Abstract
Introduction. The risk of death after coronary revascularization is markedly higher for dialysis patients than for the general population and the cause is inadequately explained. We analyzed cause-specific mortality of dialysis patients after coronary revascularization.
Methods. This was a retrospective analysis of dialysis patients hospitalized for first surgical coronary revascularization after renal replacement therapy initiation from 1 January 1999 to 31 December 2002. Patients were identified from the US Renal Data System database (n = 1 516 251) by the International Classification of Diseases, Ninth Edition, Clinical Modification code for coronary artery bypass (CAB) surgery (36.1×). Endpoints were deaths due to all causes, all cardiac causes, cardiac arrest or arrhythmia, myocardial infarction, infection and other causes. Cause-specific mortality information was obtained from Centers for Medicare & Medicaid Services End-Stage Renal Disease Death Notification form (CMS 2746-U3).
Results. For CAB patients (n = 5830), the all-cause mortality rate was 290 per 1000 patient-years and the rate for arrhythmically mediated deaths was 76 per 1000 patient-years. The largest cause of attributable mortality is cardiac arrest or arrhythmia, accounting for approximately one-fourth of all-cause mortality.
Conclusions. The risk of arrhythmically mediated death may contribute to poor long-term outcomes after coronary revascularization in dialysis patients. A treatment strategy employing coronary revascularization and other interventions to reduce the sudden cardiac death risk might improve long-term survival.
Keywords: coronary artery bypass surgery, mortality, renal dialysis
Introduction
The death rate for dialysis patients is extraordinarily high. The rate of all-cause mortality for US dialysis patients in 2004 was 230 deaths per 1000 patient-years [1]. Cardiac disease is the major cause of death, accounting for 43% of all-cause mortality [1]. The single largest cause of death among dialysis patients is linked to arrhythmic mechanisms, with 62% of all cardiac deaths ascribed to cardiac arrest or arrhythmia [1]. Although the mortality hazard is markedly lower in the general population, the relative proportion of arrhythmically mediated deaths is qualitatively similar, as 63% of all cardiac deaths in the United States from 1989 to 1998 were reportedly due to sudden cardiac death [2]. Obstructive coronary artery disease is the most common cause of sudden cardiac death in the general population [3], and myocardial revascularization is a primary therapy for the reduction of sudden cardiac death [3,4].
Dialysis patients are vulnerable to sudden cardiac death. Factors including obstructive coronary artery disease, left ventricular hypertrophy, electrolyte shifts and abnormal myocardial ultrastructure and function [5–7] have been implicated in the heightened risk of arrhythmically mediated death. The relative contribution of these individual factors to the overall hazard of sudden cardiac death is controversial; HEMO study data point to ischaemic heart disease as the single largest cause of sudden cardiac death [8,9]. If ischaemic heart disease were indeed the largest cause of sudden cardiac death in dialysis patients, coronary revascularization should play a key role in reducing the hazard of arrhythmically mediated death.
We previously reported on long-term survival of US dialysis patients after percutaneous and surgical coronary revascularization procedures. Two-year mortality for dialysis patients who had their first coronary artery revascularization procedure in 1995 to 1998 was 52% after both percutaneous transluminal coronary angioplasty and coronary artery stenting, and 44% after coronary artery bypass (CAB) surgery [10]. In contrast, in the Bypass Angioplasty Revascularization Investigation (BARI) Study in the general population, 5-year mortality for patients with multivessel coronary artery disease was 13.7% after percutanueous transluminal coronary angioplasty (PTCA) and 11.7% after CAB. Even among the high-risk subset of BARI patients with diabetes mellitus, 5-year mortality was 34.5% after PTCA and 19.4% after CAB [11]. The risk of death after coronary revascularization is therefore markedly higher for dialysis patients compared with the general population, and the cause is not adequately explained.
The purpose of the present study was to analyze cause-specific mortality of dialysis patients after coronary revascularization. We hypothesized that, in contrast to the general population of non-dialysis patients undergoing coronary revascularization, the hazard of arrhythmically mediated death may not be attenuated even among dialysis patients undergoing primary therapy for ischaemic heart disease. To reduce the potential confounding influence of inadequate or incomplete long-term coronary revascularization for the amelioration of myocardial ischaemia, we focus our analysis on patients receiving the ‘best’ (from a survival standpoint) long-term revascularization strategy for treatment of myocardial ischaemia: surgical coronary artery revascularization including the use of the internal mammary artery as a graft conduit [12–14]. Using the US Renal Data System (USRDS) database, we examined outcomes for chronic dialysis patients after surgical coronary revascularization (CAB).
