double immunolabeling for bromodeoxyuridine (BrdU) incorporation (red, proliferating cells) and glial fibrillary acidic protein (GFAP; green, mesenchymal and lens epithelial cells) at E18.5. A group of putative mesenchymal cells immunolabeled by GFAP is absent in the mutant eyelids (H). The vitreous body (fills the space behind the lens) is also defective in the mutant eyes (D,F,H). ON, optic nerve. Note the ventral 45° rotation approximately of the lens in the mutant eye (shown with yellow dashed arrow). Dashed lines in C–H indicate the horizontal midline of the eye. Axes: d, dorsal; v, ventral; a, anterior; p, posterior; l, lateral; m, medial.
Defective Dorsoventral Neuroretina Patterning in the Eye of Lrp6-KO Embryos
To dissect the causal mechanism for the retinal coloboma in the ventral eyes of Lrp6-KO embryos, we examined the expression of ventral and dorsal neuroretinal marker genes during early patterning (Fig. 3). Vax2 is a homeodomain protein expressed in the ventral neuroretina (Fig. 3A), and gain-of-function studies have demonstrated that Vax2 is capable of ventralizing the neuroretina (Barbieri et al., 1999). Unexpectedly, Vax2 expression was preserved in the neuroretina of Lrp6-KO embryos but we noticed a dorsal shift of the Vax2-expressing domains compared with the Vax2 expression in the control embryo (Fig. 3A,B). By contrast, Tbx5, a member of the T-box transcription factor family (Koshiba-Takeuchi et al., 2000) that is normally expressed in dorsal retina precursors of wild-type eyes at E10.5 (Fig. 3C,C1), was completely absent in the mutant eyes at the same age (Fig. 3D,D1). These data suggest that retinal coloboma in the ventral eyes of Lrp6 mutants is caused by defective dorsal but not ventral neuroretinal patterning (also refer to Fig. 7). To determine whether neuroretinal fate is properly specified in Lrp6-KO embryos, we analyzed the expression of Pax6 (Fig. 3E–F1), a transcriptional factor playing a key-regulatory role in the eye and neuroretina development (Schwarz et al., 2000; Chow and Lang, 2001). We observed no significant differences in Pax6 expression in the eyes (including neuroretina and lens) of Lrp6-KO vs. wild-type control embryos. This result (particularly for the Pax6 in the lens) is consistent with previously published reports by other groups (Smith et al., 2005; Song et al., 2007). In addition, we examined Pax2 expression in the optic stalk and found no obvious difference between the mutant and control eye at E10.5 (data not shown).
Fig. 3.
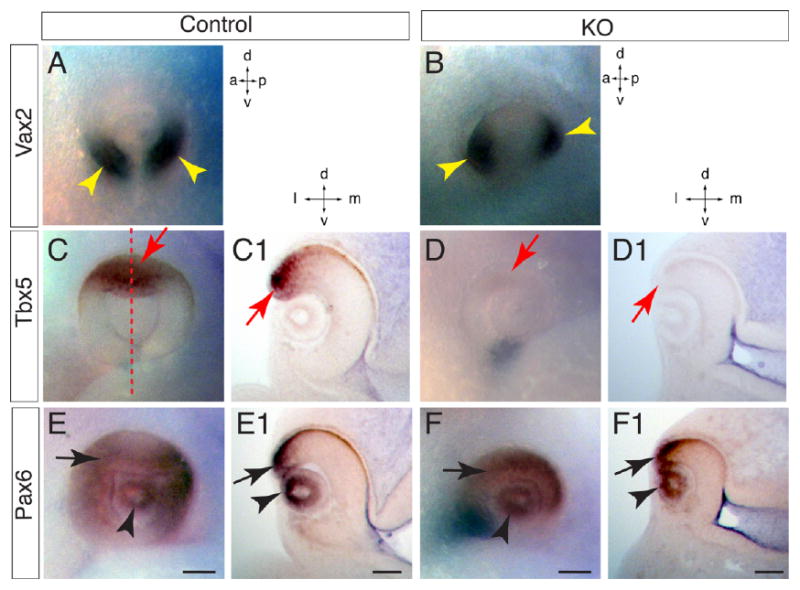
Dorsoventral neuroretina patterning defects in Lrp6-KO (knockout) embryos at embryonic day (E) 10.5. Whole-mount in situ hybridization (A–F) and corresponding Vibrotome sections (C1,D1,E1,F1) through dorsoventral midline of eye territory are shown. A: Vax2 mRNA is expressed in ventral retina in the normal eyes (arrowheads). B: Vax2 mRNA expression domain shifts dorsally in the eyes of Lrp6-KO embryos (arrowheads). C,C1: Tbx5 mRNA is expressed in dorsal neuroretina (arrows) of wild-type embryos. Dashed line in C indicates the dorsoventral midline where the section in C1cut through. D,D1: Tbx5 mRNA expression is completely absent in the dorsal neuroretina (arrows) of the mutant eyes. E–F1: Pax6 expression showed no dramatic changes in both neuroretina (arrows) and lens (arrowheads) between normal control (E,E1) and mutant eyes (F,F1) except the smaller eye and larger gap in the ventral retina of the mutant compared with the control. Axes: a, anterior; p, posterior; d, dorsal; v, ventral; l, lateral; m, medial. Scale bars = 100 μm.
