Abstract
Background. Insulin resistance is associated with increased sympathetic and reduced parasympathetic activity. Resting heart rate reflects autonomic activity. Therefore, we examined the associations of resting heart rate with insulin resistance, cardiovascular events and mortality in the moderate chronic kidney disease (CKD) population.
Methods. Four hundred and sixty participants with MDRD GFR <60 ml/min/1.73 m2 in the limited access Atherosclerosis Risk in Communities (ARIC) study database were divided into four resting heart rate groups: <60, 60–74, 75–89 and ≥90/min. The prevalence of metabolic syndrome at baseline across the groups was examined. Time to cardiovascular composite (myocardial infarction or fatal coronary artery disease event or stroke or coronary revascularization procedure) and time to all-cause death were examined in multivariate Cox models.
Results. The prevalence of metabolic syndrome in the <60, 60–74, 75–89 and ≥90/min groups were 41, 44, 69 and 82% (P < 0.001), respectively. In a multivariate Cox model adjusted for demographics, comorbidity, haemoglobin and physical activity, compared to the 60–74/min group, the hazard ratios of cardiovascular composite in <60, 75–89 and ≥90/min groups were 1.27 (95% CI 0.75–2.16), 1.79 (95% CI 1.07–2.99) and 1.37 (95% CI 0.54–3.44), respectively. In a similar model, the hazard ratios of death were 1.47 (95% CI 0.85–2.53), 3.11 (95% CI 1.93–5.02) and 3.97 (95% CI 1.99–7.94), respectively.
Conclusions. Resting heart rate is associated with metabolic syndrome in moderate CKD. Higher resting heart is associated with increased mortality and possibly cardiovascular events in this population. Interventional studies to examine whether a target resting heart rate of 60–74/min improves cardiovascular outcomes and survival in moderate CKD are warranted.
Keywords: chronic kidney disease, heart rate, insulin resistance, metabolic syndrome, mortality
Introduction
Visceral adiposity is associated with several metabolic derangements including insulin resistance [1], inflammation [2,3], endothelial dysfunction [4] and dyslipidaemia [5]. Visceral adiposity [6] and its metabolic consequences of hyperinsulinaemia [7] and hyperleptinaemia [8,9] are associated with increased sympathetic and/or reduced parasympathetic activity [10,11]. Furthermore, increased sympathetic activity might contribute to insulin resistance [12]. Thus, alterations in autonomic activity might both be a cause and a consequence of insulin resistance.
Higher resting heart rate might reflect increased sympathetic and/or reduced parasympathetic activity. Earlier studies suggest that high resting heart rate, even within the normal range (<100 beats/min), is a predictor of mortality in the general population [12–15]. Recently, it has been suggested that in the general population, elevated resting heart rate should be considered as an independent risk factor of cardiovascular events, and maintaining resting heart rate substantially below the traditionally defined tachycardia threshold of 90 or 100 beats/min might be desirable [16]. However, the associations between resting heart rate and clinical outcomes are poorly defined in the chronic kidney disease (CKD) population, and there are no data in this population on whether resting heart rate is associated with insulin resistance. Therefore, we examined in the limited access, public use Atherosclerosis Risk in Communities (ARIC) study dataset, the hypothesis that resting heart rate is associated with metabolic syndrome and predicts cardiovascular outcomes and mortality in the moderate CKD population.
Subjects and methods
Study population
The ARIC Study is a large-scale, National Heart Lung Blood Institute (NHLBI) sponsored, long-term prospective study that measured the associations of established and suspected coronary artery disease (CAD) risk factors with atherosclerosis in a cohort of men and women aged 45–64 years in four US communities. A total of 15 792 participants were included in the baseline data collection. With 1987–89 as the baseline, follow-up examinations were conducted approximately every 3 years along with annual telephone interviews [17].
Assessment of baseline characteristics in ARIC study
Information on age, gender and race was based on self-report. Prevalent coronary artery disease was defined as a reported history of physician-diagnosed heart attack, cardiovascular surgery, coronary angioplasty or evidence of previous myocardial infarction on electrocardiogram. Congestive heart failure was defined as history of leg swelling associated with either orthopnoea or paroxysmal nocturnal dyspnoea. Peripheral vascular disease was defined as the presence of intermittent claudication or the absence of posterior tibialis pulse. History of physician-diagnosed stroke, chronic lung disease and malignancy were defined as prevalent cerebrovascular disease, lung disease and malignancy, respectively. Smoking and alcohol use were categorized as never, current and past.
The participants were asked to bring all medications taken during the study period to the clinic for examination, and the names of medications including beta blockers, calcium channel blockers, digoxin, diuretics and angiotensin-converting enzyme inhibitors were recorded.