Materials and methods
All data were derived from the USRDS. The accuracy of these data has been validated previously [15]. The study was a retrospective analysis of dialysis patients hospitalized for first coronary revascularization after renal replacement therapy initiation from 1 January 1999 to 31 December 2002. Patients were identified from the USRDS database (n = 1 516 251) by International Classification of Diseases, Ninth Edition, Clinical Modification code for CAB surgery (36.1×). Internal mammary graft use in CAB surgery was determined from codes 36.15 and 36.16. As the best surgical coronary revascularization outcomes in the general population are obtained with procedures that include use of internal mammary grafts [12–14], we stratified our survival analysis by the use of internal mammary grafts. Thus, patients with internal mammary grafts are the primary focus, and an additional group of patients undergoing CAB without the use of internal mammary grafts was analyzed separately.
Eligible patients received renal replacement therapy for ≥90 days and were on dialysis for ≥60 days before revascularization. Patient demographic data included age, gender, race, renal diagnosis and prior end-stage renal disease (ESRD) duration.
Time to event was calculated from the time of coronary revascularization to censoring or endpoint. Study endpoints were deaths due to all causes, all cardiac causes, cardiac arrest or arrhythmia (i.e. ‘arrhythmic mechanisms’), myocardial infarction, infection and other causes (not due to cardiac arrest or arrhythmia, myocardial infarction or infection). Cause-specific mortality information was obtained from the Centers for Medicare & Medicaid Services ESRD Death Notification form (CMS 2746-U3). Patients were censored at renal transplantation or loss to follow-up. The probability of mortality was estimated by the Kaplan–Meier method. Unadjusted cause-specific mortality rates were calculated for each group of interest.
Results
Among dialysis patients, 5830 underwent CAB surgery that included use of internal mammary grafts (Table 1). Patients who underwent CAB surgery without the use of internal mammary grafts (n = 2919) were initially excluded from the analysis, but we provide additional survival data for the excluded group (Table 2) to amplify the study findings. The median survival time for CAB patients was 2.55 (95% confidence interval 2.46–2.67) years. A total of 2965 CAB patients died. The all-cause mortality rate was 290 deaths per 1000 patient-years (Table 2). The corresponding rate for deaths attributable to arrhythmic mechanisms (cardiac arrest or arrhythmia identified as cause of death on form CMS-2746-U3) was 76 per 1000 patient-years, accounting for approximately one-fourth of all-cause deaths. Among patients undergoing CAB without the use of internal mammary artery grafting (IMG-), the all-cause mortality rate was 400 deaths per 1000 patient-years, and the mortality rate ascribed to arrhythmic mechanisms was 104 deaths per 1000 patient years. Table 3 summarizes the cause-specific event-free survival of patients after CAB including the use of internal mammary grafts.
Table 1.
Demographic characteristics
| Characteristics | CAB IMG+ | |
|---|---|---|
| n | Percent | |
| 5830 | ||
| Age (years) | ||
| <45 | 437 | 7.5 |
| 45–64 | 2724 | 46.7 |
| 65–74 | 1911 | 32.8 |
| ≥75 | 758 | 13.0 |
| Gender | ||
| Male | 3685 | 63.2 |
| Female | 2145 | 36.8 |
| Race | ||
| White | 3974 | 68.2 |
| Black | 1500 | 25.7 |
| Other | 356 | 6.1 |
| Cause of renal failure | ||
| Diabetes | 3125 | 53.6 |
| Hypertension | 1341 | 23.0 |
| Other | 1353 | 23.2 |
| ESRD duration (years) | ||
| <1 | 1096 | 18.8 |
| 1–2 | 1207 | 20.7 |
| 2–5 | 2250 | 38.6 |
| ≥5 | 1271 | 21.8 |
CAB, coronary artery bypass surgery; ESRD, end-stage renal disease; IMG+, with internal mammary artery graft.
Table 2.
Unadjusted estimated cause-specific mortality rates per 1000 patient-years
| Cause of death | Coronary revascularization | |
|---|---|---|
| CAB IMG+ | CAB IMG− | |
| All-cause | 290.3 | 400.0 |
| All cardiac | 138.9 | 199.4 |
| Cardiac arrest/arrhythmia | 76.1 | 104.2 |
| Infection | 39.6 | 48.8 |
| Other | 111.7 | 151.9 |
CAB, coronary artery bypass surgery; IMG+, with internal mammary artery graft; IMG−, without internal mammary artery graft.
Table 3.
