Abstract
Purpose
Single-agent gemcitabine (GEM) is standard treatment of metastatic pancreatic cancer. Fixed-dose rate (FDR) GEM and GEM plus oxaliplatin have shown promise in early clinical trials. E6201 was designed to compare overall survival (OS) of standard weekly GEM 1,000 mg/m2/30 minutes versus GEM FDR 1,500 mg/m2/150 minutes or GEM 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days (GEMOX).
Methods
This trial included patients with metastatic or locally advanced pancreatic cancer, normal organ function, and performance status of 0 to 2. The study was designed to detect a 33% difference in median survival (hazard ratio [HR] ≤ 0.75 for either of the experimental arms) with 81% power while maintaining a significance level of 2.5% in a two-sided test for each of the two primary comparisons.
Results
Eight hundred thirty-two patients were enrolled. The median survival and 1-year survival were 4.9 months (95% CI, 4.5 to 5.6) and 16% for GEM, 6.2 months (95% CI, 5.4 to 6.9), and 21% for GEM FDR (HR, 0.83; stratified log-rank P = .04), and 5.7 months (95% CI, 4.9 to 6.5) and 21% for GEMOX (HR, 0.88; stratified log-rank P = .22). Neither of these differences met the prespecified criteria for significance. Survival was 9.2 months for patients with locally advanced disease, and 5.4 months for those with metastatic disease. Grade 3/4 neutropenia and thrombocytopenia were greatest with GEM FDR. GEMOX caused higher rates of nausea, vomiting, and neuropathy.
Conclusion
Neither GEM FDR nor GEMOX resulted in substantially improved survival or symptom benefit over standard GEM in patients with advanced pancreatic cancer.
INTRODUCTION
Pancreatic adenocarcinoma is the fourth leading cause of cancer death in the United States with an anticipated 37,700 new patients and 34,300 deaths in 2008.1 It is the eighth most common cause of death from cancer worldwide.2
Gemcitabine (GEM) is the currently accepted standard treatment for pancreatic cancer,3 as no chemotherapy combination has demonstrated statistical improvement in survival, when compared to GEM alone. However, two recent trials did suggest benefit.
A randomized phase II for patients with pancreatic cancer showed improved time to treatment failure for fixed-dose rate GEM (FDR) at 10 mg/m2/min compared to GEM 30 minute infusion.4 The second, a phase III of the combination GEM FDR and oxaliplatin (GEMOX), demonstrated a higher response rate, and progression-free survival (PFS), but not overall survival (OS) compared to GEM.5
Eastern Cooperative Oncology Group (ECOG) 6201 was developed to compare standard GEM, GEM FDR, and GEMOX. Different than the prior two studies, the primary end point was overall survival.
METHODS
Eligibility Criteria
Patients age ≥ 18 years were required to have locally advanced or metastatic pancreatic adenocarcinoma with measurable or assessable disease. Patients could not have had prior chemotherapy for metastatic disease but could have had prior adjuvant chemotherapy. Any prior radiation must have been completed at least 4 weeks previously, and there had to be evidence of disease outside the radiation fields or radiologically confirmed progression of disease within the radiation fields. ECOG performance status of 0 to 2 was required. Patients had to have adequate baseline organ function including WBC ≥ 3,500/mm3, absolute neutrophil count ≥ 1,500/mm3, platelets ≥ 125,000/mm3, bilirubin lower than 2.0 mg/dL, AST lower than 3.0× upper limit of normal, creatinine ≤ 1.5× upper limit of normal. Women could be neither pregnant nor breast feeding. Patients could not have had another malignancy within the prior 5 years except for nonmetastatic, nonmelanoma skin cancers, carcinoma in situ of cervix, or cancer cured by surgery or small field radiotherapy. Patients with other active illnesses were excluded as well as those with symptomatic peripheral neuropathy ≥ grade 2. Institutional review board approval was required, and all patients signed informed consent.
Treatment
Patients were randomly assigned to treatment using a dynamic balancing algorithm that stratified for performance status, 0 to 1 and versus 2, and for locally advanced versus metastatic disease. Patients were randomly assigned to either GEM (the first cycle of GEM at 1,000 mg/m2 as a 30-minute infusion weekly for 7 weeks followed by 1 week of rest; for the subsequent cycles, patients received cycles of GEM 1,000 mg/m2/30 minutes weekly for 3 weeks followed by 1 week rest), GEM FDR (1,500 mg/m2 administered as a 150-minute infusion [10 mg/m2/min] days 1, 8, and 15 every 28 days cycle), or GEMOX (GEM 1,000 mg/m2 over 100 minutes [10 mg/m2/min] day 1 and oxaliplatin 100 mg/m2 day 2 over 120 minutes every 14 days cycle).
All patients completed a symptom assessment before therapy, and after 8 and 16 weeks.
