Abstract
Background. Adiponectin (ADPN) levels are consistently elevated among patients with advanced chronic kidney disease, but its relationship with cardiovascular outcomes in this population remains controversial.
Methods. We measured baseline and yearly plasma ADPN in 182 prevalent haemodialysis patients recruited to the Haemodialysis (HEMO) Study from two Boston centres. Plasma ADPN at baseline and during follow-up was studied in relation to prevalent cardiovascular disease (CVD) and cardiovascular and all-cause mortality.
Results. Baseline plasma ADPN levels were found to be approximately twofold higher than in the general population and correlated inversely with (log-transformed) CRP levels and (log-transformed) body mass index (BMI). Levels measured over time showed a gradual increase (0.95 μg/mL, 95% CI = 0.12–1.78 μg/mL; P = 0.03) by year, although this difference became non-significant after adjustment for covariates. Baseline ADPN levels were lower among patients with pre-existing CVD (adjusted OR of 0.67; P = 0.03). They also predicted all-cause mortality (P < 0.01) and the composite outcome of ‘cardiovascular events/cardiovascular mortality’ (P < 0.01); levels measured over time predicted the composite outcome of ‘cardiovascular events and all-cause mortality’ (P < 0.01). These relationships were non-linear (quadratic) with the hazard for each outcome increasing in the lower and upper ranges of the distribution of ADPN, and strengthened after adjustment for baseline covariates including serum albumin, CVD and the flux and dialysis dose categorization of the HEMO study.
Conclusions. In summary, low plasma levels of ADPN were associated with inflammation and pre-existing CVD; ADPN levels predicted cardiovascular and mortality outcomes, the relationship being extensively confounded by multiple patient-related factors.
Keywords: adiponectin, cardiovascular disease, ESRD, inflammation, mortality
Introduction
Adiponectin (ADPN) is a multifunctional adipokine with favorable effects on glucose and lipid metabolism, insulin resistance and inflammation, and has been shown to play a protective role in experimental models of vascular injury [1]. Levels are consistently elevated among patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) [2–4], despite which a markedly increased risk of cardiovascular morbidity and mortality exists [5]. While ADPN may be a potential modulator of cardiovascular risk, both directly and through the metabolic processes that elevate this risk, epidemiological evidence has not consistently supported elevated levels being protective for adverse outcomes. Given the multiplicity of biologic pathways impacted upon by this adipokine extensive confounding in differing subject populations, especially by other cytokine responses, may be a major reason for varying results. Moreover, prior epidemiological studies have relied on single measurements as a predictor of outcome and the potential variability of ADPN over time in the context of an inflammatory state has not been explored.
We measured ADPN levels at study enrolment and annually in a cohort of patients undergoing haemodialysis (HD), who were enrolled into the baseline phase of the NIH-sponsored HEMO study. We examined the ability of ADPN levels at baseline and repeated over time to predict prevalent and incident cardiovascular disease, as well as cardiovascular and all-cause mortality.
Patients and methods
Subjects
The study cohort consisted of 182 patients with ESRD on maintenance HD recruited to the baseline phase of the Haemodialysis (HEMO) Study from two Boston centres. This ancillary study was approved by the Human Investigation Review Committee and all participants provided written, informed consent.
The details of the NIH-sponsored HEMO Study have been published elsewhere [6,7]. Briefly, this study, initiated in 1995, was sponsored by the U.S. National Institute of Diabetes, Digestive and Kidney Diseases, and was a multi-centre, prospective, randomized clinical trial designed to evaluate the effect of dialyzer urea and β2-microglobulin clearances on morbidity and mortality. Eligible patients were between the ages of 18 and 80 years, were receiving chronic HD three times per week, and had residual renal urea clearance <1.5 mL/min/35 L of urea distribution volume. Patients in acute or chronic care hospitals, with active malignancy, decompensated cardiac, hepatic, or pulmonary disease, serum albumin <2.5 g/dL, pregnancy, or a scheduled or recently (<6 months) failed transplant were excluded. Eligible patients were randomly assigned in a 1:1 ratio with a two-by-two factorial design to either a standard dose (single-pool Kt/V of 1.25) or a high dose (single-pool Kt/V of 1.65) goal and to dialysis with either a low-flux (minimum values for ultrafiltration coefficient ≤14 mL/h/mmHg and first use β2-microglobulin clearance <10 mL/min) or a high-flux dialyzer (minimum values for ultrafiltration coefficient >14 mL/h/mmHg and first use β2-microglobulin clearance >20 mL/min). The planned follow-up ranged from 1 to 6.5 years depending on the time of randomization.
Data procurement
Clinical data
Demographic, medical and socioeconomic information were obtained in the baseline phase of the study. HD prescription and monitoring of routine laboratory parameters followed the protocol of the HEMO study. Comorbidities were catalogued using the Index of Co-Existing Diseases (ICED) [8]. The highest scores of IDS (Index of Disease Severity) and IPI (Index of Physical Impairment) were combined to create the ICED score, from 0 to 3 (0 indicating the absence of disease and increasing values indicating the increasing severity of the disease), and diabetes-related scores were excluded from the final severity scores.
Characterization of vascular disease definition at baseline
Characterization of vascular disease definition at baseline has been previously described [9]. Briefly, the IDS scores for ischaemic heart disease (IHD), peripheral vascular disease (PVD) and cerebrovascular disease (CeVD) scores were considered on a scale of 0–3. The presence of any cardiovascular disease (CVD) was defined by the presence of any grade of IHD, PVD or CeVD, and the extent of atherosclerotic vascular disease by the total number of coexistent vascular diseases in the individual patient was expressed as a numerical score of 0–3.