Estimated event-free survival after coronary artery bypass surgery (IMG+) by mortality cause over time
| Cause of death | Years | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| All-cause | 0.712 | 0.571 | 0.445 |
| All cardiac | 0.842 | 0.759 | 0.684 |
| Cardiac arrest/arrhythmia | 0.912 | 0.862 | 0.808 |
| Myocardial infarction | 0.963 | 0.940 | 0.924 |
| Infection | 0.954 | 0.926 | 0.890 |
| Other | 0.888 | 0.812 | 0.731 |
IMG+, with internal mammary artery graft.
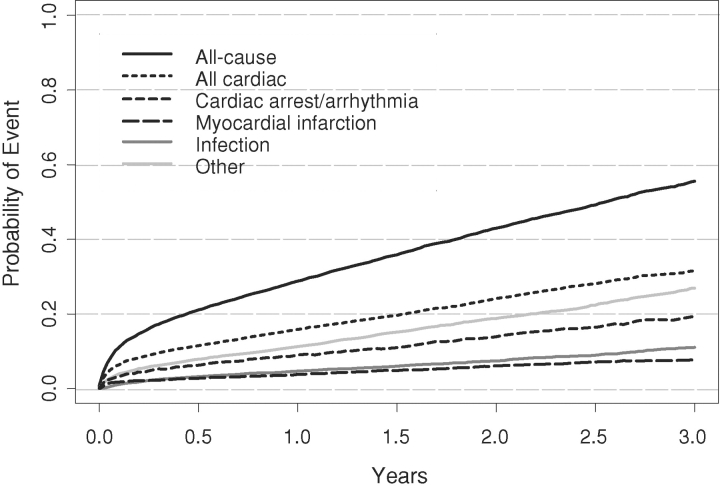
Figure 1 graphically displays the Kaplan–Meier estimates for the probability of all-cause and cause-specific mortality for CAB patients. There is an early peri-operative (<30 days) hazard of all-cause death.
Fig. 1.
Kaplan-Meier estimates for the probability of all-cause and cause-specific mortality after coronary artery bypass surgery.
Discussion
Our data indicate that the single largest cause of death among dialysis patients after surgical coronary revascularization is attributable to arrhythmic mechanisms. In this population of patients with ischaemic heart disease, treated with optimal surgical coronary revascularization (CAB including the use of internal mammary grafts), the probability of arrhythmically mediated death was not lower than that reported for the entire US dialysis population. In 2002, the 2-year probability of all-cause death was 40% and sudden cardiac death 14% in the prevalent US dialysis population [1]. In the present study, for dialysis patients receiving CAB surgery with internal mammary artery graft use, 2-year all-cause mortality was 43% and mortality attributed to arrhythmic mechanisms was 14%. Our data suggest that the persistent risk of arrhythmically mediated death is a major contributor to poor long-term outcomes after surgical coronary revascularization in dialysis patients.
Prior studies of coronary revascularization have suggested a reduction in sudden death after surgical coronary revascularization, but studies attempting to further analyze the potential additional benefit of defibrillators have yielded mixed results. Prior clinical trials [the Coronary Artery Surgery Study (CASS) and the European Coronary Surgery Study] [16,17] indicate that the risk of sudden death is reduced after surgical coronary revascularization compared with medical therapy. In the Coronary Artery Bypass Graft Patch trial [18], prophylactic defibrillator therapy in patients with impaired left ventricular systolic function did not further improve the outcome for patients receiving surgical coronary revascularization. This lack of detectible additional benefit with defibrillators is attributable to the significant reduction of sudden death after surgical coronary revascularization [19]. Makikallio et al. [4] reported in an observational study that myocardial revascularization is the treatment strategy associated with the greatest reduction in the risk of sudden cardiac death (compared with medical treatment with beta-blockers, aspirin, statins and angiotensin-converting enzyme inhibitor agents). In contrast, in the Antiarrhythmics versus Implantable Defibrillators registry [20], the survival benefit of defibrillators was not attenuated by surgical coronary revascularization. In a retrospective analysis of patients receiving implantable cardioverter defibrillators, Brockes et al. [21] reported similar rates of delivered shocks and mortality in patients with and without coronary revascularization, implying that the risk of sudden death is not always nullified by coronary revascularization.