Fig. 7.
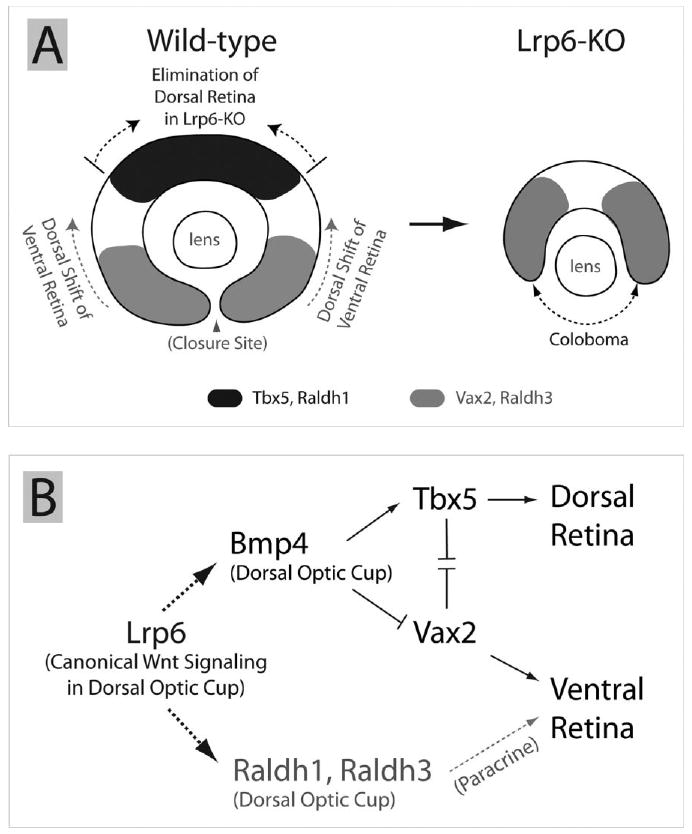
Proposed coloboma mechanism in Lrp6-null eyes and a possible signaling network during early eye development. A: Genetic deletion of Lrp6 severely reduces expression of the dorsal retina marker genes and leads to substantial reduction or elimination of the dorsal retina territory in Lrp6-null embryos, resulting in dorsal expansion/shift of ventral neural retina territory. The dorsal shifting of the ventral retina may lead to coloboma in the ventral eye region. Arrowhead indicates the closure site of the optic cup/optic fissure at embryonic day (E) 10.5. B: We propose a possible molecular mechanism or a signaling network for early eye development that Lrp6 (and its mediated canonical Wnt signaling pathway) is acting upstream of the Bmp4 and RA signaling pathways in the dorsal eye region and plays significant roles in establishing dorsoventral retina boundaries and related morphogenetic movements. The known roles of Bmp4 and retinoic acid (RA) signaling pathways for early eye development are summarized from the literature. Dashed arrows indicate direct or indirect regulations. Solid arrows indicate the direct regulations.