Mortality and cardiac outcomes during follow-up
The primary outcome was death from any cause (all-cause mortality, ACM). Two secondary end points were as follows: time to a composite of the first hospitalization for cardiac causes or death from cardiac causes (‘cardiovascular event or cardiovascular mortality’, CVE/CVM), and time to a composite of the first hospitalization for cardiac causes or death from any cause (‘cardiovascular event or all-cause mortality’, CVE/ACM). Hospitalization for cardiac causes included those for angina, congestive heart failure, myocardial infarction, arrhythmia, or other heart disease. Cardiac death referred to deaths due to IHD, CHF, arrhythmias or other heart disease. The composite outcomes therefore represented new onset cardiac hospitalization events as well as cardiac death events. Use of ACM in the last composite outcome maximized the number of events captured. Both these composite outcomes were also the secondary outcomes defined for the HEMO study [6].
Blood samples
Baseline blood samples were obtained pre-dialysis within 1 month of enrollment and follow-up blood samples at yearly intervals (range ± 2 months to allow for intercurrent illness or other causes for inability to provide a blood sample or with potential to alter plasma cytokine levels). Heparinized blood samples (30 mL) were immediately placed on ice, plasma separated within 30 min of sample collection, aliquoted and stored at −80°C. ADPN levels were measured using a commercially available enzyme immunoassay kit (R&D Systems, Minneapolis, MN, USA). The coefficients of variation for intra- and inter-assay precision were <5% and <7%, respectively. IL-6 levels were measured using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) (Quantikine® HS human IL-6 assay, R&D Systems, Minneapolis, MN, USA) with a lower limit of detection for IL-6 of 0.094 pg/mL. C-reactive protein (CRP) levels were measured using a high-sensitivity immunoassay (Hemagen Diagnostics Inc., Columbia, MD, USA). For each individual patient, samples from each time-point were analysed in the same assay. All samples for a given assay were tested simultaneously, in duplicate and in appropriate dilutions.
Statistical analysis
Analysis was performed using SAS software version 9.1. Data were expressed as means and standard deviations for continuous variables that were normally distributed and as medians and ranges for non-normally distributed data. Log transformation was carried out where appropriate [plasma IL-6 and CRP levels, duration of HD and body mass index (BMI)]. Categorical data were expressed as proportions.
The factors contributing to the variability of ADPN levels were examined by linear regression for baseline ADPN estimations and linear mixed regression models for ADPN levels repeated over time. The latter incorporated random effects to account for correlated observations on the same subject and were robust to missing data. As multiple measurements of plasma levels of CRP and IL-6 were also available at the same time points as plasma ADPN levels, they were also included in the models as repeated measures.
The relationship between ADPN and prevalent CVD was examined using logistic regression. Covariates included age, gender, race (Caucasian versus African American), diabetes, smoking history (current or past smokers versus non-smokers) and BMI (biomarkers of inflammation) (plasma albumin, CRP and IL-6 levels). Serum cholesterol was not evaluated in the final model, since observations were unavailable for ∼15% of the cohort and its relationship with vascular disease in ESRD is undetermined [10] (see explanation below regarding handling of missing data). Ordinal regression was used to examine the relationship between plasma ADPN and the extent of vascular disease expressed as an ordinal outcome with three categories (0, 1 and ≥2).
Cox proportional hazards regression was used to evaluate the effect of plasma ADPN on cardiovascular and mortality outcomes. Data were censored at the time of transplantation but in keeping with the intention-to-treat principle of the parent study, data were not censored when patients left the study due to transfer to a non-participating centre or alternative method of dialysis. The proportional hazards assumption was tested using Schoenfeld residuals, a time-varying coefficient model and by examination of log (-log survival) curves for parallelism and was met for individual covariates except diabetes. The same covariates outlined above were explored in multivariate models in addition to pre-existing vascular disease, and the dialysis dose and flux grouping of randomization and the final models arrived at by backward selection. As models obtained with and without stratification by diabetes were similar, diabetes was retained within the models. As there was significant non-linearity in the relationship of ADPN with cardiovascular and mortality outcomes, the functional form of ADPN was tested by fitting parametric cubic splines with 3 degrees of freedom while controlling for the covariates. Models with a quadratic transformation of plasma ADPN provided a better fit than models with the linear form, by comparison of the Akaike information criterion, the Δ −2 log-likelihood, and by analysis of Martingale residuals. The final models were arrived at by backward selection of variables to limit the number of variables and avoid over-fitting in the models.
A time-dependent model was used to examine the association between repeated measurements of plasma ADPN and time for outcomes. In this analysis, the counting process style of input was used, where multiple records were created for each patient, one record for each distinct pattern of the time-dependent measurements. If ADPN values were missing for a given segment of time, the preceding ADPN value was used as a predictor for the following segment of time. As multiple measurements of plasma levels of CRP and IL-6 were also available at the same time intervals, they were included into the models as time-varying covariates, in a similar fashion. The advantage of using time-dependent Cox regression was the ability to predict survival while allowing the covariate value to change over time.
In instances of missing values for baseline covariates (serum cholesterol, plasma CRP and IL-6) the technique of multiple imputations was applied. Logistic regression models were re-run with imputed baseline data for vascular disease as the outcome and Cox regression models were re-run for the outcomes of ACM, CVE/CVM and CVE/ACM. Estimates were compared to those from the models without imputed data.
All tests were two-tailed and P-values <0.05 were considered significant. All confidence intervals were calculated at the 95% level.
Results
Plasma ADPN measurements of 182 patients were available of whom 176 patients were randomized, forming four roughly equal groups by the 2×2 combinations of dialysis dose and flux categories. The mean age of the cohort was 62.2 ± 12.3 years, and median (IQR) duration of HD was 2.1 (0.78, 4.94) years; 47% (85/182) were male, 58% (105/182) Caucasian, 41% (75/182) diabetic, and CVD was present in 66% (117/182). The mean (±SD) serum albumin was 3.6 ± 0.34 g/dL (36.0 ± 4.0 g/L) and haematocrit (Hct) 33.0 ± 4.4%.
Baseline and annual plasma ADPN levels and patient characteristics
The distribution of plasma ADPN levels at baseline was normal with a mean of 17.2 ± 8.8 μg/mL (range 0.95– 57.7 μg/mL). Baseline plasma ADPN levels did not differ by age, gender, race, number of years spent on HD, diabetes or serum albumin (Table 1). However, they showed a weak but significant inverse correlation to log-transformed CRP levels (r = −0.15, P = 0.05) and log-transformed BMI values (r = −0.15, P = 0.04) at baseline, both adjusted for age, gender, race, number of years on HD and diabetes.