Baecke physical activity questionnaire is a 16-item self-administered questionnaire that assesses physical activity in the domains of work, sport and leisure time. Items are scored on a five-point scale ranging from ‘Never’ to ‘Always’ or ‘Very Often’. Each response is given a numerical score. The physical activity score for each domain was calculated from these numerical scores [17].
Trained technicians measured blood pressure in the sitting position three times by using a random-zero sphygmomanometer, and the average of last two readings was used.
Resting heart rate was obtained from a standard supine 12-lead resting electrocardiogram and 2-min rhythm strip that was recorded at baseline after a 12-h fast followed by a light snack and at least 1 h after smoking or ingestion of caffeine.
Anthropometry measures
Height was measured to the nearest centimetre using a metal rule attached to a wall and a standard triangular headboard. Weight was measured in pounds using a beam balance with the subject standing in a scrub suit and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Waist circumference was determined by the horizontal measurement of maximum girth at the umbilicus.
Laboratory data
The participants were asked to fast for 12 h before the clinical examination. Processed fasting blood samples were quickly frozen at −70°C until analysis, which was performed within a few weeks.
The ARIC Central Laboratories measured serum glucose by using the hexokinase method, serum creatinine by an alkaline picrate colorimetric assay (Coulter Diagnostics, Hialeah, FL, USA), serum insulin by a radio-immuno assay (Cambridge 125I Insulin Kit, Cambridge Medical Diagnostics, Inc., Billerica, MA, USA), plasma von Willebrand factor by an ELISA (American Bioproducts Co., Diagnostica Stago, NJ, USA) and plasma fibrinogen by the thrombin-time titration method. Enzymatic methods were used to measure plasma total cholesterol and triglycerides. High-density lipoprotein (HDL) cholesterol was measured after dextran–magnesium precipitation of non-HDL lipoproteins. Low-density lipoprotein (LDL) cholesterol values were calculated from these measurements. The coefficients of variation% ranged from 1.3 to 6% for these assays. Laboratories in each study community performed white blood cell counts by using cell counters, and haemoglobin values were measured using the cyanomethaemoglobin method.
Definition of CKD
Estimated glomerular filtration rate (eGFR) was calculated from the four-variable Modification of Diet in Renal Disease (MDRD) equation [eGFR = 186.3 × serum creatinine−1.154 × age−0.203 × 0.742 if female × 1.21 if black] [18]. Serum creatinine concentration was calibrated with Cleveland Clinic measurement standards by the subtraction of 0.24 mg/dl [19]. Those with eGFR >150 ml/min/1.73 m2 were excluded [19]. CKD was defined as eGFR <60 ml/min/1.73 m2, and non-CKD was defined as eGFR ≥60 ml/min/1.73 m2.
Definition of metabolic syndrome
The National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) considered metabolic syndrome present if any three of the following five conditions were present [20]: abdominal obesity (waist circumference ≥102 cm in men and 88 cm in women), elevated serum triglycerides (≥150 mg/dl after 12-h fasting), reduced levels of serum HDL cholesterol (<40 mg/dl in men and <50 mg/dl in women), hypertension (systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or use of antihypertensive medications or a self-reported history of hypertension) and insulin resistance (fasting glucose ≥110 mg/dl or use of anti-diabetic agents or self-reported history of diabetes).
In addition, the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated by the following formula: [fasting serum insulin (mmol/l) × fasting serum glucose (μU/ml)]/22.5 [21].
Follow-up of cardiovascular events and mortality
The follow-up included annual telephone interviews (to identify hospitalizations and deaths), examinations every 3 years in 1990–92, 1993–95 and 1996–98 and survey of death certificates and discharge lists from local hospitals. Out-of-hospital deaths were traced by using death certificate data and, in most cases, an interview with next of kin and questionnaires completed by the patients’ physicians. Coroner reports and autopsy reports were obtained, when available, for use in validation. The follow-up data until 1998 are used in the current analyses.
ARIC study definition of coronary events
An ARIC Morbidity and Mortality Classification Committee reviewed and adjudicated all potential clinical CAD events that occurred during the follow-up [22]. Hospitalized definite/probable myocardial infarction (MI) was defined by the ARIC Morbidity and Mortality Classification Committee based upon the presence or the absence of cardiac pain, ECG findings and enzymes using published criteria. Further, this committee defined definite fatal CAD event as the lack of evidence to diagnose definite fatal MI and the absence of another lethal non-atherosclerotic or non-cardiac atherosclerotic process with either one or both of the following: history of chest pain within 72 h of death and history of ever having had chronic ischaemic heart disease in the absence of valvular heart disease or non-ischaemic cardiomyopathy. Coronary revascularization procedures were identified from hospital admissions.