Down-regulated Bmp4 Signaling in the Eye of Lrp6-KO Embryos
Bone morphogenetic protein (Bmp) 4 is expressed in the dorsal neuroretina, and is essential for the normal eye development (Adler and Belecky-Adams, 2002; Behesti et al., 2006). Because Bmp4 plays a crucial role in dorsoventral patterning of the neuroretina acting upstream of Tbx5, we examined the expression of Bmp4 in the eyes of Lrp6-KO embryos at E10.5 (Fig. 4). Bmp4 was strongly expressed in the dorsal neuroretina of the control embryo (Fig. 4A,C) but was not detected in the mutant eyes (Fig. 4B,D) at this age. In addition, we immunolabeled coronal frozen sections through the eye territory of normal and Lrp6-KO embryos at E10.5 with antibodies to phosphorylated (p) Smad 1/5/8 proteins (Fig. 4E,F), which are known to be phosphorylated in response to Bmp4 signaling, and found that pSmad1/5/8 immunolabeling was significantly decreased in the dorsal neuroretina of Lrp6-KO embryos (Fig. 4F). However, both Bmp4 and pSmad1/5/8 were relatively conserved in adjacent orofacial primordia of the mutants (asterisks in Fig. 4A–F). These suggest that Lrp6-mediated canonical Wnt signaling pathway plays a primary role in dorsal neuroretinal patterning through regulation of Bmp4 signaling activity.
Fig. 4.

Bmp4 signaling is attenuated in the dorsal neuroretina of Lrp6-KO (knockout) embryos at embryonic day (E) 10.5. A,C: Bmp4 mRNA expression in dorsal neuroretina of the wild-type control embryos at E10.5. B,D: Bmp4 mRNA expression is lost in the dorsal neuroretina of the Lrp6-KO embryos at E10.5. E: Phosphorylated (p) Smad1/5/8 immunoreactivity is observed in dorsal neuroretina of the wild-type embryos. F: pSmad1/5/8-immunoreactivity is significantly reduced in the mutant neuroretina. Arrows, dorsal neuroretina. Note that Bmp4/pSmad signaling is not obviously affected in the orofacial processes of Lrp6-KO embryos at E10.5 (asterisks in B,D,F). Axes: a, anterior; p, posterior; d, dorsal; v, ventral; l, lateral; m, medial. Scale bars = 100 μm.
Altered Expression of Retinoic Acid-Synthesizing Enzymes in the Eye of Lrp6-KO Embryos
Because the retinoic acid-generating enzyme Raldh1 and Raldh3 are uniquely expressed in distinct domains of normal neuroretina (Molotkov et al., 2006), we asked whether these RA signaling genes were disrupted in the mutant eyes. As expected, Raldh1, normally expressed in the dorsal neuroretina (Fig. 5A,C), was dramatically lost in the mutant eye at E10.5 (Fig. 5B,D). By contrast, Raldh3 was expressed in the dorsal RPE starting from E9.75, and was also expressed in the ventral neuroretina starting from E10.0 (Molotkov et al., 2006) (Fig. 5E,G). However, Raldh3 expression was decreased in dorsal eye but relatively conserved in the ventral neuroretina of Lrp6-KO embryos at E10.5 (Fig. 5F,H). Similar to Vax2 expression, this ventral Raldh3 expression domain was shifted dorsally (yellow arrowheads in Fig. 5F). In addition, the expression of Raldh3 in the adjacent orofacial region was notably increased in the mutants at E10.5 (asterisks in Fig. 5G,H), suggesting a repression role of Lrp6 for Raldh3 expression in this region during normal development. To determine whether RPE is defective in the mutant eyes, we examined the gene expression of an RPE patterning factor, Mitf (the mouse microphthalmia gene), and found no significant alterations in the mutant eyes (data not shown).
Fig. 5.
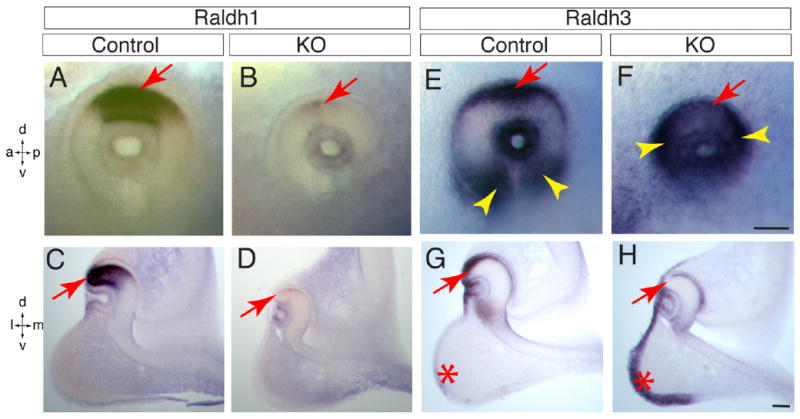
RA signaling is altered in the dorsal neuroretina of Lrp6-KO embryos at embryonic day (E) 10.5. A,C: Raldh1 mRNA is expressed in the dorsal neuroretina of normal control embryos. B,D: Expression of Raldh1 mRNA is nearly absent in the mutant eyes. E,G: Raldh3 mRNA is expressed in both dorsal eye cup (arrows) and ventral retina (arrowheads) in the wild-type embryos. F: Raldh3 mRNA expression domain in the ventral retina shifts dorsally in the mutant eyes (arrowheads). H: Raldh3 mRNA expression is decreased in dorsal retinal pigment epithelium (RPE; arrow). Note the altered Raldh3 expression pattern in orofacial region (asterisks in G,H) of the mutants. Axes: a, anterior; p, posterior; d, dorsal; v, ventral; l, lateral; m, medial. Scale bars = 100 μm.