Table 1.
Baseline plasma ADPN levels in relation to patient and dialysis characteristics
| Patient characteristic | n | Mean ± SD (μg/mL) | P-value |
|---|---|---|---|
| Gender | |||
| Male | 85 | 16.8 ± 7.3 | 0.6 |
| Female | 97 | 17.5 ± 10.0 | |
| Race | |||
| Caucasian | 105 | 16.8 ± 8.1 | 0.5 |
| African-American | 77 | 17.7 ± 9.8 | |
| Diabetes | |||
| No | 107 | 17.6 ± 8.4 | 0.5 |
| Yes | 75 | 16.6 ± 9.4 | |
| Vascular disease | |||
| No | 60 | 18.9 ± 10.2 | 0.06 |
| Yes | 117 | 16.2 ± 8.0 | |
| Smoker | |||
| No | 83 | 16.5 ± 8.8 | 0.4 |
| Yes | 99 | 17.7 ± 8.8 | |
| Dialysis dose randomization | |||
| Standard dose | 89 | 17.5 ± 9.8 | 0.6 |
| High dose | 87 | 16.8 ± 7.8 | |
| Dialysis membrane randomization | |||
| Low flux | 91 | 17.6 ± 8.0 | 0.5 |
| High flux | 85 | 16.7 ± 9.7 |
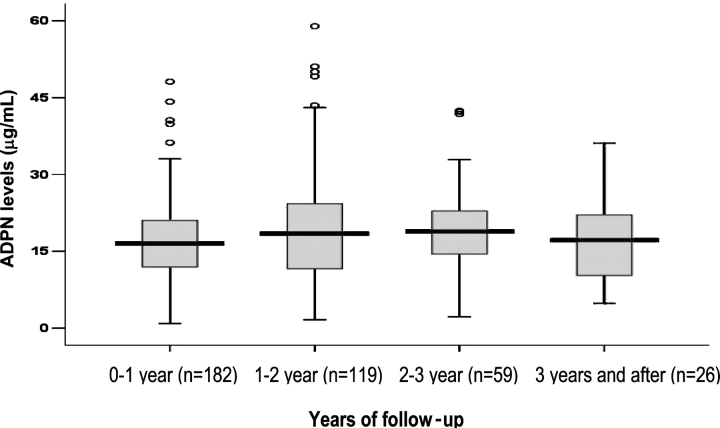
Figure 1 shows inter-quartile ranges for yearly plasma ADPN. ADPN levels measured over time showed a gradual increase (0.95 μg/mL, 95% CI = 0.12–1.78 μg/mL; P = 0.03) by year, although this difference became non-significant after the adjustment for covariates (Table 2). The multivariable analysis was adjusted for a random intercept and included (a) the dialysis and flux randomization of the parent study and (b) multiple measurements of plasma levels of CRP and IL-6 that were also available at the same time intervals as plasma ADPN levels. The model showed that women had higher levels (3.0 μg/mL, 95% CI = 0.45–5.49 μg/mL) of ADPN over time and each log increase in BMI was associated with a 10 μg/mL (95% CI = −17.10 to −4.52 μg/mL) decrease in ADPN; however, there was no relationship with DM or serum albumin. While each log increase in CRP levels was associated with a 0.84 μg/mL (95% CI = −1.65 to −0.04 μg/mL) decrease in plasma ADPN levels, each log increase in IL-6 levels was associated with a 1.26 μg/mL (95% CI = 0.35–2.17 μg/mL) increase when adjusted for (log) CRP. A strong correlation was demonstrable between (log) CRP and (log) IL-6 after similar accounting for correlated observations on the same subject (unadjusted Beta = 0.48 per (log) IL-6; 95% CI 0.39–0.58).
Fig. 1.
Box plots of plasma ADPN levels measured annually, showing the number of patients with repeated measurements (ranging from two to five or more) each year.
Table 2.
Relationship of yearly plasma ADPN levels with time and other patient and dialysis-related variables using a linear mixed regression model
| Predictor | Estimate | 95% CI of estimate | P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Unadjusted | ||||
| Year of study (per year) | 0.95 | 0.12 | 1.78 | 0.02 |
| Adjusteda | ||||
| Year of study (per year) | 0.68 | −0.25 | 1.61 | 0.15 |
| Sex (female) | 3.00 | 0.45 | 5.49 | 0.02 |
| BMI (kg/m2) | −10.81 | −17.10 | −4.52 | <0.001 |
| IL-6 (per log increase) | 1.26 | 0.35 | 2.17 | 0.007 |
| CRP (per log increase) | −0.84 | −1.65 | −0.04 | 0.04 |
aVariables in the model that did not reach significance: age, smoking, diabetes, race, duration on HD and serum albumin, and the flux and dose grouping of the parent study.