Treatment modifications were mandated for myelosuppression or grade 3/4 toxicity. Patients requiring doses to be withheld on two or more consecutive occasions were removed from study. Patients requiring a decrease in GEM dose to lower than 500 mg/m2 were removed from study. Oxaliplatin was held for patients with persistent grade 3 or 4 neuropathy or other oxaliplatin-related symptoms, and such patients then could continue to receive 30-minute infusion GEM alone weekly for 3 weeks followed by 1 rest week.
All patients who received a single dose of assigned chemotherapy were assessable for efficacy and toxicity. Patients who progressed during the first 8 weeks of study were considered nonresponders. Patients were removed from study at the time of progressive disease. Patients could withdraw or be removed from study at the discretion of the treating physician for unacceptable toxicity. Patients removed from study for any reason were observed for 4 weeks after the last dose of chemotherapy for toxicity assessment and until death for survival duration. Patients with stable disease, or partial or complete remission were eligible to continue therapy on study until disease progression or intolerable toxicity occurred.
National Cancer Institute Common Toxicity Criteria version 2.0 was used initially during the study and, later, version 3.0 was used for toxicity reporting. Version 2.0 toxicities were mapped to version 3.0 toxicities according to Cancer Therapy Evaluation Program specifications.
Response Evaluation Criteria in Solid Tumors were utilized for response assessment at 8-week intervals.6 All responses had to be confirmed by repeat assessment at ≥ 4 weeks. Patients who had global deterioration of health status but without imaging evidence of disease progression were classified as symptomatic deterioration.
Symptom Assessment
Assessment of patient-reported pancreatic cancer symptoms was a secondary end point in the trial. Symptom severity was measured using the 8-item Functional Assessment of Cancer Therapy-Hepatobiliary Symptom Index,7 which queried pain (three items), fatigue (two items), nausea, weight loss and jaundice. The study investigators added four questions to the Functional Assessment of Cancer Therapy-Hepatobiliary Symptom Index to include appetite, malaise, everyday functional ability, and bother with treatment adverse effects.
Pharmacokinetics
Investigators from 18 centers contributed 23 sample sets after the first dose of GEM. Five time points over 4 hours were sampled from start of infusion. GEM and its metabolite difluorodeoxyuridine were quantified from acetonitrile-deproteinized plasma after perchloric acid extraction by gradient elution reverse phase high-performance liquid chromatography.8,9 Gemcitabine triphosphate was quantified by gradient elution ion-exchange high-performance liquid chromatography in neutralized peripheral mononuclear cells after removal of ribonucleotide triphosphates. Data were fit to nonlinear models (WINNonlin pro version 4.1; Scientific Consultant, Apex, NC) and comparisons among dosing groups employed the nonparametric, one-sided Mann-Whitney U-test.10
Statistical Considerations
The primary objective of this study was to compare survival of GEM FDR and GEMOX each to GEM using pair-wise comparisons. Secondary end points were the comparison of survival between the two experimental regimens and the assessment of toxicity, objective response to therapy, patterns of failure, PFS, and symptom severity across the three regimens.
Due to rapid accrual, the data monitoring committee approved the accrual expansion from 666 to 789 patients. This expanded trial was designed to be able to detect a 33% difference in median survival with 81% power while maintaining a significance level of 2.5% in a two-sided test for each of the two primary comparisons, assuming exponential failure and a median survival of 6 months for the GEM and 8 months for the GEM FDR or GEMOX.
OS and PFS curves were obtained using the Kaplan-Meier method.11 OS was defined as the time from random assignment to death, or censored at last known date of survival. PFS was defined as the time from random assignment to progression, or death without evidence of progression. For patients without documentation of progression, follow-up was censored at the date of last disease assessment without progression. Patients dying within 4 months of last disease assessment were considered to have treatment failure, with date of death the date utilized for PFS. Cox regression models12 of OS and PFS were utilized to provide adjusted treatment comparisons and identify simultaneous significant prognostic factors. Comparisons were made by fitting Cox models with the use of a stratified two-sided Wald test.13 (stratified by ECOG performance status and locally advanced versus metastatic disease). Objective tumor response rates, categoric patient characteristics, as well as toxicity, were compared among the three arms using χ2 tests with a two-sided significance level of .05. Where cell frequencies were small, Fisher's exact tests14 were used. Baseline lab and continuous patient characteristic were compared among the three arms using Kruskall-Wallis15 tests with a two-sided significance level of .05.