ARIC study definitions of ischaemic stroke
Using symptoms, diagnostic procedures performed and autopsy evidence, cases were classified as definite stroke, probable stroke, possible stroke of undetermined type or no stroke by the computer algorithm and by a physician reviewer [22]. Another physician adjudicated differences between the computer and physician diagnoses.
Statistical methods
Predictor variable
For statistical analyses, resting heart rate was subdivided into four clinically defined categories as follows: the resting heart rate <60, resting heart rate 60–74, resting heart rate 75–89 and resting heart rate ≥90/min. These categorical groups were used to examine the relationship between baseline heart rate and baseline descriptive data and follow-up outcomes. Recognizing that categorical cut-offs could affect the results, we also examined the spline curves (as described below) to examine the associations of heart rate with outcomes.
Baseline data
Baseline characteristics across the resting heart rate groups were examined using the chi-squared test for categorical variables and the Kruskal–Wallis test for continuous variables.
Examination of cardiovascular events and mortality
Definition of composite cardiovascular outcome
Based on the ARIC study classification of events, we defined a composite cardiovascular outcome as the first occurrence of any one of the following events: definite/probable MI or definite/probable fatal CAD event, or definite/probable incident stroke or the performance of a coronary revascularization procedure.
The participants were censored at the first occurrence of cardiovascular composite, death, loss to follow-up or on 31 December 1998. Cox proportional hazards regression analysis was used to examine the association of the time to the composite cardiovascular outcome with the resting heart rate category. The resting heart rate group of 60–74/min was used as the reference group as heart rate of <60/min is considered abnormal. First, a basic Cox regression model was fitted without covariate adjustment to obtain unadjusted hazard ratios, and then the Cox model was expanded by adding covariates to adjust for demographics (age, gender and race), baseline comorbidities (coronary artery disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, diabetes mellitus, lung disease, malignancy, smoking and alcohol use), haemoglobin, physical activity indices and baseline eGFR. These covariates were specified prior to the analysis, based on clinical and biological considerations as factors that may act as confounders for the effects of heart rate on subsequent cardiovascular events and mortality. In these models, examination of scaled Shoenfeld residuals and evaluation of interaction terms between baseline factors and follow-up time showed no evidence of violations of proportional hazards assumptions.
For graphical presentations, we modified the above Cox-regression analyses by using a 4-degree of freedom natural cubic regression spline basis matrix [23], with internal knot points at the heart rate tertiles, to model the relationship between mortality and heart rate as a continuous variable while allowing for the possibility of nonlinearity. Because the cubic splines provided smooth functions over the range of the pulse rates in the dataset, the results are relatively insensitive to the selection of the knot points. This analysis was run with and without adjustment for the covariates listed above to depict unadjusted and adjusted hazard ratios, using the median heart rate of 67 beats/min as the reference.
Examination of all-cause mortality
The participants were censored at death, loss to follow-up or on 31 December 1998. The above analyses were repeated with time to death from any cause as the outcome.
Sensitivity analyses
As medications could potentially affect the heart rate, the above analyses were repeated, adding medications (beta blockers, diuretics, calcium channel blockers, ACE inhibitors and digoxin) to the models.
Because the presence of diabetes could modify the relationships between heart rate and outcomes, the associations of heart rate with the CV composite and all-cause mortality were examined in the subgroup without diabetes (n = 341). Since only nine individuals without diabetes had a heart rate of ≥90/min, three heart rate groups <60/min (n = 170), 60–74/min (n = 102) and ≥75/min (n = 69) were used for this sensitivity analysis.
All analyses were carried out using STATA version 11 or S-Plus version 7.0. Two-sided P-values and 95% confidence limits are provided without adjustment for multiple comparisons.
Results
We included 460 ARIC participants with CKD (defined as eGFR <60 ml/min/m2) in this analysis. The mean (±SD) age of the CKD cohort was 57 ± 6 years, 26% were African American and 36% were men. The mean resting heart rate was 68 ± 11/min and the mean eGFR was 50 ± 12 ml/min/1.73 m2.
Associations of resting heart rate with baseline demographic, clinical, nutritional and metabolic characteristics
Associations of resting heart rate with baseline characteristics are summarized in Tables 1 and 2. Higher resting heart rate was associated with the greater prevalence of congestive heart failure (Table 1). Even more striking was the higher prevalence of diabetes mellitus with higher resting heart rate (12% in the lowest resting heart rate group versus 68% in the highest resting heart rate group). The use of beta blockers was significantly more in patients with lower heart rate, and diuretic use was higher in the higher resting heart rate group. On the other hand, increased physical activity was associated with lower heart rate.