Plasma ADPN and prevalent vascular disease
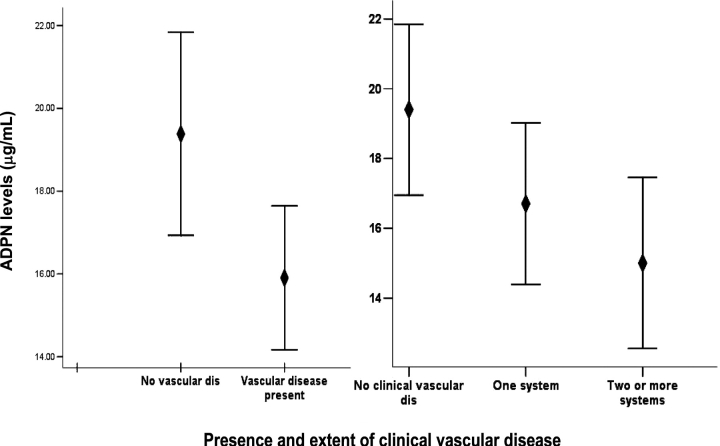
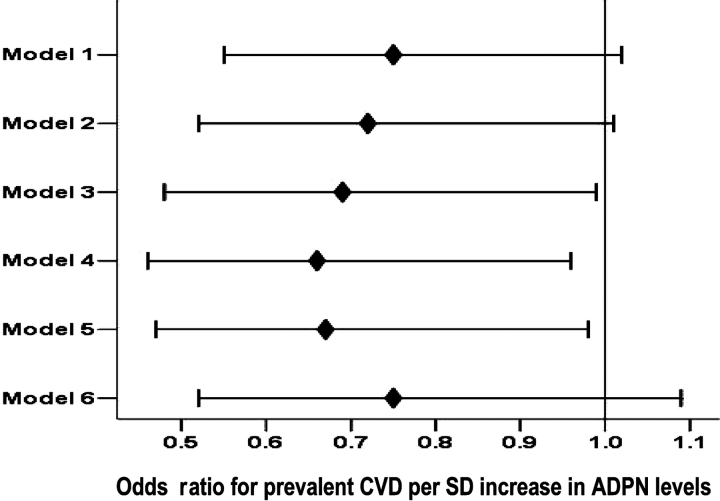
Plasma ADPN was lower among patients with prevalent CVD and among patients with more extensive CVD (18.9 ± 10.2 μg/mL in those without vascular disease and 16.8 ± 9.3 and 15.6 ± 6.4 μg/mL in those with one and two or more vascular systems involved, respectively; P = 0.13). Although these unadjusted differences did not reach statistical significance, Figure 2 shows the adjusted means that differ significantly among patients with and without vascular disease and among patients with more extensive vascular disease. The unadjusted logistic regression model showed that one standard deviation increase in plasma ADPN was associated with a 26% decrease in the odds for vascular disease (OR = 0.74, 95% CI = 0.54–1.02). Progressive adjustment for patient-related variables increased the strength of the association providing an adjusted OR of 0.67 (95% CI = 0.46–0.96) for the model without the adjustment for inflammatory markers. Adjustment for (log) CRP weakened the association while the adjustment for (log) IL-6 did not (Figure 3). This relationship was also consistent for the extent of CVD tested using ordinal regression. On univariate analysis, one standard deviation increase in ADPN levels was associated with a 24% decrease in the odds for more extensive vascular disease (OR = 0.76, 95% CI = 0.58–1.01). Again, progressive adjustment for patient-related variables except (log) CRP levels increased the strength of the association providing an adjusted OR of 0.70 (95% CI = 0.51–0.95).
Fig. 2.
Error bars (estimated mean ± SEM) ADPN levels among patients with and without clinical CVD and with increasing extent of clinical CVD.
Fig. 3.
Odds ratio for a one-SD increase in plasma ADPN for prevalent CVD for a univariate model and after sequential adjustment for covariates. The multivariate analysis sequentially adjusted models for covariates as follows: Model 1, unadjusted; Model 2, adjusted for age, gender, race and duration on HD; Model 3, adjusted in addition for diabetes, (log)BMI, serum albumin; Model 4, adjusted in addition for smoking; Model 5, adjusted in addition for (log)IL-6; Model 6, adjusted in addition for (log)CRP but not (log)IL-6.
Baseline plasma ADPN and relationship with mortality and CVD outcomes
Information on survival and outcomes was available for 176 patients who underwent randomization. A total of 107 patients (61%) died, 36% (38/107) of all deaths were due to cardiovascular causes and 86/176 (49%) had cardiac hospitalization events. The median overall survival was 1165 days (95% CI: 1010–1319) and overall actuarial survival in the cohort was 89% at 1 year, 69% at 2 years and 55% at 3 years. The median time of the composite outcome of ‘cardiac hospitalization or cardiac death’ was 736 days (95% CI: 552–920) and number of events was 96/176 (55.0%). The median time of the composite outcome of ‘cardiac hospitalization or death from any cause’ was 599 days (95% CI: 475–723) and number of events was 126/176 (72.0%).
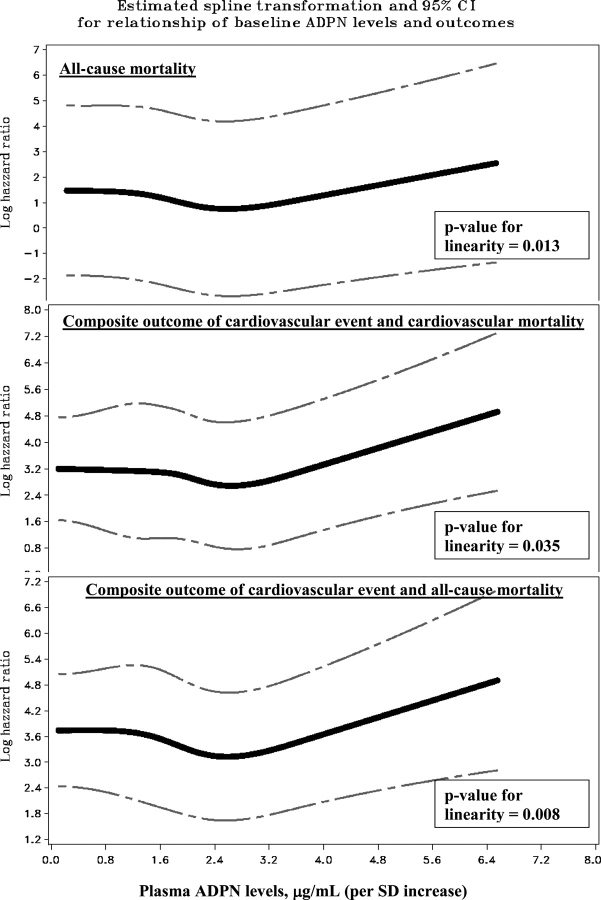
The relationship between plasma ADPN and all-cause mortality was non-linear (test of linear hypothesis: P = 0.013 at 3 degrees of freedom) and the best fit was obtained with a quadratic transformation. Although the relationship between ADPN and mortality did not reach significance in the unadjusted Cox regression model, progressive adjustment for patient-related variables including inflammatory markers, increased the strength of the association. A similar relationship was seen with the composite end points of ‘cardiac hospitalization or cardiac mortality’ (test of linear hypothesis: P = 0.035 at 3 degrees of freedom) and ‘cardiac hospitalization or all-cause mortality’ (test of linear hypothesis: P = 0.008 at 3 degrees of freedom), the association being strongest for the latter composite outcome (Table 3, Figure 4).