Baseline Patient Characteristics
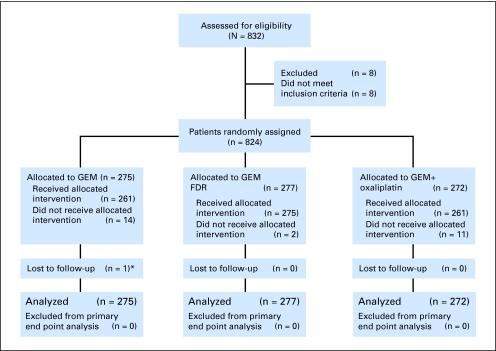
This study accrued 832 patients between March 2003 and March 2005 (Fig 1) of whom 784 patients have died. There were 30 people who never started assigned therapy, most often because of early progression or death or withdrawal from study. There was no pattern to the reasons for patients' not receiving their assigned therapy across three treatment arms (P = .35). Eight ineligible patients were excluded, leaving an analyzable set of 824 patients.
Fig 1.
CONSORT diagram. (*) One patient, case 62256, withdrew consent, did not receive allocated intervention, and was lost to follow-up. GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate.
Table 1 presents patient demographics and basic patient characteristics at entry onto the study by treatment arms.
Table 1.
Baseline Characteristics Among Eligible Patients
| Characteristic | Treatment |
|||||
|---|---|---|---|---|---|---|
| GEM (n = 275) |
GEM FDR (n = 277) |
GEMOX (n = 272) |
||||
| No. | % | No. | % | No. | % | |
| Mean age, years* | 63 | 62 | 63 | |||
| Standard deviation | 11 | 11 | 11 | |||
| Median | 64 | 61 | 63 | |||
| Range | 31-88 | 36-87 | 29-96 | |||
| Under 55 | 57 | 20.7 | 88 | 31.8 | 59 | 21.7 |
| 55-69 | 141 | 51.3 | 123 | 44.4 | 136 | 50.0 |
| 70+ | 77 | 28.0 | 66 | 23.8 | 77 | 28.3 |
| Sex† | ||||||
| Male | 155 | 56.4 | 160 | 57.8 | 124 | 45.6 |
| Female | 120 | 43.6 | 117 | 42.2 | 148 | 54.4 |
| Race | ||||||
| Hispanic | 9 | 3.3 | 12 | 4.4 | 12 | 4.4 |
| Non-Hispanic white | 235 | 85.8 | 236 | 86.1 | 231 | 85.6 |
| Non-Hispanic black | 24 | 8.8 | 22 | 8.0 | 23 | 8.5 |
| Other | 6 | 2.2 | 4 | 1.5 | 4 | 1.5 |
| Previous 6-month weight loss | ||||||
| < 5% of body weight | 100 | 36.6 | 100 | 36.8 | 107 | 39.8 |
| 5-< 10% of body weight | 71 | 26.0 | 64 | 23.5 | 65 | 24.2 |
| 10-< 20% of body weight | 78 | 28.6 | 79 | 29.0 | 65 | 24.2 |
| 20% or more of body weight | 24 | 8.8 | 29 | 10.7 | 32 | 11.9 |
| Histology grade | ||||||
| Well differentiated | 14 | 5 | 16 | 6 | 22 | 8 |
| Moderately differentiated | 82 | 30 | 71 | 26 | 56 | 21 |
| Poorly differentiated/undifferentiated | 76 | 28 | 77 | 28 | 78 | 29 |
| Missing/unknown | 103 | 38 | 113 | 41 | 116 | 43 |
| Prior RT | ||||||
| No | 254 | 92.4 | 253 | 91.3 | 250 | 91.9 |
| Yes | 21 | 7.6 | 23 | 8.3 | 21 | 7.7 |
| Prior adjuvant chemotherapy | ||||||
| No | 260 | 94.5 | 259 | 93.5 | 261 | 96.0 |
| Yes | 15 | 5.5 | 17 | 6.1 | 10 | 3.7 |
| Prior surgery | ||||||
| No | 230 | 83.6 | 234 | 84.5 | 239 | 87.9 |
| Yes | 43 | 15.6 | 42 | 15.2 | 32 | 11.8 |
| History of DVT or prior embolus | ||||||
| No | 236 | 85.8 | 240 | 86.6 | 243 | 89.3 |
| Yes | 39 | 14.2 | 36 | 13.0 | 28 | 10.3 |
| Disease measurable or not | ||||||
| Measurable only | 70 | 25.5 | 67 | 24.2 | 84 | 30.9 |
| Nonmeasurable only | 13 | 4.7 | 13 | 4.7 | 16 | 5.9 |
| Both | 192 | 69.8 | 194 | 70.0 | 172 | 63.2 |
| PS on study | ||||||
| 0 | 94 | 34.2 | 86 | 31.0 | 73 | 26.8 |
| 1 | 147 | 53.5 | 157 | 56.7 | 168 | 61.8 |
| 2 | 34 | 12.4 | 33 | 11.9 | 30 | 11.0 |
| Disease status on study | ||||||
| Locally advanced | 27 | 9.8 | 30 | 10.8 | 29 | 10.7 |
| Metastatic | 248 | 90.2 | 246 | 88.8 | 243 | 89.3 |
| Median CA19-9, U/mL | 1,961 | 1,148 | 1,077 | |||
| 25%-75% quantile | 167-12,024 | 136-9,651 | 90-9,301 | |||
| Median CEA, ng/dL | 5.7 | 5.9 | 6.3 | |||
| 25%-75% quantile | 2.3-30.9 | 2.4-30.1 | 2.4-35.5 | |||
Abbreviations: GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate; GEMOX, gemcitabine 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days; RT, radiotherapy; DVT, deep vein thrombosis; PS, performance status.
