Abstract
Purpose
Merkel cell carcinoma (MCC) is a rare, aggressive neuroendocrine cutaneous malignancy. Current recommendations include offering regional lymph node evaluation by either sentinel lymph node biopsy (SLNB) or complete lymph node dissection (CLND) to all patients with MCC; however, we hypothesized a cohort of low-risk patients may exist for whom regional nodal metastasis would be unlikely.
Methods
A retrospective review of the Department of Veterans Affairs national health care database was performed. Patients undergoing resection of primary MCC were identified; and demographic, medical, and social history; tumor characteristics; nodal status; and recurrence events were recorded.
Results
Between 1995 and 2006, 346 patients were diagnosed with MCC. Of these, 213 underwent resection of the primary lesion and evaluation of the draining lymph node basin. Fifty-four patients (25%) had tumors ≤ 1.0 cm in diameter. Average tumor diameter was 0.7 cm, and 63% were located on the head or neck. Only two patients (4%) with tumors ≤ 1.0 cm had regional lymph node metastasis, compared with 51 (24%) of 213 patients with tumors more than 1.0 cm (P < .0001). Both patients had clinically evident nodal disease at presentation and underwent CLND. Both have remained recurrence-free for 40 months. Thirteen (25%) of 51 patients with nodal metastasis and tumors more than 1 cm had occult nodal metastasis.
Conclusion
In this series, patients with MCC ≤ 1.0 cm were unlikely to have regional lymph node metastasis, suggesting that regional nodal evaluation may reasonably be avoided in these patients. However, these data support SLNB for MCC more than 1 cm in diameter.
INTRODUCTION
Merkel cell carcinoma (MCC) is an aggressive neuroendocrine malignancy of the skin first described by Toker in 1972.1 When compared with other malignancies of the skin, MCC is extremely rare, 100 times less prevalent than melanoma.2 However, its incidence has tripled recently, increasing from 0.15 per 100,000 in 1986 to 0.44 per 100,000 in 2001.3 Some of the increase in the incidence of MCC may be due to increased use of cytokeratin-20 staining, which has been a valuable diagnostic tool for diagnosing MCC since the mid 1990s.4–7 Factors that may have contributed to actual increases in MCC include an increasing elderly population, increase in sun exposure, and a greater number of immunosuppressed patients.3,8,9
The overall prognosis for patients with MCC is poor. MCC is characterized by its high incidence of local recurrence and propensity for regional lymph node and distant metastasis. In a review of the Surveillance, Epidemiology, and End Results (SEER) Program database, Agelli et al reported 5-year survival rates of 75%, 59%, and 25% for localized, regional, and distant MCC, respectively.10 Although MCC survival rates are known to worsen in correlation with disease progression, no standardized staging system currently exists specifically for MCC, though some have been proposed. Previous studies have used the American Joint Committee on Cancer staging system for skin cancer, whereas the most popular system currently used is a three-tiered system proposed by Yiengpruksawan et al,11 listed in Table 1. This scheme expands on other staging systems in that it recognizes the importance of tumor size as a prognostic factor and subdivides stage I disease into IA and IB based on tumors being smaller or larger than 2 cm in diameter, respectively.
Table 1.