Table 3.
Univariate and multivariate Cox regression models for the relationship between baseline ADPN levels and outcomes. The final multivariable models were arrived at by backward selection
| Outcomes | Death from any cause | Cardiovascular hospitalization event/cardiovascular death | Cardiovascular hospitalization event/death from any cause | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor variable | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P |
| Quadratic form | Unadjusted analyses | ||||||||
| ADPN levels (per SD)a | 1.02 | 0.83–1.24 | 0.86 | 1.08 | 0.87–1.32 | 0.49 | 1.01 | 0.83–1.22 | 0.94 |
| ADPN levels (per SD)b | 0.71 | 0.45–1.12 | 0.14 | 0.73 | 0.44–1.21 | 0.22 | 0.68 | 0.43–1.08 | 0.10 |
| ADPN * ADPNb | 1.07 | 0.99–1.16 | 0.07 | 1.08 | 0.99–1.17 | 0.09 | 1.08 | 1.00–1.17 | 0.06 |
| Other covariates | |||||||||
| Age (per year) | 1.03 | 1.01–1.05 | <0.01 | 1.03 | 1.01–1.05 | <0.01 | 1.03 | 1.01–1.05 | <0.01 |
| Sex (versus male) | 0.84 | 0.58–1.20 | 0.33 | 0.84 | 0.57–1.23 | 0.37 | 0.95 | 0.68–1.33 | 0.75 |
| Race (versus AA) | 1.31 | 0.91–1.90 | 0.15 | 2.03 | 1.35–3.04 | <0.01 | 1.53 | 1.09–2.15 | 0.01 |
| Duration on HD (years) (per log) | 1.21 | 1.04–1.41 | 0.12 | 1.06 | 0.89–1.24 | 0.53 | 1.10 | 0.95–1.26 | 0.20 |
| BMI (per log) | 0.63 | 0.25–1.58 | 0.32 | 1.00 | 0.38–2.62 | 0.99 | 0.98 | 0.43–2.28 | 0.97 |
| Diabetes | 1.18 | 0.82–1.70 | 0.36 | 0.89 | 0.60–1.31 | 0.55 | 0.97 | 0.69–1.36 | 0.85 |
| Smoking (versus never) | 1.47 | 1.01–2.13 | 0.04 | 1.53 | 1.04–2.26 | 0.03 | 1.52 | 1.08–2.13 | 0.02 |
| Vascular disease | 1.56 | 1.02–2.37 | 0.04 | 1.75 | 1.13–2.71 | 0.01 | 1.61 | 1.11–2.33 | 0.01 |
| Serum albumin (per g/dL) | 0.74 | 0.43–1.28 | 0.28 | 1.02 | 0.59–1.76 | 0.96 | 0.98 | 0.61–1.58 | 0.94 |
| Serum CRP levels (mg/dL) (per log) | 1.18 | 1.02–1.37 | 0.03 | 1.15 | 1.00–1.33 | 0.06 | 1.15 | 1.01–1.30 | 0.03 |
| Serum IL-6 levels (pg/mL) (per log) | 1.24 | 1.05–1.46 | 0.01 | 1.15 | 0.97–1.36 | 0.11 | 1.13 | 0.98–1.31 | 0.10 |
| Kt/V group (high versus standard) | 1.03 | 0.72–1.48 | 0.88 | 1.19 | 0.81–1.75 | 0.38 | 1.22 | 0.87–1.71 | 0.25 |
| Flux group (high versus low) | 0.86 | 0.59–1.23 | 0.41 | 0.64 | 0.43–0.94 | 0.02 | 0.70 | 0.50–0.98 | 0.04 |
| Final model adjusted for convariates | |||||||||
| ADPN levels (per SD)b | 0.44 | 0.26–0.73 | <0.01 | 0.50 | 0.29–0.88 | 0.01 | 0.48 | 0.29–0.79 | <0.01 |
| ADPN * ADPNb | 1.16 | 1.07–1.27 | <0.01 | 1.15 | 1.05–1.26 | <0.01 | 1.15 | 1.05–1.25 | <0.01 |
| Age (per year) | 1.04 | 1.02–1.07 | <0.01 | 1.04 | 1.02–1.07 | <0.01 | 1.04 | 1.02–1.06 | <0.01 |
| Race (versus AA) | 1.70 | 1.08–2.67 | <0.01 | ||||||
| Duration on HD (years) (per log) | 1.39 | 1.17–1.66 | <0.01 | 1.26 | 1.04–1.53 | 0.02 | 1.33 | 1.13–1.57 | <0.01 |
| Smoking (versus never) | 1.72 | 1.14–2.60 | 0.01 | 1.92 | 1.24–2.98 | <0.01 | 1.92 | 1.30–2.83 | <0.01 |
| Serum IL-6 levels (pg/mL) (per log) | 1.20 | 1.02–1.41 | 0.03 | ||||||
| Flux group (high versus low) | 0.58 | 0.37–0.89 | 0.01 | 0.53 | 0.36–0.77 | <0.01 | |||
Tests of linear hypothesis at 3 degrees of freedom: ACM: P = 0.013; CVE/CVM: P = 0.035; CVE/ACM: P = 0.008.
aADPN shown as a linear term–without transformation of the variable.
bADPN shown as a quadratic (polynomial) term. The model requires the inclusion of both the lowest order term and the higher power (squared) terms. This functional form of ADPN indicates that its effect on ACM or CVE/CVM or CVE/ACM varies at different values of ADPN. The negative coefficient of the lower order term and positive coefficient of the squared term are consistent with the shape of the relationship being similar to an upright parabola (see Figure 4).