Prior publications on percutaneous and surgical coronary artery revascularization in the general population provide a frame of reference for the long-term risk of sudden cardiac death after coronary revascularization. Holmes et al. [22], using the 1985 to 1986 National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty (NHLBI PTCA) Registry of 2127 patients, reported an overall 5-year cardiac mortality of 5.3%, noncardiac mortality of 4.8% and 5-year sudden death incidence of 2.0%. In the higher risk subset of patients with a history of congestive heart failure, 5-year cardiac mortality was 27.2%, noncardiac mortality 15.7% and the 5-year sudden death incidence 10.5%. In patients with severe concomitant noncardiac disease, 5-year cardiac mortality was 14.7%, noncardiac mortality 12.9% and 5-year sudden death incidence 3.2%. Finally, in a subset analysis of 951 patients receiving coronary revascularization (CAB surgery or percutaneous coronary intervention) before being enrolled in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II), Goldenberg et al. [23] reported a significant time-dependent relationship of prior coronary revascularization and implantable cardioverter defibrillator benefit: 36% reduction in the all-cause mortality risk and 68% reduction in the sudden cardiac death risk in patients undergoing coronary revascularization more than 6 months before MADIT-II enrollment, but no benefit in patients enrolled within 6 months of coronary revascularization.
The magnitude of the sudden death risk in the CASS study is considerably lower than in our study. For the highest risk quartile in the CASS study, the 5-year incidence of sudden cardiac death was 16% for medically treated and 5% for surgically treated patients. In the entire study, there was nearly a threefold relative risk of sudden death in the medical group compared with the CAB surgery group [16]. In contrast, we report a 14% 2-year incidence of arrhythmically mediated death in dialysis patients after CAB surgery.
Our study is limited in several ways. The USRDS database consists of predominantly administrative data. Clinical data including coronary anatomy, left ventricular ejection fraction and anatomic adequacy of coronary revascularization cannot be determined from this source. Cause-specific mortality is not based on adjudication, as would occur in a clinical trial, but from information obtained from the Centers for Medicare & Medicaid Services ESRD Death Notification form. Our definition of ‘arrhythmically mediated death’, obtained from the USRDS database, may not be identical to ‘sudden death’ or ‘sudden cardiac death’ reported in clinical trials. Our estimate of the frequency of arrhythmically mediated death (as an equivalent to sudden death), however, is likely to be accurate, as the proportion of mortality attributed to arrhythmic mechanisms in the USRDS database (about 27%) is similar to the 25–26% sudden death (as a percentage of all-cause mortality) reported in the 4D [24] and HEMO [9] trials.
Our study does not provide any information on the potential benefits of surgical coronary revascularization in improving survival. It is plausible (but not studied) that our study patients who received coronary revascularization for the treatment of obstructive coronary artery disease could have had worse outcomes with a more conservative approach. We do not suggest that coronary revascularization in dialysis patients is not efficacious. Rather, a significant residual hazard of arrhythmically mediated death remains that is not nullified by coronary revascularization. Sudden cardiac death is a major manifestation of ischaemic heart disease [3]. Although myocardial ischaemia is likely a trigger for sudden cardiac death in dialysis patients, our data suggest that other mechanisms (such as left ventricular hypertrophy and myocardial fibrosis) may be paramount in the ESRD population [25].
Coronary revascularization may be a particularly incomplete therapy for cardiac disease in ESRD patients, as a large untreated hazard of arrhythmic death may remain. In this special population, additional treatment strategies targeting the ‘non-ischaemic’ contributors to sudden cardiac death may be necessary.
Acknowledgments
The authors thank Nan Booth, MSW, MPH, and Shane Nygaard, BA, of the USRDS Coordinating Center, for manuscript editing and preparation, respectively. The Cardiovascular Special Studies Center of the United States Renal Data System is supported by Contract No. HHSN267200715003C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland). This study was performed as a deliverable. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Conflict of interest statement. C.A.H. has received research support from Medtronic, has served as a consultant to Medtronic and Guidant Corporation, and has equity interest in Cambridge Heart. J.S., A.J.C. and D.T.G. have no conflict of interest with the subject matter of this manuscript.
References
- 1.U.S. Renal Data System. USRDS 2006 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2006.