Table 1.
Associations of resting heart rate with demographics and clinical characteristics in CKD (N = 460)
| Characteristic | <60/min (N = 116) | 60–74/min (N = 226) | 75–89/min (N = 90) | ≥90/min (N = 28) | P-value |
|---|---|---|---|---|---|
| Age (year) | 56 + 6 | 57 + 6 | 57 ± 5 | 57 ± 6 | 0.58 |
| Black race (%) | 22 | 24 | 31 | 39 | 0.13 |
| Male gender (%) | 40 | 36 | 39 | 21 | 0.32 |
| Coronary artery disease (%) | 13 | 9 | 17 | 4 | 0.15 |
| Cerebrovascular disease (%) | 9 | 8 | 14 | 18 | 0.14 |
| Peripheral vascular disease (%) | 7 | 5 | 5 | 7 | 0.79 |
| Congestive heart failure (%) | 16 | 17 | 26 | 43 | 0.004 |
| Diabetes mellitus (%) | 12 | 25 | 33 | 68 | <0.001 |
| Malignancy (%) | 8 | 6 | 4 | 18 | 0.12 |
| Lung disease (%) | 4 | 6 | 11 | 18 | 0.04 |
| Current or past smoking (%) | 61 | 62 | 50 | 57 | 0.27 |
| Current or past alcohol use (%) | 70 | 73 | 63 | 57 | 0.18 |
| eGFR, ml/min/1.73 m2 | 51 ± 10 | 51 ± 12 | 49 ± 13 | 45 ± 15 | 0.03 |
| Beta blockers (%) | 40 | 17 | 16 | 7 | <0.001 |
| Calcium channel blockers (%) | 13 | 9 | 10 | 10 | 0.76 |
| ACE inhibitors (%) | 7 | 9 | 16 | 14 | 0.14 |
| Diuretics (%) | 45 | 38 | 46 | 64 | 0.04 |
| Digoxin (%) | 2 | 4 | 7 | 7 | 0.21 |
| Haemoglobin (g/dl) | 13.5 ± 1.7 | 13.4 ± 2.0 | 13.3 ± 2.3 | 12.8 ± 2.3 | 0.43 |
| Work index | 2.0 ± 1.0 | 1.8 ± 0.9 | 1.7 ± 0.9 | 1.7 ± 0.9 | 0.03 |
| Sport index | 2.4 ± 0.9 | 2.3 ± 0.8 | 2.1 ± 0.7 | 2.1 ± 0.6 | 0.14 |
| Leisure index | 2.3 ± 0.6 | 2.3 ± 0.6 | 2.1 ± 0.6 | 2.1 ± 0.5 | 0.01 |
Table 2.
Associations of resting heart rate with nutritional and metabolic characteristics in CKD (N = 460)
| Characteristic | <60/min (N = 116) | 60–74/min (N = 226) | 75–89/min (N = 90) | ≥90/min (N = 28) | P-value |
|---|---|---|---|---|---|
| BMI (kg/m2) | 28.1 ± 5.0 | 28.6 ± 5.0 | 29.6 ± 6.3 | 31.4 ± 6.9 | 0.07 |
| Waist circumference (cm) | 98 ± 14 | 100 ± 14 | 104 ± 15 | 106 ± 16 | 0.02 |