Restricted Activation of Canonical Wnt Signaling in Dorsal RPE and Ubiquitous Expression of Lrp6 During Early Eye Development
To evaluate canonical Wnt signaling activity during normal eye development, we examined the Wnt reporter TOPgal mice that carry a β-galactosidase reporter gene (lacZ) under the control of Tcf-optimal promoter, which is induced by interaction of β-catenin with Tcf/Lef1 transcriptional factors (DasGupta and Fuchs, 1999; Fig. 6A). TOPgal embryos stained with the X-gal substrate showed the characteristic labeling representing canonical Wnt signaling activity in the dorsal optic vesicle at E9.5 and in the dorsal RPE precursor at E10.5 (Fig. 6C–E). However, we did not see obvious X-gal staining in the developing neuroretina and nascent lens at these early stages (E9.25–E10.5). It is known that Lrp6 is broadly or ubiquitously expressed in many embryonic tissues (Kelly et al., 2004; Zhou et al., 2004a), and was also examined in the eye tissue sections (Smith et al., 2005). Using either in situ hybridization or X-gal staining for the lacZ gene activation in heterozygous Lrp6 embryos, we found that Lrp6 is dominantly activated in the early developing eyes, and ubiquitously expressed in E10.5 embryos (data not shown).
Fig. 6.

Expression patterns of the Wnt reporter TOPgal. A: TOPgal construct. B–E: Localization of Wnt signaling activity in the eyes of TOPgal embryos during early development. X-gal staining (blue) of coronal section through the optic vesicle at embryonic day (E) 9.25 (B), E9.5 (C), and E10.5 (E) and an E10.5 whole embryo (D) are shown. Black arrows in C–E indicate TOPgal activation restricted to the dorsal optic vesicle and retinal pigment epithelium (RPE). Arrowhead in E indicates no obvious TOPgal activation observed in the dorsal neural retina. *optic vesicle.
Discussion
Lrp6 Signaling Controls Dorsal Neuroretina Patterning Upstream of the Bmp Signaling Pathway
Our finding that, the down-regulation of the dorsal neuroretina territory markers (Bmp4, Tbx5, and Raldh1) with simultaneous dorsal shift of the ventral retina markers (Vax2 and Raldh3) in the Lrp6-KO embryos (Fig. 7A), suggests a primary role of Lrp6 in establishing the identity of the dorsal retina. Lrp6 has been well-demonstrated to be a key factor in conducting canonical Wnt signaling (He et al., 2004). To date, no demonstrations regarding Lrp6 signaling in other pathways are reported. Thus, our data also suggest that Lrp6 mediates canonical Wnt signaling pathway acting upstream of the Bmp signaling pathway in retinal development. It has been reported that canonical Wnt/β-catenin signaling can directly regulate Bmp4 expression positively or negatively in different biological processes (Baker et al., 1999; Kim et al., 2002; Shu et al., 2005). Our results suggest a novel interaction of Wnt and Bmp signaling pathways during early eye development. Within the optic cup, Bmp signaling controls the patterning of the dorsoventral axis in the developing retina (Murali et al., 2005). Genetic evidence demonstrates that inactivation of Bmp4 signaling during eye development affects retina dorsoventral polarity and causes coloboma (Adler and Belecky-Adams, 2002; Behesti et al., 2006). Ectopic expression of Bmp4 up-regulates Tbx5 expression and down-regulates Vax2 expression in the optic cup (Koshiba-Takeuchi et al., 2000). The signaling network and molecular interactions are summarized (Fig. 7B). Of interest, we observed no obvious changes in Bmp4 signaling in the orofacial region of the Lrp6-null mice on the same tissue sections where Bmp4 signaling was dramatically attenuated in the eye, suggesting that distinct molecular mechanisms exist simultaneously in the signaling interactions between canonical Wnt and Bmp4 signaling pathways in different developmental processes.