Age different among three treatment arms, P = .03 (Pearson's χ2 test).
Sex different among three treatment arms, P < .01 (Pearson's χ2 test).
Treatment
Table 2 provides a summary of treatment administered by study arm. Of the 824 eligible patients, 96% of them received at least one cycle of chemotherapy. Patients came off treatment primarily for progressive disease, but also for toxicity, the distribution of which varied significantly among the three arms (P = .03). On GEMOX, a lower proportion of patients discontinued treatment due to disease progression (48%), but a higher proportion of patients experienced treatment-terminating toxicity/adverse effects (26%). Fifty-four patients (7%) were categorized as off study for death occurring before the first assessment, presumably disease related.
Table 2.
No. of Cycles Received and Off Treatment Reason by Arm
| Parameter | Treatment |
|||||
|---|---|---|---|---|---|---|
| GEM (n = 275) |
GEM FDR (n = 277) |
GEMOX (n = 272) |
||||
| No. | % | No. | % | No. | % | |
| Mean No. of total cycles* | 3 | 3 | 5 | |||
| Standard deviation | 3 | 4 | 6 | |||
| Median | 2 | 3 | 4 | |||
| Range | 0-21 | 0-18 | 0-32 | |||
| Mean duration on treatment, days | 87 | 99 | 79 | |||
| Standard deviation | 94 | 105 | 88 | |||
| Median | 43 | 63 | 43 | |||
| Range | 0-607 | 0-883 | 0-434 | |||
| Off treatment reason | ||||||
| Disease progression/symptom deterioration | 161 | 59 | 157 | 57 | 131 | 48 |
| Toxicity/adverse effects/complications | 42 | 15 | 52 | 19 | 70 | 26 |
| Death ≤ 4 weeks after beginning protocol therapy | 19 | 7 | 18 | 7 | 17 | 6 |
| Physician/patient withdrawal | 26 | 10 | 33 | 12 | 42 | 15 |
| Alternative therapy/other complicating disease/treatment delay or cancelled/other/unknown | 27 | 10 | 17 | 6 | 12 | 4 |
NOTE. GEM: first cycle 8 weeks; subsequent cycles 4 weeks. GEM FDR: 4-week cycles. GEMOX: 2-week cycles.
Abbreviations: GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate; GEMOX, gemcitabine 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days.
Toxicity
Results are presented by treatment arm for all randomly assigned patients who received any treatment (Table 3). The most significant toxicity was myelosuppression, which was worse for GEM FDR. Grade 3 sensory neuropathy occurred in 10% of patients receiving GEMOX (P = .001).
Table 3.
Toxicity by Arm
| Toxicity Type | Treatment by Grade (%) |
|||||
|---|---|---|---|---|---|---|
| GEM (n = 264) |
GEM FDR (n = 275) |
GEMOX (n = 263) |
||||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| Allergic reaction | — | — | — | — | 2 | — |
| Hemoglobin | 8 | 2 | 16 | 3 | 5 | < 1 |
| Leukocytes* | 15 | 1 | 32 | 7 | 11 | 1 |
| Neutrophils* | 19 | 14 | 29 | 30 | 11 | 11 |
| Platelets* | 12 | 1 | 29 | 4 | 10 | 1 |
| Fatigue | 18 | 1 | 18 | 1 | 15 | 2 |
| Anorexia | 8 | — | 6 | — | 7 | < 1 |
| Dehydration | 5 | — | 3 | < 1 | 4 | — |
| Diarrhea without prior colostomy | 3 | < 1 | 1 | < 1 | 6 | — |
| Nausea and vomiting | 7 | — | 10 | 1 | 15 | 1 |
| Infection w/grade 3-4 neutropenia | 1 | < 1 | — | — | < 1 | < 1 |
| AST | 3 | — | 5 | — | 5 | < 1 |
| Bilirubin | 6 | 2 | 7 | 2 | 5 | 2 |
| Neuropathy, sensory* | 0 | — | 1 | — | 25 | — |
Abbreviations: GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate; GEMOX, gemcitabine 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days.
Grade 3 and 4 toxicities different among three treatment arms (P < .001).
Response
Two patients experienced a complete response, one receiving GEM FDR and the other on GEMOX. Partial responses were noted in 6% of patients on GEM, 10% on GEM FDR and 9% on GEMOX. There was a higher proportion of partial responses in patients with baseline PS 0 (11%) than for patients with baseline PS of 1 to 2 (7%; P < .01).