Current Staging Systems for Patients With Merkel Cell Carcinoma
| Staging System | Criteria |
|---|---|
| Yiengpruksawan et al11 three-tiered staging system | |
| Stage I | |
| IA | Primary tumor ≤ 2 cm |
| IB | Primary tumor > 2 cm |
| Stage II | Regional lymph node metastasis |
| Stage III | Distant metastases |
| Memorial Sloan-Kettering Cancer Center TNM staging system15 | |
| T | |
| 1 | Primary tumor < 2 cm |
| 2 | Primary tumor ≥ 2 cm |
| N | |
| 0 | Negative regional lymph node involvement |
| 1 | Positive regional lymph node involvement |
| M | |
| 0 | No evidence of distant metastatic disease |
| 1 | Presence of distant metastatic disease |
| Stage | |
| I | T1, N0, M0 |
| II | T2, N0, M0 |
| III | Any T, N1, M0 |
| IV | Any T, any N, M1 |
As previously mentioned, advanced disease resulting from metastatic spread to the regional draining lymph node basins significantly worsen a patient's overall prognosis. Five-year survival rates range from 22% to 65% in patients with nodal disease as compared with 80% to 88% in patients with node-negative disease.7,12 Because of the rarity of this malignancy, the data supporting recommendations for pathologic staging and treatment remain limited. Although some features of the primary lesion (large tumors, lymphatic or vascular invasion, and small-cell subtype) may indicate a more aggressive tumor, currently there are no treatment recommendations based on these characteristics. With regard to evaluation of regional lymph nodes, current guidelines and staging systems recommend performing sentinel lymph node biopsies (SLNB) or complete lymph node dissection (CLND) on all patients with MCC regardless of tumor size or other tumor characteristics.11,13 Patients with positive nodal disease are offered further adjuvant therapy as a means of locoregional control. Combination of completion dissection with adjuvant radiation therapy has been reported to decrease regional failure rates from 50% to 70% down to 20% in MCC.14 This benefit of regional control is not without costs, as the morbidity associated with lymph node dissections is well known.
Previous studies have reported rates of occult lymph node metastasis observed in patients with MCC15,16; however, we were unable to find any large studies primarily examining the correlation between the size of the primary lesion and subsequent risk of occult lymph node metastasis. For the present study, we examined a cohort of 346 patients diagnosed with MCC. The aim of this study was to assess whether there is a cohort of low-risk patients in whom the risk of regional nodal metastasis would be unlikely and nodal evaluation may be reasonably and safely avoided.
METHODS
A review of the Veterans Affairs (VA) prospectively collected national health care database was performed to identify all patients with a diagnosis of MCC. Patients seen at VA inpatient and outpatient settings for a histologically confirmed diagnosis of MCC between 1995 and 2006 were included in this study. Patients undergoing treatment for skin malignancies other than MCC were excluded from this study. Approval from the VA institutional review board was obtained before initiation of database review.
Characteristics of each patient, tumor, treatment modalities, and follow-up were reviewed and analyzed. Patient characteristics examined include age at diagnosis, sex, race, alcohol and tobacco use, and family history. Tumor features included number of primary lesions, tumor size based on pathologic measurements, and primary tumor location. Tumor size is reported as diameter of lesion measured during pathologic examination. Presence of clinically and/or pathologically evident nodal metastatic disease was recorded, as well as evidence of distant metastasis. Treatment strategies were recorded with regards to type of surgical management of the primary tumor, evaluation of the regional lymph node basin, and administration of adjuvant chemotherapy and radiation therapy. Treatment was not standardized among disease stages or across individual VA centers, but instead was left to the discretion of the individual patient and physician. However, only patients who underwent definitive surgical therapy were included in the final analysis. Each patient in the final analysis also had the regional lymph node basins evaluated by either SLNB or CLND, at the discretion of the treating physician.
For the purposes of this study, patient disease stages are reported in the results according to Memorial Sloan-Kettering Cancer Center's (MSKCC) proposed staging system as published by Allen et al.16 The criteria for the MSKCC staging system are listed in Table 1. MSKCC's criteria differs from the commonly used three-tiered system proposed by Yiengpruksawan et al11 in that they expand the scheme into a four-stage system more consistent with other TNM staging schemes.
Statistical Analysis
Data were analyzed using the GraphPad Prism software (GraphPad Prism version 5 for Macintosh, GraphPad Software, San Diego, CA) and SAS software (SAS/STAT version 8 for MacOS, SAS Institute, Cary, NC) packages. Categoric variables were compared using the χ2 test and Fisher's exact test. Contiguous variables, such as age, were analyzed using a two-tailed, unpaired t test with Welch correction when comparing two groups, whereas a one-way analysis of variance was performed when comparing three or more groups. Disease-free recurrence curves were generated by the Kaplan-Meier method17 and compared using the log-rank test. P values of ≤ .05 were considered to be statistically significant.