- 2.Zheng ZJ, Croft JB, Giles WH, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 3.Rourke R. Role of myocardial revascularization in sudden cardiac death. Circulation. 1992;85(Suppl 1):1112–1117. [PubMed] [Google Scholar]
- 4.Makikallio TH, Barthel P, Schneider R, et al. Frequency of sudden cardiac death among acute myocardial infarction survivors with optimized medical and revascularization therapy. Am J Cardiol. 2006;97:480–484. doi: 10.1016/j.amjcard.2005.09.077. [DOI] [PubMed] [Google Scholar]
- 5.Amann K, Ritz E. Cardiac disease in chronic uremia: pathophysiology. Adv Ren Replace Ther. 1997;4:212–224. doi: 10.1016/s1073-4449(97)70030-x. [DOI] [PubMed] [Google Scholar]
- 6.Ritz E, Amann K, Tornig J, et al. Some cardiac abnormalities in renal failure. Adv Nephrol Necker Hosp. 1997;27:85–103. [PubMed] [Google Scholar]
- 7.Amann K, Buzello M, Simonaviciene A, et al. Capillary/myocyte mismatch in the heart in renal failure—a role for erythropoietin-grou? Nephrol Dial Transplant. 2000;15:964–969. doi: 10.1093/ndt/15.7.964. [DOI] [PubMed] [Google Scholar]
- 8.Rocco MV, Yan G, Gassman J, et al. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration. Am J Kidney Dis. 2002;39:146–153. doi: 10.1053/ajkd.2002.29905. [DOI] [PubMed] [Google Scholar]
- 9.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65:2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 10.Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 2002;106:2207–2211. doi: 10.1161/01.cir.0000035248.71165.eb. [DOI] [PubMed] [Google Scholar]
- 11.The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson TB Jr, Peterson ED, Coombs LP, et al. Use of continuous quality improvement to increase use of process measures in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. JAMA. 2003;290:49–56. doi: 10.1001/jama.290.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Cameron A, Davis KB, Green G, et al. Coronary bypass surgery with internal-thoracic-artery grafts—effects on survival over a 15-year period. N Engl J Med. 1996;334:216–219. doi: 10.1056/NEJM199601253340402. [DOI] [PubMed] [Google Scholar]
- 14.Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med. 1986;314:1–6. doi: 10.1056/NEJM198601023140101. [DOI] [PubMed] [Google Scholar]
- 15.U.S.Renal Data System. USRDS 1992 Annual Data Report. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 1992.
- 16.Holmes DR Jr, Davis KB, Mock MB, et al. The effect of medical and surgical treatment on subsequent sudden cardiac death in patients with coronary artery disease: a report from the Coronary Artery Surgery Study. Circulation. 1986;73:1254–1263. doi: 10.1161/01.cir.73.6.1254. [DOI] [PubMed] [Google Scholar]
- 17.European Coronary Surgery Study Group. Long-term results of prospective randomised study of coronary artery bypass surgery in stable angina pectoris. Lancet. 1982;2:1173–1180. [PubMed] [Google Scholar]
- 18.Bigger JT Jr. Coronary Artery Bypass Graft (CABG) Patch Trial Investigators. Prophylactic use of implanted cardiac defibrillators in patients at high risk for ventricular arrhythmias after coronary-artery bypass graft surgery. N Engl J Med. 1997;337:1569–1575. doi: 10.1056/NEJM199711273372201. [DOI] [PubMed] [Google Scholar]
- 19.Veenhuyzen GD, Singh SN, McAreavey D, et al. Prior coronary artery bypass surgery and risk of death among patients with ischemic left ventricular dysfunction. Circulation. 2001;104:1489–1493. doi: 10.1161/hc3801.096335. [DOI] [PubMed] [Google Scholar]
- 20.Cook JR, Rizo-Patron C, Curtis AB, et al. Effect of surgical revascularization in patients with coronary artery disease and ventricular tachycardia or fibrillation in the Antiarrhythmics Versus Implantable Defibrillators (AVID) Registry. Am Heart J. 2002;143:821–826. doi: 10.1067/mhj.2002.121732. [DOI] [PubMed] [Google Scholar]
- 21.Brockes C, Rahn-Schonbeck M, Duru F, et al. ICD implantation with and without combined myocardial revascularisation—incidence of ICD therapy and late survival. Thorac Cardiovasc Surg. 2002;50:333–336. doi: 10.1055/s-2002-35741. [DOI] [PubMed] [Google Scholar]
- 22.Holmes DR, Jr, Kip KE, Kelsey SF, et al. Cause of death analysis in the NHLBI PTCA Registry: results and considerations for evaluating long-term survival after coronary interventions. J Am Coll Cardiol. 1997;30:881–887. doi: 10.1016/s0735-1097(97)00249-0. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg I, Moss AJ, McNitt S, et al. Time dependence of defibrillator benefit after coronary revascularization in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:1811–1817. doi: 10.1016/j.jacc.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 24.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 25.Mark PB, Johnston N, Groenning BA, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69:1839–1845. doi: 10.1038/sj.ki.5000249. [DOI] [PubMed] [Google Scholar]