There were no significant differences in the objective response rates (complete response plus partial response) among the three arms (P = .11, χ2 test). Two hundred ninety-six patients (36%) had a best response of stable disease, and 222 patients (27%) experienced progressive disease at the time of first tumor reevaluation. An additional 222 patients (27%) were unassessable for RECIST-defined response: 89 who died within 4 months of random assignment and were coded as progression; and an additional 100 who had no response coded but had physician-determined progression. Often, rapid clinical deterioration or logistic impediments confounded the acquisition of mandated imaging studies. An additional 33 had insufficient information provided to assess response and date of progression.
OS and PFS
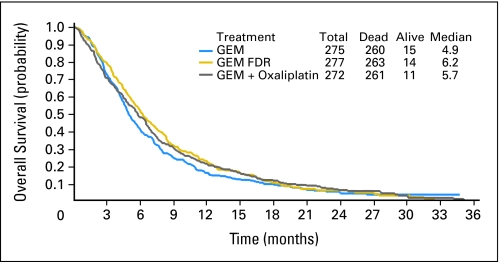
For all eligible patients, median OS was 5.6 months (95% CI, 5.2 to 6.0; Fig 2). Median survival was 4.9 months for GEM (95% CI, 4.5 to 5.6), 6.2 months for GEM FDR (95% CI, 5.4 to 6.9), and 5.7 months for GEMOX (95% CI, 4.9 to 6.5). The 1-year survival rates were 16% for GEM (SE, 2%), 22% for GEM FDR (SE, 3%); and 21% for GEMOX (SE, 3%). The 2-year survival rates were 4% (SE, 1%), 6% (SE, 2%), and 6% (SE, 2%) for GEM, GEM FDR, and GEMOX, respectively (Fig 2). The death HR for GEM FDR versus GEM was 0.83 (95% CI, 0.69 to 1.00) and 0.88 for GEMOX versus GEM (95% CI, 0.73 to 1.05). Stratified log-rank P values for GEM versus GEM FDR and for GEM versus GEM FDR were .04 and .22, respectively. Neither is statistically significant given the parameters of the study (P < .025 for statistical significance).
Fig 2.
Overall survival. GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate; GEMOX, gemcitabine 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days.
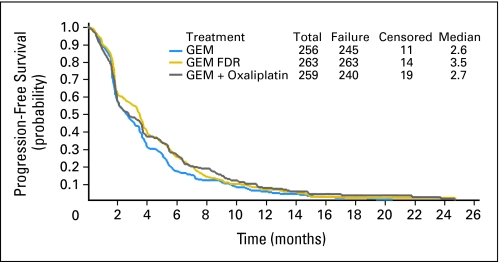
The median PFS for all eligible patients was 2.9 months (95% CI, 2.5 to 3.4; Fig 3). Median PFS for GEM, GEM FDR, and GEMOX were 2.6, 3.5, and 2.7 months, respectively. Stratified log-rank P values for GEM versus GEM FDR and for GEM versus GEMOX were .04 and .10, respectively. Neither comparison demonstrates a statistically significant difference.
Fig 3.
Progression-free survival. GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate; GEMOX, gemcitabine 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days.
Median survival was 9.2 months for patients presenting with locally advanced disease and 5.4 months for those with metastatic disease (P < .01). Similarly, median survival was better for patients with better baseline performance status (PS 0; 6.8 months) than it was for patients with PS 1 (5.3 months) and PS 2 (3.9 months; P < .01). PFS followed a similar pattern, better for patients with locally advanced and for those with better performance status. Median CA19-9 was 1,313 U/mL. Elevated CA19-9 was a significant predictor for poor OS and PFS (P < .001; data not shown).
By univariate analysis, the two experimental regimens were not found to be statistically significantly different from the control arm in terms of OS or PFS (Table 4). Multivariable proportional hazards regression models were fit to OS and PFS to confirm the univariate results after adjusting for prognostic demographic and clinical features. No substantive differences in treatment comparisons resulted from the covariate adjusted models. No statistically significant interactions with regard to PFS or survival between treatment and age, sex, or race were noted.
Table 4.