RESULTS
Three hundred forty-six patients were evaluated for MCC in the VA health care system between 1995 and 2006 (Table 2). The median age of patients was 75 years. Only three patients (0.9%) were female, and seven patients (2%) were nonwhite. Only one patient, who presented with stage III disease, was documented to have had exposure to the combination of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid (Agent Orange).
Table 2.
Demographics of the Patients Evaluated for Merkel Cell Carcinoma in the Veterans Affairs Health System, 1995 to 2006 (N = 346)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 75 | |
| Range | 42-90 | |
| Sex, male | 343 | 99 |
| Race, white | 339 | 98 |
| Current or past history, family history of cancer | 34 | 10 |
Abbreviations: MCC, Merkel cell carcinoma; VA, Veterans Affairs.
Tumor characteristics and treatment profiles for the 346 patients diagnosed with MCC are shown in Table 3. Sun-exposed areas represented the most common sites of occurrence. The extremities contained nearly one quarter of all lesions (n = 101, 29%) followed closely by the face, head, and neck (n = 73, 21%). One hundred fifty-one patients (71%) presented with clinically negative nodal involvement (stage I and II disease), 24% presented with regional nodal metastasis (stage III disease), and 5% had evidence of distant metastatic disease (stage IV). Tumor size for 31 patients was not recorded in the database. The median diameter of the primary tumor in the remaining 315 patients was 1.8 cm (range, 0.2 to 12.0 cm). Two hundred forty-four patients underwent surgical treatment consisting of at least a biopsy of their tumors. The remaining 64 patients either were not candidates for surgery secondary to existing comorbidities or were offered surgery and declined. Excisional biopsy was the surgical procedure performed most often, with wide local excision as the next most common. Few patients (12%) underwent a diagnostic biopsy procedure before definitive resection. Thirty-seven patients (11%) received adjuvant chemotherapy, 104 patients (30%) were treated with adjuvant radiation therapy, and 22 patients (6%) received both modalities.
Table 3.
Tumor Characteristics and Treatment Profile of Patients Diagnosed With Merkel Cell Carcinoma in the Veterans Affairs Health System, 1995 to 2006
| Characteristic* | No. | % |
|---|---|---|
| Location of primary, n = 346 | ||
| Face | 45 | 13 |
| Head/neck | 28 | 8 |
| Upper extremity | 34 | 10 |
| Trunk | 68 | 20 |
| Lower extremity | 67 | 19 |
| Primary tumor diameter, cm (n = 315) | ||
| Median | 1.8 | |
| Range | 0.2-12.0 | |
| Stage at diagnosis per MSKCC system, n = 213 | ||
| I | 95 | 45 |
| II | 56 | 26 |
| III | 52 | 24 |
| IV | 10 | 5 |
| Type of surgical resection, n = 308 | ||
| Excisional biopsy only | 101 | 29 |
| Wide local excision | 95 | 27 |
| Mohs surgery | 8 | 2 |
| Biopsy followed by definitive resection | 40 | 12 |
| No surgery | 64 | 18 |
| Adjuvant therapy, n = 163 | ||
| Chemotherapy | 37 | 11 |
| Radiation therapy | 104 | 30 |
| Both | 22 | 6 |
| Recurrence of disease, n = 187 | 40 | 21 |
| Follow-up, months | ||
| Median | 38.5 | |
| Range | 2-139 | |
Abbreviation: MSKCC, Memorial Sloan-Kettering Cancer Center.
n = patients evaluated.