Fig. 4.
Estimated spline transformation and confidence interval for the relationship of increasing plasma ADPN levels with cardiovascular and mortality outcomes, while controlling for covariates (the covariates shown to be significant in the adjusted models for each outcome in Table 3) showing that the relationship between increasing levels of ADPN and outcomes is non-linear. The spline reflects only the non-linearity and not the actual magnitude of the association for increasing ADPN levels scaled per SD.
The other variables that were independent predictors of outcomes were plasma IL-6, older age, longer duration on dialysis and a history of smoking. African American patients and those patients randomized to the high-flux limb of the parent study had a lower risk of cardiovascular outcomes.
Plasma ADPN measured over time and relationship with mortality and CVD outcomes
The relationship of plasma ADPN measured over time, with the composite outcome of ‘cardiac hospitalization or all-cause mortality’ was also non-linear (test of linear hypothesis: P = 0.04 at 1 degree of freedom). Here again, the best fit was obtained with a quadratic transformation, and while the unadjusted Cox regression model did not show a significant association between ADPN and the composite outcome, progressive adjustment for patient-related variables including inflammatory markers, increased the strength of the association (Table 4). Plasma ADPN measured over time was a more powerful marker of this outcome than either (log) CRP or (log) IL-6, both measured over time. Both (log) CRP and (log) IL-6, measured over time, were also independent predictors of all-cause mortality.
Table 4.
Time-dependent Cox regression for the relationship of ADPN levels measured over time with mortality and cardiovascular outcomes. Multiple measurements of plasma CRP and IL-6 at the same time points were available and included in the model as time-dependent covariates. The final multivariable models were arrived at by backward selection
| Outcome variable | All-cause mortality and cardiovascular events | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Final model adjusted for covariates | |||||
| Predictor variable | HR | 95% CI | P | HR | 95% CI | P |
| ADPN levels (per μg/mL)a | 1.01 | 0.99–1.03 | 0.12 | |||
| ADPN levels (per μg/mL)b | 0.87 | 0.67–1.15 | 0.33 | 0.73 | 0.55–0.98 | 0.03 |
| ADPN * ADPNb | 1.02 | 1.00–1.05 | 0.11 | 1.04 | 1.01–1.07 | <0.01 |
| Age (per year) | 1.03 | 1.01–1.05 | <0.01 | 1.04 | 1.02–1.06 | <0.01 |
| Sex (versus male) | 0.95 | 0.68–1.33 | 0.75 | |||
| Race (versus AA) | 1.53 | 1.09–2.15 | 0.01 | |||
| Duration on HD (years) (per log) | 1.10 | 0.95–1.26 | 0.21 | 1.31 | 1.11–1.55 | <0.01 |
| BMI (per log) | 0.99 | 0.63–2.28 | 0.97 | |||
| Diabetes | 0.97 | 0.69–1.36 | 0.85 | |||
| Smoking (versus never) | 1.52 | 1.08–2.13 | 0.02 | 1.69 | 1.16–2.47 | <0.01 |
| Vascular disease | 1.61 | 1.11–2.33 | 0.01 | |||
| Serum albumin (per g/dL) | 0.98 | 0.61–1.59 | 0.94 | |||
| Serum CRP levels (mg/dL) (per log)c | 1.19 | 1.04–1.36 | 0.01 | |||
| Serum IL-6 levels (pg/mL) (per log)c | 1.21 | 1.03–1.41 | 0.02 | |||
| Kt/V group (high versus standard) | 1.22 | 0.87–1.71 | 0.25 | |||
| Flux group (high versus low) | 0.70 | 0.50–0.98 | 0.04 | 0.53 | 0.36–0.78 | <0.01 |
aADPN shown as a linear term—without transformation of the variable.
bADPN shown as a quadratic term, with concomitant adjustment for the linear term.
cSeparate models were constructed with either CRP or IL-6 included. Except for their respective coefficients, estimates for other covariates were similar.
Sensitivity analysis for missing data
Plasma ADPN was available in 81% of patients at risk at the end of the first year; 53% of patients at risk at the end of the second year and 33% of patients at risk at the end of the third year. Patients with missing values for annual plasma ADPN measurements did not differ significantly with regard to baseline variables from those with available measurements. Morover, the model ensured that outcomes at each interval depended upon the most recently preceding plasma ADPN measurement. Further, the technique of multiple imputations was used to impute values that were missing at baseline for serum cholesterol (15%), plasma CRP (3%) and IL-6 (1%). Logistic regression models were re-run with imputed data for vascular disease as the outcome and Cox regression models were re-run for the outcomes of ACM, CVE/CVM and CVE/ACM. Estimates obtained after inclusion of imputed values did not differ from the original results.
Discussion
The present study confirms earlier reports that plasma ADPN is elevated among patients with ESRD compared to the general population. The results indicate that lower baseline levels of plasma ADPN are independently associated with both the presence and extent of pre-existing vascular disease. ADPN levels at baseline predicted all-cause mortality and the composite of ‘cardiovascular events and cardiovascular mortality’. The association was further supported by the significant association of repeated measurements of ADPN levels over time with the composite of ‘all-cause mortality and cardiovascular events’. A notable aspect of models incorporating ADPN levels was that all the associations were extensively confounded by other covariates and became stronger after adjustment. Moreover, the relationship of plasma ADPN with prospective outcomes was non-linear (quadratic), the risk increasing at extremes of the range of plasma ADPN concentrations. Plasma ADPN was inversely related to BMI and plasma CRP and women had higher levels over time, associations noted previously in other studies [1,2]. However, unlike plasma CRP that was inversely associated with ADPN, plasma IL-6 and ADPN measured over time showed a positive correlation after adjusting for CRP. While this observation may be explained by the collinearity between CRP and IL-6, it is pertinent that both ADPN and IL-6 are adipocyte-derived products and their concordance could represent a co-secretory response after accounting for the variability in IL-6 levels that is represented by CRP.