| Serum glucose (mg/dl) | 107 ± 26 | 117 ± 56 | 154 + 104 | 182 + 103 | <0.001 |
| Serum insulin (uU/l) | 14 ± 13 | 21 ± 41 | 44 + 105 | 66 + 102 | <0.001 |
| Log HOMA | 0.93 ± 0.88 | 1.18 ± 0.89 | 1.58 ± 1.05 | 2.28 ± 1.43 | <0.001 |
| Serum triglycerides (mg/dl) | 143 ± 87 | 149 ± 89 | 212 + 172 | 181 + 117 | <0.001 |
| Serum HDL (mg/dl) | 49 ± 18 | 50 ± 18 | 46 ± 15 | 46 ± 15 | 0.58 |
| Serum LDL (mg/dl) | 145 ± 42 | 144 ± 42 | 144 ± 42 | 167 ± 71 | 0.60 |
| Plasma fibrinogen (mg/dl) | 316 ± 64 | 334 ± 88 | 354 ± 79 | 392 ± 95 | <0.001 |
| Plasma vWF (% standard) | 143 ± 61 | 143 ± 64 | 154 ± 63 | 190 ± 73 | <0.001 |
| White blood cell count (1 × 103/μl) | 6.7 ± 6.3 | 6.4 ± 2.2 | 6.9 ± 1.9 | 8.3 ± 2.8 | <0.001 |
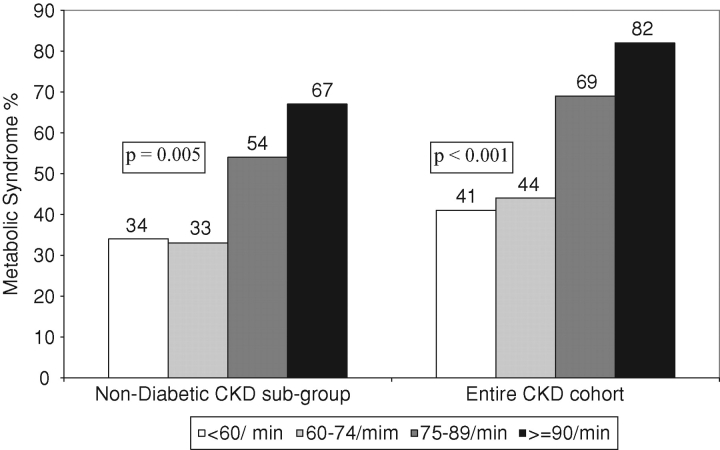
Higher heart rate was associated with the greater prevalence of metabolic syndrome in the entire CKD cohort and the non-diabetic subgroup (Figure 1). Furthermore, higher resting heart rate was associated with higher fasting serum glucose and insulin levels (Table 2). In addition, higher resting heart rate was associated with higher white blood cell count (marker of inflammation), plasma fibrinogen (marker of thrombotic tendency) and plasma von Willebrand factor (marker of endothelial function) as shown in Table 2.
Fig. 1.
Prevalence of metabolic syndrome in resting heart rate groups.
Association of resting heart rate with cardiovascular events
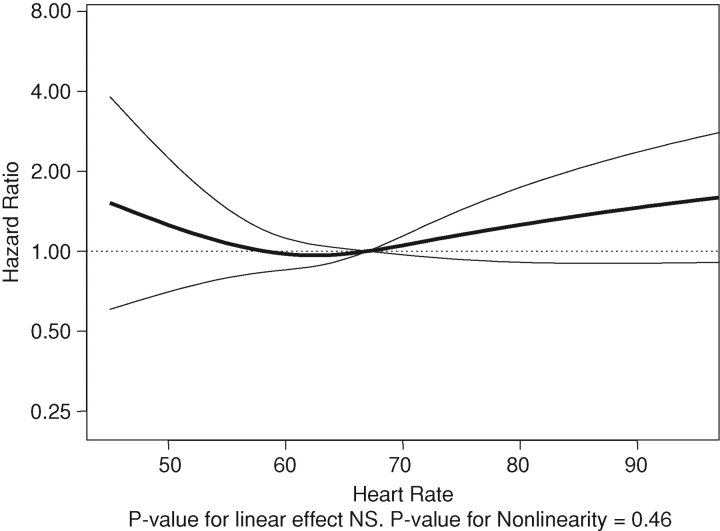
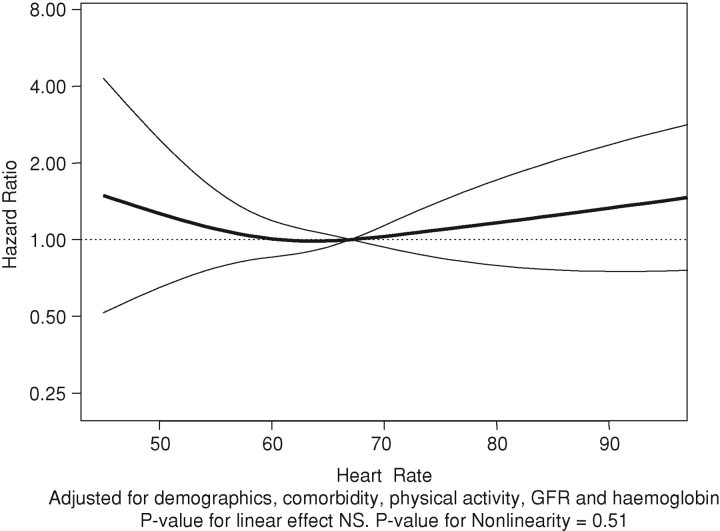
There were 2.89 cardiovascular events per 100 years of follow-up (a total of 110 cardiovascular events over 3797 years of follow-up). The associations of heart rate as a continuous variable with cardiovascular events are shown in Figures 2 and 3. Compared to the 60–74/min group, resting heart rate in the 75–89/min group was associated with statistically significant 1.79-fold higher hazard of the composite cardiovascular outcome (Table 3) but not in the ≥90/min group.