Regional lymph node status was available for analysis in 213 patients who underwent surgery, and a comparison with respect to tumor size is listed in Table 4. Fifty-four patients (25%) presented with tumors ≤ 1 cm, 77 patients (36%) had tumors between 1 and 2 cm, and the remaining 82 patients (39%) had tumors more than 2 cm. Demographics for the three groups were similar. Location of the primary tumor was also similar between both groups, with the most common sites occurring on the head, neck, and extremities, consistent with the sun-exposed pattern seen in the overall cohort. Patients with tumors between 1 and 2 cm and those with tumors more than 2 cm were more likely to present with node-positive disease than patients with tumors ≤ 1 cm in diameter. In fact, only two patients with tumors ≤ 1 cm had evidence of regional lymph node disease at the time of diagnosis, compared with 22% of patients with tumors 1 to 2 cm and 41% of patients with tumors more than 2 cm in diameter (P < .0001). Both patients who had tumors ≤ 1 cm and regional node metastasis presented with clinical stage III disease, with clinically evident (not occult) nodal involvement discovered during physical examination. Thus they underwent therapeutic CLND. One of the patients elected to receive adjuvant chemotherapy after surgery. Neither patient received adjuvant radiation therapy. Thus considering the presentation of patients with primary MCC and clinically negative nodes, rates of lymph node metastases identified at initial surgical staging were 0%, 5%, and 12% for tumors 0 to 1, 1 to 2, and more than 2 cm diameter, respectively (Table 4).
Table 4.
Comparison of 213 Surgically Treated Patients
| Characteristic | Tumor ≤ 1 cm |
Tumor > 1 cm and ≤ 2 cm |
Tumor > 2 cm |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| No. of patients | 54 | 77 | 82 | ||||
| Age, years | .07 | ||||||
| Median | 77 | 75 | 75 | ||||
| Range | 55-97 | 44-90 | 53-89 | ||||
| Primary tumor location | |||||||
| Head/neck | 34 | 63 | 44 | 57 | 42 | 51 | .61 |
| Extremities | 17 | 31 | 25 | 33 | 24 | 29 | .97 |
| Trunk | 3 | 6 | 8 | 10 | 12 | 15 | .20 |
| Positive lymph nodes | 2 | 4 | 17 | 22 | 34 | 41 | < .0001 |
| Clinically positive nodes | 2 | 4 | 13 | 17 | 25 | 30 | < 0.0001 |
| Clinically negative/histologically positive nodes | 0 | 0 | 4 | 24 | 9 | 26 | 1.000 |
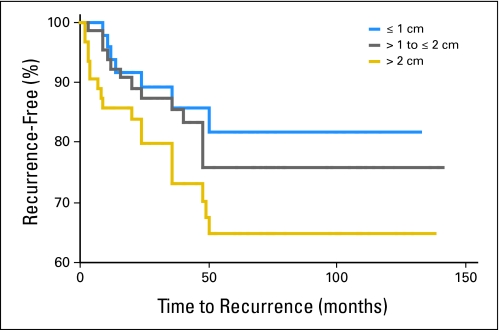
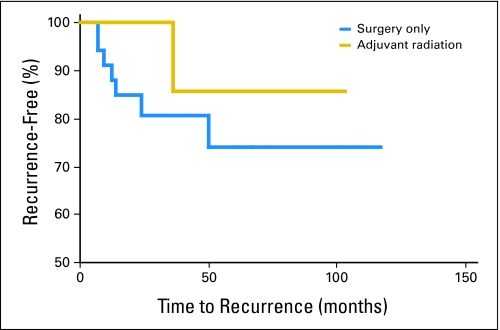
Follow-up data regarding recurrence were available for 187 patients. Data regarding disease-specific cause of death were not available from the data set, and only qualitative (not site-specific) information regarding recurrences were available. Forty patients (21%) experienced a recurrence, and 50% of these occurred within 2 years. Using Kaplan-Meier analysis, we estimated the impact of tumor size on the incidence of local recurrence after surgical resection. The recurrence-free interval curves are shown in Figure 1. There was a trend for patients with tumors larger than 1 cm to have a higher recurrence rate than patients with tumors less than 1 cm (23% v 17%, respectively; P = .43). Five-year recurrence-free rate estimates were 82%, 76%, and 64% for patients with tumors ≤ 1 cm, 1 to 2 cm, and more than 2 cm, respectively. The median follow-up was 38.5 months (range, 2 to 139 months). The median time to recurrence for patients with tumors less than 1 cm was 20 months, compared with 9 months for patients with tumors larger than 2 cm. Local recurrence occurred in seven (20.5%) of 34 patients who did not receive radiation therapy and in one (7.7%) of 13 patients who did receive radiotherapy (P = .413). Figure 2 shows the effects of adjuvant radiation therapy on the relapse-free rates among patients with tumors ≤ 1 cm. In this cohort, there was no significant difference in time to recurrence between these two patient groups, though a small trend suggesting a benefit of radiation therapy cannot be completely ruled out.