Univariate Analyses of Progression-Free and Overall Survival Among Eligible Patients
| Parameter | Progression-Free Survival |
Overall Survival |
||
|---|---|---|---|---|
| Median (months) | Log-Rank P | Median (months) | Log-Rank P | |
| All eligible patients | 2.9 | — | 5.6 | — |
| Treatment* | ||||
| GEM | 2.6 | .09 | 4.9 | .15 |
| GEM FDR | 3.5 | 6.2 | ||
| GEM + oxaliplatin | 2.7 | 5.7 | ||
| Age, years | ||||
| 55-69 | 2.8 | .65 | 5.6 | .63 |
| 70+ | 3.0 | 5.5 | ||
| Under 55 | 3.3 | 5.7 | ||
| Sex | ||||
| Female | 2.7 | .40 | 5.6 | .40 |
| Male | 3.3 | 5.7 | ||
| Race | ||||
| Hispanic | 2.6 | .69 | 6.0 | .80 |
| Non-Hispanic black | 2.2 | 4.8 | ||
| Non-Hispanic white | 3.2 | 5.7 | ||
| Other | 2.0 | 4.3 | ||
| ECOG performance status | ||||
| 0 | 3.6 | < .01 | 6.8 | < .01 |
| 1 | 2.8 | 5.3 | ||
| 2 | 2.1 | 3.9 | ||
| Disease status | ||||
| Locally advanced | 5.4 | < .01 | 9.2 | < .01 |
| Metastatic | 2.7 | 5.4 | ||
| Previous 6-month weight loss | ||||
| < 5% | 2.4 | .02 | 5.3 | .27 |
| 5-< 10% | 3.4 | 6.1 | ||
| 10-< 20% | 3.4 | 5.5 | ||
| 20% or more | 2.8 | 6.0 | ||
| Prior RT | ||||
| No | 5.5 | 0.52 | 2.9 | .53 |
| Yes | 6.9 | 3.1 | ||
| Prior adjuvant chemotherapy | ||||
| No | 5.5 | .10 | 3.0 | .14 |
| Yes | 7.3 | 2.9 | ||
| Prior surgery | ||||
| No | 5.5 | < .01 | 2.8 | .06 |
| Yes | 7.2 | 3.4 | ||
| History of DVT or prior embolus | ||||
| No | 5.8 | < .01 | 3.1 | .02 |
| Yes | 4.5 | 2.5 | ||
| Disease measurable or not | ||||
| Both | 5.3 | < .01 | 2.7 | .12 |
| Measurable | 6.8 | 3.6 | ||
| Nonmeasurable | 5.4 | 2.8 | ||
Abbreviations: GEM, gemcitabine; GEM FDR, gemcitabine fixed-dose rate; GEMOX, gemcitabine 1,000 mg/m2/100 minutes/day 1 plus oxaliplatin 100 mg/m2/day 2 every 14 days; ECOG, Eastern Cooperative Oncology Group; RT, radiotherapy; DVT, deep vein thrombosis.
Stratified by strata at random assignment.
Symptom Severity
There were 787 questionnaires completed at baseline, but only 501 at 8 weeks and 276 at 16 weeks. At baseline, lack of energy, loss of appetite, fatigue, and inability to do usual activities were the most prominent symptoms noted by 85% to 90% of patients. Pain was present at baseline in 81% of patients. There were no differences in symptom severity between groups observed at baseline. The severity of fatigue, loss of appetite loss, and weight loss did not change with time for patients remaining on study, although pain severity lessened.
Pharmacokinetics
For GEM, GEM FDR, and GEMOX the plasma GEM (mean ± standard deviation) area under the time-concentration curves (AUCs) were: 4,678 ± 2,472 (n = 9), 9,720 ± 2,608 (n = 8), and 11,276 ± 8,788 (n = 6) ng/mL/hr, respectively. The difference between GEM and GEM FDR (P = .0008) and GEM and GEMOX (P = .025) were statistically different. PBMC intracellular dFdCTP AUCs for GEM, GEM FDR, and GEMOX were: 1,958.7 ± 794 (n = 8); 6,804 ± 7,763 (n = 8), and 4,501 ± 2,113 μM/L, and significantly different for both GEM versus GEM FDR (P = .025) and GEM versus GEMOX (P = .033). These data support the finding that GEM FDR yields higher plasma GEM and PBMC dFdCTP levels than those achieved with 30-minute GEM infusion.
DISCUSSION
GEM has been the only cytotoxic drug with proven and consistent activity against advanced pancreatic cancer. Tempero and colleagues4 administered EM at 10 mg/m2/min to maximize the phosphorylation of GEM and the incorporation of dFdCTP into newly synthesized DNA, with the goal of improving response for patients. In that randomized phase II trial of GEM 2,200 mg/m2/30 minutes versus GEM FDR 1,500 mg/m2/150 minutes, the median times to failure (primary end point) were 1.8 months and 2.1 months, respectively. Median survival times for all patients were 5.0 months for GEM and 8.0 months for GEM FDR (P = .013). The phase III Groupe Cooperateur Multidisciplinaire en Oncologie/Gruppo italiano per lo studio dei carcinomi dell' apparato digerente study conducted by Louvet et al compared the combination of GEMOX to GEM alone in 313 eligible patients with advanced pancreatic cancer.5 The median OSs were 9.0 and 7.1 months, respectively (P = .13). However, whether any advantage of GEMOX was provided by the slower FDR infusion of GEM or the addition of oxaliplatin could not be determined in this smaller study.