Fig. 2.
Unadjusted associations of resting heart rate with cardiovascular composite.
Fig. 3.
Adjusted associations of resting heart rate with the cardiovascular composite.
Table 3.
Association of resting heart rate with the composite cardiovascular outcome in CKD
| Resting heart | Unadjusted hazard | Adjusteda hazard |
|---|---|---|
| rate | ratio (95% CI) | ratio (95% CI) |
| <60/min | 1.20 (0.76–1.90) | 1.27 (0.75–2.16) |
| 60–74/min | Reference | Reference |
| 75–89/min | 1.77 (1.10–2.84) | 1.79 (1.07–2.99) |
| ≥90/min | 1.29 (0.55–3.02) | 1.37 (0.54–3.44) |
aAdjusted for age, gender, race, coronary artery disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, diabetes mellitus, lung disease, malignancy, smoking, alcohol use, physical activity (work, leisure and sports indices) and haemoglobin.
Association of resting heart rate with mortality
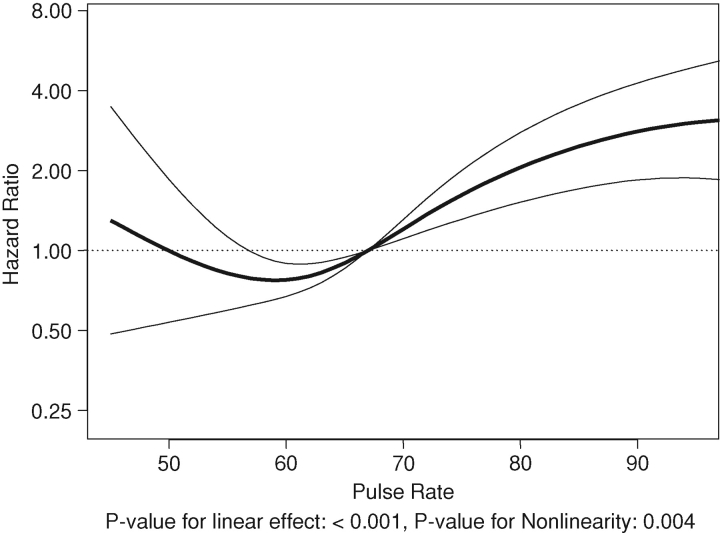
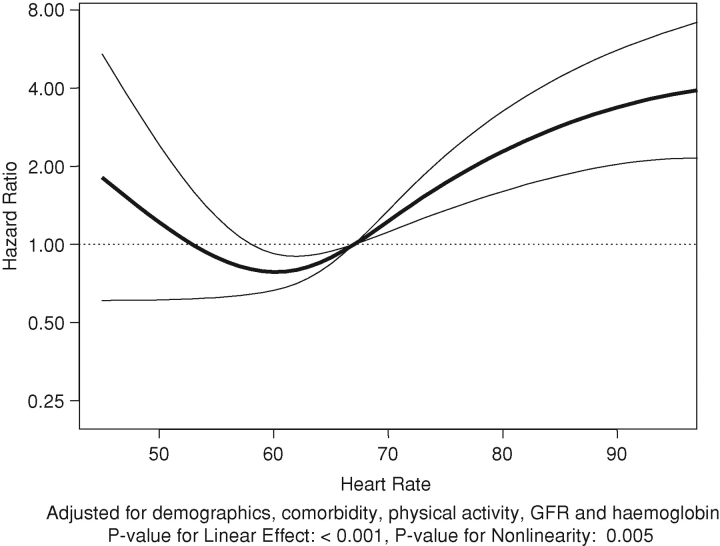
There were 3.12 deaths per 100 years of follow-up (a total of 126 deaths over 4033 years). The association of resting heart rate with death is summarized in Table 4 and Figures 4 and 5. There appears to be a J-shaped association of resting heart rate with death (Figures 4 and 5) when heart rate was used as a continuous variable. Compared to the 60–74/min group, resting heart rate in the < 60/min group was associated with statistically non-significant increased hazard of death (Table 4). However, compared to the reference group, resting heart rate groups of 75–89/min and ≥90/min were associated with statistically significant 3.11- and 3.97-fold higher hazards of death (Table 4), respectively.
Table 4.