Lrp6 Signaling Regulates Retinoic Acid Pathway in Eye Development
Our finding that, in addition to Bmp4, Lrp6 signaling upstream of Raldh1 and Raldh3 expression in the dorsal optic cup suggests a novel regulation of the retinoic acid pathway by Wnts. Retinoic acid plays major roles during vertebrate embryonic development (Niederreither et al., 1999), and genetic inactivation of retinoic acid synthesis in the eye impairs optic cup formation and causes coloboma (Matt et al., 2005; Molotkov et al., 2006; Halilagic et al., 2007). Also, deletion of the retinoid receptor RXRa causes congenital hypertrophy/hyperplasia of the RPE (Perrault et al., 2004). In an attempt to rescue the retina defects in Lrp6-KO embryos, we gave dietary retinoic acid to the pregnant female mice. Although this approach has been proven to be effective in rescuing developmental eye defects caused by retinoic acid deficiency (Molotkov et al., 2006), we failed to rescue the retina defects in Lrp6-KO embryos. Therefore, our findings suggest a model in which the Lrp6-mediated canonical Wnt pathway serves dual roles in regulating both retinoic acid and Bmp4 signaling (Fig. 7B). Thus, rescue of retinoic acid signaling might not be sufficient to restore normal eye development in Lrp6-KO embryos.
Wnt Signaling Molecules and Activity During Early Eye Development
Prior studies demonstrated the expression of Wnt2b in the dorsal optic vesicle of E9.5 mouse embryo, where it was colocalized with the TOPgal reporter activity (Liu et al., 2006), and Wnt2b was shown to control cell differentiation at the ciliary marginal zone (Kubo et al., 2003; Cho and Cepko, 2006). Similar data demonstrating Wnt activity in the dorsal optic vesicle at E9.5 and subsequently in the dorsal RPE precursor at E10.5 but not in the developing neuroretina were recently reported using BATgal Wnt reporter mouse line (Kreslova et al., 2007) and confirmed by us in this study using TOPgal mice. In addition, Axin2, a general target of canonical Wnt signaling, was demonstrated to be restrictively expressed in the dorsal RPE at E10.5 (Burns et al., 2008), which is consistent with the activation site of the Wnt reporter TOPgal or BATgal in the early developing eyes.
The evident role of canonical Wnt signaling in the expression of the dorsal retina marker genes is puzzling considering the absence of the general Wnt target Axin2 expression and Wnt reporter X-gal staining in the neuroretina at the early stage. One possible explanation for this is the expression of several secreted frizzled-related proteins (Sfrp 1, 2, and 4) in the neuroretina (Leimeister et al., 1998). Sfrps bind to Wnts, preventing Wnt proteins from binding to Fzd receptors, and thus inhibiting Wnt signaling. High levels of Sfrps expressed in retina may decrease activation of the Wnt reporter in retina, and it is believed that the TOPgal and BATgal reporters only reveal areas of high level Wnt signaling but may miss regions with lower signaling activity. An alternative explanation may be that the dorsal RPE precursor with Wnt signaling activation is a signaling center that controls dorsal neuroretina patterning by Bmp4 and RA production under the control of Wnt signaling in the dorsal optic cup. We examined the promoter regions of Bmp4, Raldh1, and Raldh3 for putative Tcf/Lef binding sites and identified multiple Tcf/Lef-binding sites in the immediate upstream of each gene (data not shown), which will serve as the basis for future studies. Thus, there is a measure of support for either a model of direct Wnt regulation of dorsal neuroretinal genes or for the RPE signaling center hypothesis. Nevertheless, it still cannot be excluded the possibility that Lrp6 regulating Bmp4 and RA signaling pathways in the dorsal eye is independent of Wnt/β-catenin pathway through undetermined mechanisms.
Of interest, one additional Wnt reporter Tcf/Lef-lacZ showed signaling activities in a small group of cells restricted in the central neural retina at E11.5, and predominant in inner retina and the apical side of the neuroblast layer by E12.5 onward (Liu et al., 2006). We also observed a similar pattern of the TOPgal reporter activity in the developing eyes from E11.5 onward (data not shown). These observations suggest that canonical Wnt signaling pathway is not only important for dorsoventral neuroretinal patterning at the early stage that was addressed in the current study, but may also play a role in neuroretinal cell differentiation at later developmental stages.