Our findings point toward the conflicting literature relating ADPN levels to clinical outcomes. ADPN has a range of physiological effects on insulin sensitivity and lipid profile, as well as anti-atherogenic and anti-inflammatory properties [1]. Several epidemiological studies in the general population have shown that higher ADPN levels are associated with a favorable cardiovascular risk factor profile [11]. In the ARIC study, high ADPN levels in non-smokers and patients without inflammation were associated with reduced risk of developing type II diabetes [12]. In the Health Professionals Follow-Up Study, low levels of adiponectin were associated with progression of coronary artery calcification in diabetic and non-diabetic subjects [13] and higher risk of incident coronary heart disease events in diabetic and non-diabetic men [14,15]. Other studies have either not concurred or shown contrary findings. No significant relationship with cardiovascular outcomes was seen in the Strong Heart Study, conducted in a large cohort of American Indians [16], and the British Women's Heart and Health Study, in a population-based sample of women [17]. Kistorp et al. showed that high rather than low ADPN levels predicted mortality in a cohort of patients with chronic heart failure, an association they ascribed to confounding from cachexia [18]. Two other recent studies among patients with coronary artery disease also identified elevated ADPN levels as a marker of poor prognosis [19,20]. Among patients with CKD, despite the presence of an insulin resistant state and more adverse cardiovascular risk profile, plasma levels of ADPN are consistently elevated [21,22]. Levels are known to decrease after successful kidney transplantation [23] suggesting a role for diminished renal clearance. A compensatory response to inflammation and/or malnutrition, or to down-regulation of the mechanisms that protect against atherogenesis and a right-shift of the relationship, cannot be excluded. Zoccali and colleagues showed that lower ADPN levels were an independent predictor for the composite outcome of fatal and non-fatal CVD events but not for all-cause mortality among ESRD patients [2], an association not observed in a smaller study by Diez et al. [24]. Becker and colleagues found that lower ADPN levels were associated with insulin resistance and prevalent CVD among patients with CKD [25], as did Tentolouris et al. [26]. However a recent study among CKD patients in the Modification of Diet in Renal Disease (MDRD) study showed a direct relationship between ADPN levels and all-cause mortality, independent of underlying CVD, metabolic risk factors, CRP and GFR [27]. Thus, despite the existence of strong experimental evidence, prospective epidemiological studies have demonstrated inconsistent results for a positive association between adiponectin and reduced risk of CVD and/or associated mortality. Several explanations exist for these conflicting results including population characteristics (diabetes and CKD), case mix, confounding influences of covariates including inflammation and nutritional status, possibly, variants in the gene encoding ADPN (ApM1, adipose most abundant gene transcript) [4] and possible differential retention of the high-molecular-weight ADPN isoform in kidney disease [28,29]. There are two circulating forms of ADPN, a low-molecular-weight hexamer and a high-molecular-weight complex of 12–18 subunits, the latter being associated with biological activity which may be differentially retained when GFR declines [33,34]. The ratio between these two forms also seems to determine the insulin sensitivity to thiazolidinedione treatment [28,30]. Perhaps the contrary effects of different thiazolidinediones (rosiglitazone versus pioglitazone), a class of drugs that are known to increase ADPN levels, upon cardiovascular outcomes may relate to differences in the ratio between high- and low-molecular-weight isoforms [31–33]. The present study highlights the likelihood that the effects of ADPN on clinical variables may be extensively confounded given its role in a wide range of metabolic pathways. Our results also suggest that the relationship between plasma ADPN and mortality outcomes may be non-linear with a quadratic fit, suggesting that poor outcomes occur with very low and very high ADPN levels, the latter possibly occurring as a cellular response to malnutrition and declining BMI, or to pro-atherogenic inflammatory stimuli that are associated with malnutrition [34,35]. Indeed ADPN was a more powerful predictor of outcomes than either CRP or IL-6. The finding of the inverse correlation between ADPN levels and BMI and CRP lends further support to this argument.
The strengths of our study included a fairly representative cohort of stable HD patients with long-term follow-up and detailed and accurate ascertainment of both baseline variables and outcomes. We believe that this is the very first study that measured ADPN at repeated time points during longitudinal observation and the only one using ADPN as a time-dependent covariate. Multiple measurements of plasma ADPN, CRP and IL-6 were available, and appropriate modeling approaches allowed us to examine their relative strengths as prognostic markers, as well as examine the independent effect of ADPN on outcomes after accounting for markers of the inflammatory state at baseline as well as over time. We have previously reported associations between plasma IL-6 and outcomes with both single and repeated measurements [36] underscoring the robustness of these associations. However the study was a secondary analysis on a cohort of prevalent, albeit relatively stable chronic haemodialysis patients enrolled in an interventional study; hence it may not be representative of the overall haemodialysis population. A similar criticism may also be applied to other studies [27] and underscores the context dependence of the effect of ADPN on outcomes. As fasting samples were unavailable in the HEMO study, insulin resistance could not be assessed. To offset any bias that may have occurred due to missing data, we applied mixed models and time-dependent analyses, and used the statistical technique of multiple imputations to impute missing values for baseline covariates. Estimates obtained after inclusion of imputed values did not differ from the original results.
In summary, we found a significant relationship between plasma ADPN and CVD and mortality outcomes in a cohort of HD patients, the relationship becoming apparent and stronger after the extensive confounding by multiple patient-related factors was accounted for. The non-linearity of the relationship suggests that there may be lower and upper thresholds defining an optimal range of levels associated with improved outcomes. The clinical utility of plasma ADPN measurements and the therapeutic potential of increasing ADPN or isoform concentrations by pharmacological intervention [21–23] is thus likely to be extremely complex and context sensitive.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (DK 45609) to Dr Balakrishnan. Additional support was provided by a grant from Satellite Healthcare, Inc. Dr Rao is supported by a grant from the National Institutes of Health (DK66992-01). This study was presented at the American Society of Nephrology, San Diego, CA, 2006.
Conflict of interest statement. None declared.
