Abstract
Background. Recent evidence suggests that treatment of type 2 diabetes with thiazolidinediones [peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists], ameliorates glomerulosclerosis and tubulointerstitial fibrosis in the rat kidney. In the current work, we have investigated whether these drugs, and specifically pioglitazone (PGT), act by preventing fibrosis and kidney dysfunction mainly through antioxidant and anti-inflammatory effects, independently of glycaemic control.
Methods. Male 2- to 3-month-old obese Zucker fa/fa (OZR) and ZDF fa/fa rats (ZDFR), and their control the lean Zucker rat (LZR), were used. Diabetic rats were given either a low dose (0.6 mg/kg/day) or a high dose (12 mg/ kg/day) of PGT in the chow for 2 or 4–5 months. Glycaemia, blood pressure, creatinine clearance and proteinuria were determined, and the underlying histopathology was defined with markers of fibrosis, glomerular damage, oxidative stress and inflammation by immunohistochemistry/ quantitative image analysis in tissue sections, and western blots and ad hoc assays in fresh tissue.
Results. PGT at low doses given for 4–5 months considerably reduced blood pressure, proteinuria and creatinine clearance. This was associated with amelioration of renal tissue damage and fibrosis, evidenced by the glomerulosclerosis, tubulointerstitial fibrosis, tubular atrophy and podocyte injury indexes, and of oxidative stress and inflammation, as shown by the decrease in the respective markers, although glycaemia remained high and obesity was not affected.
Conclusions. These results indicate that low doses of PGT ameliorate renal fibrosis and preserve renal function in this animal model of metabolic syndrome, independently of glycaemic control or effects on body weight.
Keywords: diabetic nephropathy, fibrosis, inflammation, oxidative stress, thiazolidinediones
Introduction
Diabetic nephropathy is the most common cause of end-stage kidney disease, and the leading cause of death in diabetic patients [1,2]. Hyperglycaemia, and the metabolic syndrome manifestations of obesity, hyperlipidaemia and hypertension, are strongly associated with chronic kidney disease [3]. The fundamental mechanism responsible for nephropathy in type 2 diabetes involves tubulointerstitial fibrosis and glomerulosclerosis, caused among other processes by oxidative stress, activation of the aldosterone–renin–angiotensin system, inflammation, release of pro-fibrotic factors such as transforming growth factor β1 (TGFβ1) and plasminogen activator inhibitor 1 (PAI-1), collagen cross-linking and epithelial mesenchymal transition [4–7].
A widely used animal model of nephropathy in type 2 diabetes and metabolic syndrome is the obese Zucker fa/fa rat (OZR) [8,9]. Homozygous recessive males develop considerable obesity, moderate fasting hyperglycaemia, mild hypertension, hypertriglyceridaemia, hypercholesterolaemia and hyperinsulinaemia, while the lean genotypes remain normoglycaemic and normotensive. The fa/fa genotype is characterized by impaired glucose tolerance caused by the obesity gene mutation that leads to insulin resistance. Another related strain, the Zucker diabetic fatty fa/fa rat (ZDFR), presents mild obesity but severe hyperglycaemia, and progressive hypoinsulinaemia. Studies in these strains have clarified several aspects of the pathophysiology of glomerulosclerosis and of tubulointerstitial fibrosis in relation to hyperglycaemia (e.g. 10–16). We previously showed in the male OZR at 6 months of age, in comparison to the age-matched lean Zucker rats (LZR), that the combination of an angiotensin-converting enzyme (ACE) inhibitor and an angiotensin-II type I receptor (ATIIR-1) antagonist improved glomerulosclerosis, tubulointerstitial fibrosis, hypertension and proteinuria, [17] as well as characterized other features of diabetes-induced tissue fibrosis in the heart, liver and penis [18–22].
The thiazolidinediones are promising too for the treatment of diabetic nephropathy, since they have anti-inflammatory and antifibrotic effects that may be key in the amelioration of the cardiovascular and renal [23–25] complications of diabetes. They lower blood glucose primarily by improving insulin sensitivity in peripheral tissues via activation of the peroxisome proliferator-activated receptor γ (PPAR-γ). Chronic administration of a low dose of a thiazolidinedione (pioglitazone), ameliorated fibrosis in the corporal smooth muscle tissue of the penis in the OZR independently of glycaemic control, and in the aged Fischer 344 rats [21,26]. In diabetic nephropathy, pioglitazone at higher doses that control glycaemia enhanced the renoprotective effects of an ATIIR-1 inhibitor on glomerulosclerosis and tubulointerstitial fibrosis in the rat, but no pioglitazone alone arm was included [27], and mitigated renal glomerular changes in mice [28]. Rosiglitazone and troglitazone [29,30] exerted similar effects in the ZDFR at doses that normalize glycaemia. Thiazolidinediones in general, and pioglitazone in particular, reduce blood pressure and exert pleiotropic effects beyond glucose lowering in the human [31,32].