In summary, our data showed that genetic deletion of Lrp6 leads to defects in neuroretinal patterning and coloboma associated with down-regulation of Bmp and RA signaling pathways, demonstrating Lrp6-mediated pathway plays crucial roles in establishing dorsoventral retina patterning during early mammalian eye development.
Experimental Procedures
Animals and Sampling
The Wnt signaling reporter TOPgal mice were generated by Fuchs lab (DasGupta and Fuchs, 1999) and distributed by the Jackson Laboratory (Bar Harbor, ME) in the CD1 strain background. Lrp6-KO mice were generated by a gene-trap approach (Pinson et al., 2000) and the mutant ES cells are conserved by the Bay Genomics and the Mutant Mouse Regional Resource Center at the University of California, Davis (Davis, CA). Lrp6 mice were maintained in the C57BL/6J stain background. Both mouse lines were housed in the vivarium of the UC Davis Medical School (Sacramento, CA). Pregnant, timed-mated mice were killed with CO2 gas before cesarean section. The embryos were immersion fixed in 4% paraformaldehyde (PFA) at 4°C overnight. Embryos were collected at E9.25, E9.5, E10.5, E11.5, E12.5, E13.5, E14.5, E16.5, and E18.5; where the day of conception is designated E0. All research procedures using mice were approved by the UC Davis Animal Care and Use Committee and conformed to NIH guidelines.
Whole-Mount In Situ Hybridization
Embryos were fixed in 4% PFA. Whole-mount in situ hybridization of intact mouse embryos was performed according to standard protocols using digoxigenin-labeled antisense RNA probes as described previously (Zhou et al., 2004a; Molotkov et al., 2006).
BrdU Incorporation and Immunohistochemistry
Acute BrdU incorporation was carried out in embryos from pregnant mice that were injected by BrdU intraperitoneally (100 μg/g body weight) 1 hr before sampling as described previously (Zhou et al., 2004b, 2006). The cryostat sections were pretreated with 2 N HCl for 30 min at 37°C followed by standard immunostaining. The tissue sections were preincubated in a blocking solution and then reacted with one of the following antibodies: glial fibrillary acidic protein (GFAP; 1:100, DakoCytomation), Neurofilament (NF; 1:1,000, Zymed Laboratories), BrdU (1:20, Developmental Studies Hybridoma Bank, IA), Prox1 (1:2,000, generated in the Pleasure lab but commercially supplied by Chemicon and Covance), or pSmad1/5/8 (1:100, Cell Signaling Technology). The secondary antibodies Alexa Fluor 594 goat anti-rabbit IgG and Alexa Fluor 488 goat anti-mouse IgG were used (all secondary antibodies from Molecular Probes, Inc.).
Acknowledgments
The authors thank William Skarnes for the permission and John Rubenstein for providing founder Lrp6βgeo mice, Gregg Duester for in situ probes, and Erica Whitney for editing the early version of the manuscript.
Shriners Hospitals for Children; Grant number: SHC8610; Grant sponsor: Children's Miracle Network; Grant number: CMNCZ05; Grant sponsor: American Cancer Society; Grant number: 95-125-07-S-CCZHOU1; Grant sponsor: National Multiple Sclerosis Society; Grant number: pp1458; Grant sponsor: UC Davis Health System Research Award; Grant number: CZHS.