References
- 1.Chandran M, Philips S, Ciaraldi T, et al. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 2.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 3.Mallamaci F, Zoccali C, Cuzzola F, et al. Adiponectin in essential hypertension. J Nephrol. 2002;15:507–511. [PubMed] [Google Scholar]
- 4.Stenvinkel P, Marchlewska A, Pecoits-Filho R, et al. Adiponectin in renal disease: relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney Int. 2004;65:274–281. doi: 10.1111/j.1523-1755.2004.00370.x. [DOI] [PubMed] [Google Scholar]
- 5.Foley R. Clinical epidemiology of cardiovascular disease in dialysis patients: left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin dial. 2003;16:111–117. doi: 10.1046/j.1525-139x.2003.160271.x. [DOI] [PubMed] [Google Scholar]
- 6.Eknoyan G, Beck GJ, Cheung AK, et al. Effect of dialysis dose and membrane flux in maintenance haemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 7.Greene T, Beck GJ, Gassman JJ, et al. Design and statistical issues of the haemodialysis (HEMO) study. Control Clin Trials. 2000;21:502–525. doi: 10.1016/s0197-2456(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 8.Miskulin DC, Athienites N, Yan G, et al. Comorbidity assessment using the index of coexistent diseases in a multicenter clinical trial. Kidney Int. 2000;60:1498–1510. doi: 10.1046/j.1523-1755.2001.00954.x. [DOI] [PubMed] [Google Scholar]
- 9.Rao M, Guo D, Jaber BL, et al. Transforming growth factor-beta 1 gene polymorphisms and cardiovascular disease in haemodialysis patients. Kidney Int. 2004;66:419–427. doi: 10.1111/j.1523-1755.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- 10.Kalantar-Zadeh K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: what is next-grou? Semin Dial. 2005;18:365–369. doi: 10.1111/j.1525-139X.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsushita K, Yatsuya H, Tamakoshi K, et al. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol. 2006;26:871–876. doi: 10.1161/01.ATV.0000208363.85388.8f. [DOI] [PubMed] [Google Scholar]
- 12.Duncan BB, Schmidt MI, Pankow JS, et al. Adiponectin and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2004;53:2473–2478. doi: 10.2337/diabetes.53.9.2473. [DOI] [PubMed] [Google Scholar]
- 13.Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111:747–753. doi: 10.1161/01.CIR.0000155251.03724.A5. [DOI] [PubMed] [Google Scholar]
- 14.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 15.Schulze MB, Shai I, Rimm EB, et al. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54:534–539. doi: 10.2337/diabetes.54.2.534. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay RS, Resnick HE, Zhu J, et al. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–e16. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor DA, Davey Smith G, Ebrahim S, et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 18.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 19.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 20.Pilz S, Mangge H, Wellnitz B, et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 2006;91:4277–4286. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
- 21.Guebre-Egziabher FBJ, Funahashi T, Hadj-Aissa A, et al. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol Dial Transplant. 2005;20:129–134. doi: 10.1093/ndt/gfh568. [DOI] [PubMed] [Google Scholar]
- 22.Isobe T, Saitoh S, Takagi S, et al. Influence of gender, age and renal function on plasma adiponectin level: the Tanno and Sobetsu study. Eur J Endocrinol. 2005;153:91–98. doi: 10.1530/eje.1.01930. [DOI] [PubMed] [Google Scholar]
- 23.Chudek J, Adamczak M, Karkoszka H, et al. Plasma adiponectin concentration before and after successful kidney transplantation. Transplant Proc. 2003;35:2186–2189. doi: 10.1016/j.transproceed.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Diez JJ, Iglesias P, Fernandez-Reyes MJ, et al. Serum concentrations of leptin, adiponectin and resistin, and their relationship with cardiovascular disease in patients with end-stage renal disease. Clin Endocrinol (Oxf) 2005;62:242–249. doi: 10.1111/j.1365-2265.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- 25.Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol. 2005;16:1091–1098. doi: 10.1681/ASN.2004090742. [DOI] [PubMed] [Google Scholar]
- 26.Tentolouris N, Doulgerakis D, Moyssakis I, et al. Plasma adiponectin concentrations in patients with chronic renal failure: relationship with metabolic risk factors and ischemic heart disease. Horm Metab Res. 2004;36:721–727. doi: 10.1055/s-2004-826022. [DOI] [PubMed] [Google Scholar]
- 27.Menon V, Li L, Wang X, et al. Adiponectin and mortality in patients with chronic kidney disease. JASN. 2006;17:2599–2606. doi: 10.1681/ASN.2006040331. [DOI] [PubMed] [Google Scholar]
- 28.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 29.Shoji T, Kimoto E, Shinohara K, et al. Molecular forms of adiponectin in uraemic plasma. Nephrol Dial Transplant. 2004;19:1937–1938. doi: 10.1093/ndt/gfh259. [DOI] [PubMed] [Google Scholar]
- 30.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 31.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomized controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 32.Nissen SE. Perspective: effect of rosiglitazone on cardiovascular outcomes. Curr Cardiol Rep. 2007;9:343–344. doi: 10.1007/BF02938358. [DOI] [PubMed] [Google Scholar]
- 33.Satoh N, Ogawa Y, Usui T, et al. Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabetes Care. 2003;26:2493–2499. doi: 10.2337/diacare.26.9.2493. [DOI] [PubMed] [Google Scholar]
- 34.Pecoits-Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome—the heart of the matter. Nephrol Dial Transplant. 2002;17(Suppl 11):28–31. doi: 10.1093/ndt/17.suppl_11.28. [DOI] [PubMed] [Google Scholar]
- 35.Shoji T, Shinohara K, Hatsuda S, et al. Altered relationship between body fat and plasma adiponectin in end-stage renal disease. Metabolism. 2005;54:330–334. doi: 10.1016/j.metabol.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Rao M, Guo D, Perianayagam MC, et al. Plasma interleukin-6 predicts cardiovascular mortality in haemodialysis patients. Am J Kidney Dis. 2005;45:324–333. doi: 10.1053/j.ajkd.2004.09.018. [DOI] [PubMed] [Google Scholar]