Association of resting heart rate with death in CKD
| Unadjusted hazard | Adjusteda hazard | |
|---|---|---|
| Resting heart | ratio (95% CI) | ratio (95% CI) |
| <60/min | 1.16 (0.72–1.87) | 1.47 (0.85–2.53) |
| 60–74/min | Reference | Reference |
| 75–89/min | 2.55 (1.66–3.93) | 3.11 (1.93–5.02) |
| ≥90/min | 3.44 (1.92–6.17) | 3.97 (1.99–7.94) |
aAdjusted for age, gender, race, coronary artery disease, cerebrovascular disease, peripheral vascular disease, congestive heart failure, diabetes mellitus, lung disease, malignancy, smoking, alcohol use, physical activity (work, leisure and sports indices) and haemoglobin.
Fig. 4.
Unadjusted associations of resting heart rate with all-cause mortality.
Fig. 5.
Adjusted associations of resting heart rate with all-cause mortality.
Sensitivity analyses
When adjusted for medications (beta blockers, calcium channel blockers, diuretics, ACE inhibitors and digoxin use) in addition to demographics, comorbidity, physical activity, GFR and haemoglobin and using 60–74/min as the reference group, none of the other heart rate groups were significantly associated with cardiovascular events but heart rate groups 75–89 and ≥90/min were associated with increased mortality: hazard ratio (HR) 3.16, 95% CI (1.94–5.14) and HR 3.64, 95% CI (1.86–7.12), respectively.
Since there were only nine participants without diabetes who had a heart rate of ≥90/min, these participants were included in the ≥75/min group. In Cox models adjusted for above covariates including medications, compared to 60–74/min group, the other heart rate groups were not significantly associated with cardiovascular events. In a similar model, <60/min group was not associated with increased mortality (HR 1.14, 95% CI 0.55–2.39) but ≥75/min group was (HR 2.21, 95% CI 1.08–4.51).
Discussion
In the general population, higher resting heart rate is associated with worse outcomes [13–15,24–26]. The results of this study indicate that higher resting heart rate is associated with increased risk of cardiovascular events and mortality in the CKD population. Of note, this phenomenon is seen within the ‘normal’ heart rate < 100/min. Thus, the resting heart rate does not need to be abnormally high to be associated with cardiovascular events and mortality.
To our knowledge, there are no prior data on the associations between resting heart rate with insulin resistance, cardiovascular events and mortality in the moderate CKD population. In individuals with eGFR <75 ml/min/1.73 m2 and documented prior myocardial infarction with a left ventricular ejection fraction of <30%, Chonchol et al. [27] reported that the higher resting heart rate (>80/min) was a risk factor for sudden death. In contrast, our study examined community dwelling adults with moderate CKD (eGFR < 60 ml/min/1.73 m2), and the results suggest that resting heart rate ≥75/min predicts mortality in that population. Furthermore, the association of higher resting heart rate with mortality was also present in non-diabetic CKD.
As shown in Table 1, beta blockers were associated with lower heart rate whereas diuretics were associated with higher heart rate. However, when further adjusted for medication use, high heart rate was still associated with increased mortality.
The mechanisms for increased mortality with higher resting heart rate at the upper limit of normal range are not clear. Higher catecholamine levels in dialysis patients are associated with increased risk of death [28] and they may play a similar role in patients with CKD. Further studies will be needed to examine whether interventions that result in decreased sympathetic activation, as evidenced by resting heart rate at the lower range of normal, are associated with improvement in cardiovascular outcomes in the CKD population.
The results of our study further support the hypothesis that resting heart rate is associated with metabolic syndrome. Higher resting heart rate was associated with higher body mass index, waist circumference and greater prevalence of abdominal obesity. Higher resting heart rate was also associated with insulin resistance, higher white blood cell count (marker of inflammation), plasma fibrinogen (marker of thrombotic tendency) and plasma von Willebrand factor (marker of endothelial function) (Table 2).
The strengths of this study include very careful data collection in the ARIC study such as measurement of resting heart rate with electrocardiogram and cardiovascular outcomes data. The major limitations of this study include those of all observational studies that use existing data. The observational nature of the study limits inference beyond associations. Resting heart rate categorization was done based on the initial examination only. Nevertheless, this limitation enhances rather than diminishes the importance of resting heart rate. The fact that the predictive power of resting heart rate remained independently of multivariable adjustment indicates the robustness of the association with mortality. Categorization of resting heart rate into four groups could limit the inferences on the functional relationship between the heart rate and outcomes; however, to address this issue we used heart rate as a continuous variable in spline regression, which also suggests that high resting heart rate is associated with increased mortality. Resting heart rate was associated with increased cardiovascular events in some models but not others in this study. This might reflect the smaller number of cardiovascular events in this cohort and larger studies are warranted to address this issue. Furthermore, atrial fibrillation is not adequately coded in the public access ARIC cohort. Therefore, we were unable to perform a subgroup analysis for this and other arrhythmias.