References
- Adler R, Belecky-Adams TL. The role of bone morphogenetic proteins in the differentiation of the ventral optic cup. Development. 2002;129:3161–3171. doi: 10.1242/dev.129.13.3161. [DOI] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13:3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri AM, Lupo G, Bulfone A, Andreazzoli M, Mariani M, Fougerousse F, Consalez GG, Borsani G, Beckmann JS, Barsacchi G, Ballabio A, Banfi S. A homeobox gene, vax2, controls the patterning of the eye dorsoventral axis. Proc Natl Acad Sci U S A. 1999;96:10729–10734. doi: 10.1073/pnas.96.19.10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol. 2006;6:62. doi: 10.1186/1471-213X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, Zhang J, Brown EC, Van Bibber AM, Van Es J, Clevers H, Ishikawa TO, Taketo MM, Vetter ML, Fuhrmann S. Investigation of Frizzled-5 during embryonic neural development in mouse. Dev Dyn. 2008;237:1614–1626. doi: 10.1002/dvdy.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Cepko CL. Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development. 2006;133:3167–3177. doi: 10.1242/dev.02474. [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–296. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Abud HE, Hime GR. WNT/Frizzled signaling in eye development and disease. Front Biosci. 2006;11:2442–2464. doi: 10.2741/1982. [DOI] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans CY, Williams MJ, Halford S, Gregory-Evans K. Ocular coloboma: a reassessment in the age of molecular neuroscience. J Med Genet. 2004;41:881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dolle P, Studer M. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol. 2007;303:362–375. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, Ogura T. Tbx5 and the retinotectum projection. Science. 2000;287:134–137. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- Kreslova J, Machon O, Ruzickova J, Lachova J, Wawrousek EF, Kemler R, Krauss S, Piatigorsky J, Kozmik Z. Abnormal lens morphogenesis and ectopic lens formation in the absence of beta-catenin function. Genesis. 2007;45:157–168. doi: 10.1002/dvg.20277. [DOI] [PubMed] [Google Scholar]
- Kubo F, Takeichi M, Nakagawa S. Wnt2b controls retinal cell differentiation at the ciliary marginal zone. Development. 2003;130:587–598. doi: 10.1242/dev.00244. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Bach A, Gessler M. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech Dev. 1998;75:29–42. doi: 10.1016/s0925-4773(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Rodrigo I, Bovolenta P. Eye development: a view from the retina pigmented epithelium. Bioessays. 2004;26:766–777. doi: 10.1002/bies.20064. [DOI] [PubMed] [Google Scholar]
- Matt N, Dupe V, Garnier JM, Dennefeld C, Chambon P, Mark M, Ghyselinck NB. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Perrault I, Hanein S, Gerber S, Barbet F, Ducroq D, Dollfus H, Hamel C, Dufier JL, Munnich A, Kaplan J, Rozet JM. Retinal dehydrogenase 12 (RDH12) mutations in leber congenital amaurosis. Am J Hum Genet. 2004;75:639–646. doi: 10.1086/424889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Sanlaville D, Verloes A. CHARGE syndrome: an update. Eur J Hum Genet. 2007;15:389–399. doi: 10.1038/sj.ejhg.5201778. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Cecconi F, Bernier G, Andrejewski N, Kammandel B, Wagner M, Gruss P. Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development. 2000;127:4325–4334. doi: 10.1242/dev.127.20.4325. [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Smith AN, Miller LA, Song N, Taketo MM, Lang RA. The duality of beta-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev Biol. 2005;285:477–489. doi: 10.1016/j.ydbio.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Song N, Schwab KR, Patterson LT, Yamaguchi T, Lin X, Potter SS, Lang RA. pygopus 2 has a crucial, Wnt pathway-independent function in lens induction. Development. 2007;134:1873–1885. doi: 10.1242/dev.001495. [DOI] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B, Roth MP. Congenital eye malformations in 212,479 consecutive births. Ann Genet. 1997;40:122–128. [PubMed] [Google Scholar]
- Stump RJ, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW. A role for Wnt/beta-catenin signaling in lens epithelial differentiation. Dev Biol. 2003;259:48–61. doi: 10.1016/s0012-1606(03)00179-9. [DOI] [PubMed] [Google Scholar]
- Yang XJ. Roles of cell-extrinsic growth factors in vertebrate eye pattern formation and retinogenesis. Semin Cell Dev Biol. 2004;15:91–103. doi: 10.1016/j.semcdb.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Pinson KI, Pleasure SJ. Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants. J Neurosci. 2004a;24:7632–7639. doi: 10.1523/JNEUROSCI.2123-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Zhao C, Pleasure SJ. Wnt signaling mutants have decreased dentate granule cell production and radial glial scaffolding abnormalities. J Neurosci. 2004b;24:121–126. doi: 10.1523/JNEUROSCI.4071-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou CJ, Borello U, Rubenstein JL, Pleasure SJ. Neuronal production and precursor proliferation defects in the neocortex of mice with loss of function in the canonical Wnt signaling pathway. Neuroscience. 2006;142:1119–1131. doi: 10.1016/j.neuroscience.2006.07.007. [DOI] [PubMed] [Google Scholar]