Fig 1.
Time to recurrence by tumor size.
Fig 2.
Time to recurrence by treatment modality for patients with tumors ≤ 1.0 cm.
DISCUSSION
MCC is a rare neuroendocrine tumor of the skin. Because of its aggressive biology, MCC commonly metastasizes to regional lymph nodes or distant sites and, in such cases, is associated with a poor prognosis. Such aggressive behavior and propensity for early metastasis warrant early recognition and treatment to ensure a favorable outcome. However, the rarity of this malignancy and paucity of large studies has limited the development of standardized staging schemes and consensus recommendations for the management of MCC. Reports currently in the literature with regard to staging, surgical margins, type of surgical resection, need for SLNB, and role of adjuvant therapy remain controversial.
Accurate staging of MCC is necessary for prognostication, treatment selection, and thorough patient counseling. Unfortunately, there is no standardized staging system for MCC. Two popular staging systems are reported in the literature, each similar to the other except for the expansion from a three-stage into a four-stage system by the MSKCC group.11,16 Most reports recognize disease stage at presentation as the single most important predictor of clinical outcome.16,18–23 Five-year survival rates of 75%, 59%, and 25% have been reported for localized, regional, and distant disease, respectively, greatly underscoring the need to accurately diagnose, stage, and treat MCC early.10 Allen et al16 reported in their series of 251 patients that disease stage was the only independent predictor of survival. In a study by Eng et al,23 the 5-year overall survival rates were significantly influenced by the presence of regional disease compared with local control (42% v 68%, respectively).
Accurate staging requires evaluation of the regional lymph node basins, either by SLNB or elective lymph node dissection or by having reasonable enough evidence about the tumor biology to feel confident that no nodal metastasis are present. Many authors advocate SLNB for all patients with clinically negative lymph nodes due to its relatively low morbidity and ease and reliability of sampling the regional nodes.16,24–26 SLNB offers an attractive alternative to the more morbid elective lymph node dissection as a staging procedure and has become standard for staging in breast cancer and melanoma.14,27–29 After identification of nodal metastasis, patients may then be offered chemotherapy for disseminated disease or removal of remaining lymph nodes to prevent locoregional recurrence.
The aim of this study was to identify the risk of occult lymph node metastasis in a subset of patients with small (≤ 1 cm) MCC tumors. There is currently no literature exclusively examining the correlation between tumor size and microscopic lymph node metastasis. However, the observation of occult lymph node metastasis has been previously reported by the MSKCC group.16,30 In a study of 251 patients, Allen et al11 identified lymph node metastases in 16 (23%) of 71 patients with clinically negative nodal basins undergoing SLNB. Interestingly, positive lymph nodes were discovered in 24% of patients with tumors less than 2 cm. Patients with tumors less than 1 cm were not further separated for subgroup analysis. Our data demonstrate that patients with tumors ≤ 1 cm were significantly less likely to present with nodal metastasis. No patients with tumors ≤ 1 cm harbored occult lymph node metastasis, whereas 5% of patients with tumors between 1 and 2 cm and 12% of patients with tumors more than 2 cm in diameter did have occult lymph node metastasis. Certainly the risk of developing an occult lymph node metastasis in a patient with a tumor less than 1 cm is not zero. In a further report by Allen et al,30 five of 26 patients undergoing SLNB had lymph node metastases, one of which had a tumor of 1.0 cm. However, the present data suggest that patients with MCC less than 1 cm in diameter and clinically negative nodes have a low risk of occult nodal metastasis and may be safely observed without surgical staging of the nodal basin.