In summary, we conclude that resting heart rate is associated with metabolic syndrome and predicts cardiovascular outcomes and mortality in the CKD population. Further studies will be needed to examine whether interventions that target sympathetic activation can lead to improved outcomes in the CKD population.
Acknowledgments
This work is supported by a grant from the Dialysis Research Foundation of Utah. S.B. is the recipient of RO1-DK077298 and RO1-DK078112. The ARIC Study is conducted and supported by the NHLBI in collaboration with the ARIC Study Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the ARIC study or the NHLBI.
Conflict of interest statement. None declared.
References
- 1.Hotamisligil GS, Peraldi P, Budavari A, et al. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 2.Ramkumar N, Cheung AK, Pappas LM, et al. Association of obesity with inflammation in chronic kidney disease: a cross-sectional study. J Ren Nutr. 2004;14:201–207. [PubMed] [Google Scholar]
- 3.Beddhu S, Kimmel PL, Ramkumar N, et al. Associations of metabolic syndrome with inflammation in CKD: results From the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2005;46:577–586. doi: 10.1053/j.ajkd.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 5.Brunzell JD, Hokanson JE. Dyslipidemia of central obesity and insulin resistance. Diabetes Care. 1999;22:C10–C13. [PubMed] [Google Scholar]
- 6.Alvarez GE, Beske SD, Ballard TP, et al. Sympathetic neural activation in visceral obesity. Circulation. 2002;106:2533–2536. doi: 10.1161/01.cir.0000041244.79165.25. [DOI] [PubMed] [Google Scholar]
- 7.Anderson EA, Hoffman RP, Balon TW, et al. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87:2246–2252. doi: 10.1172/JCI115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esler M, Straznicky N, Eikelis N, et al. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48:787–796. doi: 10.1161/01.HYP.0000242642.42177.49. [DOI] [PubMed] [Google Scholar]
- 9.Eikelis N, Schlaich M, Aggarwal A, et al. Interactions between leptin and the human sympathetic nervous system. Hypertension. 2003;41:1072–1079. doi: 10.1161/01.HYP.0000066289.17754.49. [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities—the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 11.Muscelli E, Camastra S, Gastaldelli A, et al. Influence of duration of obesity on the insulin resistance of obese non-diabetic patients. Int J Obes Relat Metab Disord. 1998;22:262–267. doi: 10.1038/sj.ijo.0800580. [DOI] [PubMed] [Google Scholar]
- 12.Kuo LE, Kitlinska JB, Tilan JU, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 13.Benetos A, Rudnichi A, Thomas F, et al. Influence of heart rate on mortality in a French population: role of age, gender, and blood pressure. Hypertension. 1999;33:44–52. doi: 10.1161/01.hyp.33.1.44. [DOI] [PubMed] [Google Scholar]
- 14.Dyer AR, Persky V, Stamler J, et al. Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol. 1980;112:736–749. doi: 10.1093/oxfordjournals.aje.a113046. [DOI] [PubMed] [Google Scholar]
- 15.Gillman MW, Kannel WB, Belanger A, et al. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 16.Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 17.The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, et al. Modification of Diet in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 20.Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.National Heart, Lung and Blood Institute Atherosclerosis Risk in Communities (ARIC) Study. 1987. Operations manual. ARIC Coordinating Center, School of Public Health, University of North Carolina, Chapel Hill, NC.
- 23.Therneau T, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. pp. 102–106. Section 5.4 Regression Splines. [Google Scholar]
- 24.Goldberg RJ, Larson M, Levy D The Framingham Study. Factors associated with survival to 75 years of age in middle-aged men and women. Arch Intern Med. 1996;156:505–509. [PubMed] [Google Scholar]
- 25.Kannel WB, Kannel C, Paffenbarger RS, Jr, et al. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 26.Palatini P, Julius S. Association of tachycardia with morbidity and mortality: pathophysiological considerations. J Hum Hypertens. 1997;11:S19–S27. [PubMed] [Google Scholar]
- 27.Chonchol M, Goldenberg I, Moss AJ, et al. Risk factors for sudden cardiac death in patients with chronic renal insufficiency and left ventricular dysfunction. Am J Nephrol. 2007;27:7–14. doi: 10.1159/000098431. [DOI] [PubMed] [Google Scholar]
- 28.Zoccali C, Mallamaci F, Parlongo S, et al. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]