E6201 was designed to test these two promising approaches against standard single-agent GEM in a sufficiently sized trial using a unequivocal end point of survival. Although GEM FDR was associated with the longest OS (6.2 months), this outcome did not satisfy the protocol-specified criteria for superiority. There was less evidence for superiority of the GEMOX arm over standard GEM, with OS of 5.7 months for GEMOX-treated patients. Our findings indicate that neither GEM FDR, nor GEMOX significantly increases OS or PFS in patients with advanced pancreatic carcinoma when compared to GEM by 30-minute infusion.
Survival for patients in all three arms was shorter than anticipated, perhaps because of some differences in baseline characteristics and study conduct. E6201 had fewer patients with locally advanced disease (10%) compared to Louvet et al's study (30%). This difference, along with the use of radiation therapy for some patients with locally advanced disease in the Louvet et al study, could have contributed to the differing outcomes with GEMOX in the two studies. While E6201 allowed entry of patients with measurable and assessable disease, 95% had measurable disease. In Tempero et al's study of GEM FDR, only 46% of patients had measurable metastatic disease at the time of enrollment. This difference, suggesting higher tumor burden in E6201, may have contributed to the shorter OS for patients in both the GEM as well as the GEM FDR arms of E6201 compared to those observed in the Tempero et al study.
There were differences in dose modification strategies between Tempero's study and E6201. In E6201, patients did not receive chemotherapy if grade 3 neutropenia or grade 2 thrombocytopenia was present on a midcourse treatment day, while in Tempero's study, reduced-dose GEM was given to patients with grade 3 neutropenia or grade 2 thrombocytopenia. Thus, it is possible that patients receiving GEM FDR in Tempero's study received more dose-intense treatment compared to FDR-treated patients in E6201. It is noteworthy that FDR-treated patients in the Tempero study had an 8.0-month survival compared to E6201's FDR patients, with a survival of 6.2 months.
There was a shorter median duration of treatment for GEMOX in the ECOG study compared to that in the GECOR (6 versus 17 weeks). Twenty-six percent of patients came off the GEMOX arm of E6201 for toxicity, adverse effects, or complications whereas only 10% of patients came off GEMOX for these reasons in the GERCOR study (C. Louvet, personal communication, August 2008). ECOG 6201 was initiated in 2003, shortly before oxaliplatin entered the United States market (early 2004). Toxicity concerns with this new drug may have prompted some physicians to stop oxaliplatin earlier than physicians with more experience with the drug.
Finally, the E6201 was conducted in more than 100 centers throughout the ECOG network and the United States. Results of limited institution studies often are not duplicated in large studies, with more investigators and a wide variety of patients.
There are several important observations and implications of the results of E6201. The first is the failure of this and other recent phase III trials to confirm promising results generated by smaller trials. Phase III trials of GEM plus bevacizumab (CALGB 80303)16 and GEM plus cetuximab (S0205)17 did not confirm the efficacy results obtained in earlier trials.18,19 Therefore, perhaps, different or more stringent benchmarks for promising regimens or different trial design could be developed more predictive of benefit for new regimens. In addition, only a minority of patients appear to benefit from GEM treatment. In several recent trials, specific polymorphisms in the deoxcytidine kinase, cytidine deaminase, and/or GEM transporter genes correlated with therapeutic response to GEM.20–22 To substantially improve outcome with GEM, we should consider selecting patients based on these pharmacogenomic criteria, who may be more likely to benefit from this drug. Finally, although GEM plus erlotinib resulted in longer survival in advanced pancreatic cancer patients than GEM plus placebo, the median survival for the combination arm was only 6.4 months.23 While the result was statistically significant, our goals for our patients should be substantially longer.
A decade after the Burris trial of GEM,3 we have made little progress in the treatment of advanced pancreatic cancer. Recent genetic analysis of multiple pancreatic cancers demonstrates that each cancer has large numbers of genetic alterations, likely causing disregulation of multiple pathways.24 Additional data point to the active role of pancreatic cancer stroma.25 Perhaps, the best hope for real progress in this disease will be through the coordinated use of multiple therapeutic agents or modalities that attack the most critical of these pathways.