The cohort of patients analyzed in this study is composed almost entirely of men (three women). Previous reports15,16,31 have observed male to female incidences on the order of 1.2 to 1.5:1. The male predominance in our cohort reflects the male predominance in the VA population. We do not know of fundamental differences in the female patients; however, we acknowledge that the data reported here are most specifically relevant to male patients with MCC.
The overall recurrence rate for patients in this series was 21%. This is significantly less than the recurrence rates of 52% to 54% reported by other large series.15,16 Additionally, other series have reported 5-year disease-specific survival rates of 68%, which typically are higher than recurrence-free rates.31 The causality of this difference among recurrences is not clear. It is unlikely this discrepancy is due to shorter follow-up given our median follow-up was 38.5 months compared with 35,15 40,16 and 51 months.31 Also, patients in our series presented with node-negative disease in similar rates to other series (45% and 26%, respectively, v 52% and 19%),31 thus refuting a bias toward less advanced disease at presentation in our cohort. Because we were limited to only qualitative data regarding the occurrence of a recurrence and not the specific site, it is plausible that recurrences in the nodal basin or at distant sites were not captured and reported accurately. In the MSKCC series, local recurrence accounts for 10% to 14% of recurrences, whereas the regional nodal basin is the site for 25% to 64% of recurrent disease.15,16 Tumor size, and therefore stage, at diagnosis has been shown to be significant predictor of relapse-free and disease-specific survival.31–35 In the current study, there was a trend toward fewer recurrences in patients with smaller tumors (≤ 1 cm) compared with patients with larger tumors (> 2 cm), although this did not reach statistical significance. Although there was not a significant difference among patients with large and small tumors, in our series, patients in this study with tumors ≤ 1 cm had good long-term recurrence-free survival with surgical management only. The available retrospective data failed to provide significant evidence of better clinical outcomes with addition of radiation or chemotherapy.
We hypothesized that a cohort of patients with MCC could be identified with a low enough risk of regional lymph node metastasis that regional lymph node evaluation by SLNB or CLND could be reasonably and safely omitted. These data support our hypothesis, that patients with MCC tumors less than 1 cm in diameter may safely avoid surgical evaluation of regional lymph node basins. In future revisions of the staging schemes for MCC, it may be appropriate to consider tumor size ≤ 1 cm as a substage with recommendations for omission of routine lymph node evaluation.
Acknowledgment
We thank Renee Robertson, Tumor Registrar at the Salem VA Medical Center, for her assistance in accessing the database and in collecting the data.