Footnotes
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis), and supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA25224 and CA17145 from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jordan Berlin, sanofi-aventis (C); Mace L. Rothenberg, aanofi-aventis (C); Howard Hochster, aanofi-aventis (C); Edith Mitchell, sanofi-aventis (C); Daniel Haller, sanofi-aventis (C); Al Bowen Benson III, sanofi-aventis (C) Stock Ownership: None Honoraria: Howard Hochster, sanofi-aventis, Eli Lilly & Co; Daniel Haller, sanofi-aventis Research Funding: Elizabeth Poplin, sanofi-aventis; Mace L. Rothenberg, sanofi-aventis; Howard Hochster, sanofi-Aventis, Eli Lilly & Co; Steven Alberts, sanofi-aventis; Peter O”Dwyer, sanofi-aventis, Eli Lilly & Co.; Al Bowen Benson III, sanofi-aventis, Eli Lilly & Co. Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth Poplin, Jordan Berlin, Mace L. Rothenberg, Howard Hochster, Peter O'Dwyer, Daniel Haller, David Cella, Al Bowen Benson III
Provision of study materials or patients: Peter O'Dwyer, Daniel Haller
Collection and assembly of data: Yang Feng, Howard Hochster
Data analysis and interpretation: Elizabeth Poplin, Yang Feng, Mace L. Rothenberg, Howard Hochster, Peter O'Dwyer, David Cella
Manuscript writing: Elizabeth Poplin, Yang Feng, Jordan Berlin, Mace L. Rothenberg, Howard Hochster, Daniel Haller, David Cella
Final approval of manuscript: Elizabeth Poplin, Yang Feng, Jordan Berlin, Mace L. Rothenberg, Howard Hochster, Edith Mitchell, Steven Alberts, Peter O'Dwyer, Daniel Haller, Paul Catalano, David Cella, Al Bowen Benson III
REFERENCES
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2008. Facts and Figures 2008. [Google Scholar]
- 2.Parkin M, Bray FJ, Ferlay J, et al. Global Cancer Statistics 2002. CA: Cancer J for Clinicians. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Tempero M, Plunkett W, Ruiz van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 5.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 7.Yount S, Cella D, Webster K, et al. Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancers: The FACT Hepatobiliary Symptom Index. J Pain Symptom Management. 2002;24:32–44 S. doi: 10.1016/s0885-3924(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 8.Blackstock AW, Lightfoot H, Case LD, et al. Tumor uptake and elimination of 21,21-deoxycytidine (gemcitabine) after deoxycytidine kinase gene transfer: Correlation with in vivo tumor response. Clin Cancer Res. 2001;7:3263–3268. [PubMed] [Google Scholar]
- 9.Venook AP, Egorin MJ, Rosner GL, et al. Phase I and pharmacokinetic trial of gemcitabine in patients with hepatic or renal dysfunction: Cancer and Leukemia Group B 9565. J Clin Oncol. 2000;18:2780–2787. doi: 10.1200/JCO.2000.18.14.2780. [DOI] [PubMed] [Google Scholar]
- 10.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics. 1947;18:50–60. [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. American Statistical Association. 1958;53:457–481. [Google Scholar]
- 12.Cox DR. Regression models and life tables. Journal Royal Statistical Society B. 1972;34:187–220. [Google Scholar]
- 13.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 14.Fisher RA. ed 11. New York, NY: Hafner Publishing Company; 1950. Statistical Methods for Research Workers; p. 107. [Google Scholar]
- 15.Walpole RE, Meyers RH. ed 2. New York, NY: MacMillian; 1978. Probability and Statistics for Engineers and Scientists. [Google Scholar]
- 16.Kindler HL, Niedzwiecki D, Hollis D, et al. A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB) J Clin Oncol. 2007;25(suppl):199s. abstr 4508. [Google Scholar]
- 17.Philip PA, Benedetti J, Fenoglio-Preiser C, et al. Phase III study of gemcitabine (G) plus cetuximab (C) versus gemcitabine in patients (pts) with locally advanced or metastatic pancreatic adenocarcinoma (Pca): SWOG S0205 study. J Clin Oncol. 2007;25(suppl):199s. abstr LBA4509. [Google Scholar]
- 18.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 19.Kindler HL, Friberg G, Singh D, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 20.Giovannetti E, Del Tacca M, Mey V, et al. Transcription analysis of human equilibrative nucleoside transporter-1 predicts survival in pancreas cancer patients treated with gemcitabine. Cancer Res. 2006;66:3928–3935. doi: 10.1158/0008-5472.CAN-05-4203. [DOI] [PubMed] [Google Scholar]
- 21.Ueno H, Kiyosawa K, Kaniwa N. Pharmacogenomics of gemcitabine: Can genetics lead to tailor made therapy? Br J Cancer. 2007;97:145–151. doi: 10.1038/sj.bjc.6603860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javle MM, Okazaki T, Evans DB, et al. Polymorphisms of genes involved in gemcitabine metabolism correlate with prognosis in patients receiving neoadjuvant therapy for pancreatic cancer. J Clin Oncol. 2008;26(suppl):213s. abstr 4501. [Google Scholar]
- 23.Moore MJ, Goldstein D, Hamm J, et al. National Cancer Institute of Canada Clinical Trials Group: Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 24.Jones S, Zhang X, Williams Parsons D, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu GC, Kimmelman AC, Hezel AF, et al. Stromal biology of pancreatic cancer. J Cellular Biochemistry. 2008;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]