Footnotes
Presented at the Society of Surgical Oncology 61st Annual Cancer Symposium, March 13-16, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jayme B. Stokes, Katherine S. Graw, Lynn T. Dengel, Brian R. Swenson, Craig L. Slingluff Jr, Elihu J. Ledesma
Administrative support: Todd W. Bauer, Craig L. Slingluff Jr, Elihu J. Ledesma
Provision of study materials or patients: Jayme B. Stokes, Katherine S. Graw, Brian R. Swenson, Elihu J. Ledesma
Collection and assembly of data: Jayme B. Stokes, Katherine S. Graw, Lynn T. Dengel, Elihu J. Ledesma
Data analysis and interpretation: Jayme B. Stokes, Lynn T. Dengel, Brian R. Swenson, Todd W. Bauer, Craig L. Slingluff Jr, Elihu J. Ledesma
Manuscript writing: Jayme B. Stokes, Katherine S. Graw, Lynn T. Dengel, Todd W. Bauer, Craig L. Slingluff Jr, Elihu J. Ledesma
Final approval of manuscript: Jayme B. Stokes, Katherine S. Graw, Lynn T. Dengel, Brian R. Swenson, Todd W. Bauer, Craig L. Slingluff Jr, Elihu J. Ledesma
REFERENCES
- 1.Toker C. Trabecular carcinoma of the skin: A question of title. Am J Dermatopathol. 1982;4:497–500. doi: 10.1097/00000372-198212000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Murray T, Bolden S, et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6–29. doi: 10.3322/canjclin.48.1.6. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson NC. Merkel cell carcinoma: Changing incidence trends. J Surg Oncol. 2005;89:1–4. doi: 10.1002/jso.20167. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen M. Keratin 20: immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod Pathol. 1995;8:384–388. [PubMed] [Google Scholar]
- 5.Scott MP, Helm KF. Cytokeratin 20: A marker for diagnosing Merkel cell carcinoma. Am J Dermatopathol. 1999;21:16–20. doi: 10.1097/00000372-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Moll R, Lowe A, Laufer J, et al. Cytokeratin 20 in human carcinomas: A new histodiagnostic marker detected by monoclonal antibodies. Am J Pathol. 1992;140:427–447. [PMC free article] [PubMed] [Google Scholar]
- 7.Koljonen VS. Merkel cell carcinoma. World J Surg Oncol. 2006;4:7. doi: 10.1186/1477-7819-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RW, Rabkin CS. Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol Biomarkers Prev. 1999;8:153–158. [PubMed] [Google Scholar]
- 9.Penn I, First MR. Merkel's cell carcinoma in organ recipients: Report of 41 cases. Transplantation. 1999;68:1717–1721. doi: 10.1097/00007890-199912150-00015. [DOI] [PubMed] [Google Scholar]
- 10.Agelli M, Clegg LX. Epidemiology of primary Merkel cell carcinoma in the United States. J Am Acad Dermatol. 2003;49:832–841. doi: 10.1016/s0190-9622(03)02108-x. [DOI] [PubMed] [Google Scholar]
- 11.Yiengpruksawan A, Coit DG, Thaler HT, et al. Merkel cell carcinoma: Prognosis and management. Arch Surg. 1991;126:1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]
- 12.Senchenkov A, Barnes SA, Moran SL. Predictors of survival and recurrence in the surgical treatment of Merkel cell carcinoma of the extremities. J Surg Oncol. 2007;95:229–234. doi: 10.1002/jso.20647. [DOI] [PubMed] [Google Scholar]
- 13.Gupta SG, Wang LC, Penas PF, et al. Sentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: The Dana-Farber experience and meta-analysis of the literature. Arch Dermatol. 2006;142:685–690. doi: 10.1001/archderm.142.6.685. [DOI] [PubMed] [Google Scholar]
- 14.Gipponi M, Solari N, Di Somma FC, et al. New fields of application of the sentinel lymph node biopsy in the pathologic staging of solid neoplasms: Review of literature and surgical perspectives. J Surg Oncol. 2004;85:171–179. doi: 10.1002/jso.20031. [DOI] [PubMed] [Google Scholar]
- 15.Allen PJ, Zhang ZF, Coit DG. Surgical management of Merkel cell carcinoma. Ann Surg. 1999;229:97–105. doi: 10.1097/00000658-199901000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen PJ, Bowne WB, Jaques DP, et al. Merkel cell carcinoma: Prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Medina-Franco H, Urist MM, Fiveash J, et al. Multimodality treatment of Merkel cell carcinoma: Case series and literature review of 1024 cases. Ann Surg Oncol. 2001;8:204–208. doi: 10.1007/s10434-001-0204-4. [DOI] [PubMed] [Google Scholar]
- 19.Meeuwissen JA, Bourne RG, Kearsley JH. The importance of postoperative radiation therapy in the treatment of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1995;31:325–331. doi: 10.1016/0360-3016(94)E0145-A. [DOI] [PubMed] [Google Scholar]
- 20.Pitale M, Sessions RB, Husain S. An analysis of prognostic factors in cutaneous neuroendocrine carcinoma. Laryngoscope. 1992;102:244–249. doi: 10.1288/00005537-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Fenig E, Brenner B, Katz A, et al. The role of radiation therapy and chemotherapy in the treatment of Merkel cell carcinoma. Cancer. 1997;80:881–885. doi: 10.1002/(sici)1097-0142(19970901)80:5<881::aid-cncr8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Morrison WH, Peters LJ, Silva EG, et al. The essential role of radiation therapy in securing locoregional control of Merkel cell carcinoma. Int J Radiat Oncol Biol Phys. 1990;19:583–591. doi: 10.1016/0360-3016(90)90484-2. [DOI] [PubMed] [Google Scholar]
- 23.Eng TY, Boersma MG, Fuller CD, et al. Treatment of Merkel cell carcinoma. Am J Clin Oncol. 2004;27:510–515. doi: 10.1097/01.coc.0000135567.62750.f4. [DOI] [PubMed] [Google Scholar]
- 24.Mehrany K, Otley CC, Weenig RH, et al. A meta-analysis of the prognostic significance of sentinel lymph node status in Merkel cell carcinoma. Dermatol Surg. 2002;28:113–117. doi: 10.1046/j.1524-4725.2002.02901.x. discussion 117. [DOI] [PubMed] [Google Scholar]
- 25.Ames SE, Krag DN, Brady MS. Radiolocalization of the sentinel lymph node in Merkel cell carcinoma: A clinical analysis of seven cases. J Surg Oncol. 1998;67:251–254. doi: 10.1002/(sici)1096-9098(199804)67:4<251::aid-jso8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues LK, Leong SP, Kashani-Sabet M, et al. Early experience with sentinel lymph node mapping for Merkel cell carcinoma. J Am Acad Dermatol. 2001;45:303–308. doi: 10.1067/mjd.2001.114749. [DOI] [PubMed] [Google Scholar]
- 27.Ortin-Perez J, van Rijk MC, Valdes-Olmos RA, et al. Lymphatic mapping and sentinel node biopsy in Merkel's cell carcinoma. Eur J Surg Oncol. 2007;33:119–122. doi: 10.1016/j.ejso.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Balkissoon J, Rasgon BM, Schweitzer L. Lymphatic mapping for staging of head and neck cancer. Semin Oncol. 2004;31:382–393. doi: 10.1053/j.seminoncol.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Koops HS, Doting MH, de Vries J, et al. Sentinel node biopsy as a surgical staging method for solid cancers. Radiother Oncol. 1999;51:1–7. doi: 10.1016/s0167-8140(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 30.Allen PJ, Busam K, Hill AD, et al. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer. 2001;92:1650–1655. doi: 10.1002/1097-0142(20010915)92:6<1650::aid-cncr1491>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Andea AA, Coit DG, Amin B, et al. Merkel cell carcinoma: Histologic features and prognosis. Cancer. 2008;113:2549–2558. doi: 10.1002/cncr.23874. [DOI] [PubMed] [Google Scholar]
- 32.Koljonen V, Bohling T, Granhroth G, et al. Merkel cell carcinoma: A clinicopathological study of 34 patients. Eur J Surg Oncol. 2003;29:607–610. doi: 10.1016/s0748-7983(03)00110-0. [DOI] [PubMed] [Google Scholar]
- 33.Llombart B, Monteagudo C, Lopez-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46:622–634. doi: 10.1111/j.1365-2559.2005.02158.x. [DOI] [PubMed] [Google Scholar]
- 34.Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: A clinicopathologic study with prognostic implications. J Cutan Pathol. 2004;31:217–223. doi: 10.1111/j.0303-6987.2004.00149.x. [DOI] [PubMed] [Google Scholar]
- 35.Skelton HG, Smith KJ, Hitchcock CL, et al. Merkel cell carcinoma: Analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997;37:734–739. doi: 10.1016/s0190-9622(97)70110-5. [DOI] [PubMed] [Google Scholar]