The current study investigated the hypothesis that thiazolidinediones, and specifically pioglitazone, ameliorate glomerulosclerosis and tubulointerstitial fibrosis, by preventing fibrosis and kidney dysfunction mainly through antioxidant and anti-inflammatory effects, independently of glycaemic control. Since in clinical practice, patients with type 2 diabetes who develop nephropathy show various degrees of obesity, hyperglycaemia and insulinaemia, the use in parallel of both subtypes of Zucker rats is a preliminary approach to determine whether the response to pioglitazone may be differentially affected by one or the other of these factors.
Materials and methods
Animal treatments
The male LZR and their obese diabetic counterpart, the OZR fa/fa, were obtained at 2–3 months of age from Harlan Sprague-Dawley, Inc., San Diego, CA, USA, and maintained at the LABioMed vivarium. The ZDFR fa/fa variants (strain ZDF/Crl-Leprfa; lean+/?) were obtained from Charles Rivers Laboratories, Wilmington, MA, and maintained at the Hospital Aleman vivarium. Animals were treated according to the ‘NIH Principles of laboratory animal care’ with the respective Institutional Animal Care and Use Committee (IACUC)-approved protocols, and the final review of the Hospital Aleman approval by the LABioMed IACUC. Untreated groups were fed Purina chow, whereas the pioglitazone-treated diabetic groups were fed chow containing 0.001% (low-dose) or 0.02% (high-dose) pioglitazone. The amount of food eaten was recorded daily and the approximate doses were calculated to be 0.6 or 12 mg/kg/day, respectively.
In experiment 1, kidney tissue was excised at killing from the LZR and OZR rats used for parallel studies [21,22] in the following groups (n = 8 rats/group). (a) Untreated: (1) LZR for 5 months, (2) OZR for 2 months, (3) OZR for 5 months; (b) pioglitazone treatment: (4) OZR, high dose, for 2 months; (5) OZR, low dose, for 2 months; (6) OZR, low dose, for 5 months. In experiment 2, for functional studies, the LZR and ZDFR were divided into the following groups: (a) untreated (7) LZR for 4 months; (8) ZDFR for 4 months; (b) pioglitazone: (9) ZDFR, low dose, for 4 months. Ages at sacrifice were 5 months (groups 2, 4, and 5) or 7 months (groups 1, 3 and 6–9). We have named the 2- and 4- to 5-month treatments as ‘short-term’ and ‘long-term’, respectively.
In both experiments, the left kidneys were fixed in 10% formalin and embedded in paraffin for histochemical and immunohistochemical studies. Portions of right kidneys were either embedded in OCT or left untreated, and then frozen in liquid nitrogen and stored at −80°C.
Functional measurements
At baseline and then weekly, blood pressure was measured for experiment 2 in the LZR and ZDFR by a non-invasive pressure device using volume pressure recording, CODA 2 (Kent Scientific Co., Torrington, CT, USA), on non-anaesthetized rats restrained in a thermic plastic chamber [17]. In experiments 1 and 2, for all fa/fa rats, glycaemia was determined weekly in blood from the tails (Accu-Chek Active glucose meter, Roche, Ireland). In experiment 1, blood was also withdrawn from the hearts at killing. For experiment 2, 24-h urine was collected from the LZR and ZDFR in metabolic cages, and blood samples were withdrawn from the tails (14-h fasting) at baseline, and then every 4 weeks [17]. Aliquots of sera and urine were assayed for creatinine using the enzymatic UV method (Randox Lab, Crumlin, N. Ireland). Creatinine clearance was determined by the standard formula. Body weights were determined weekly. Proteinuria was determined by standard methods by SYSMEX-XT 1800i Roche-Diagnostic.
Determinations in renal tissue sections
Paraffin-embedded sections (3 μm) were subjected to immunohistochemical assays as reported [17]. Monoclonal antibodies were used after antigen retrieval for TGFβ1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and collagen types I and III (Biogenex, San Roman, CA, USA) to evaluate fibrosis. Other antibodies were used as follows: (A) monoclonal: α-smooth muscle actin (αSMA) (Sigma Chemical Co., St Louis, MO, USA) for myofibroblasts; and ED1 (CD68) (AbD Serotec, Raleigh, NC, USA) for macrophages (inflammation); (B) Polyclonal: PAI-1 (American Diagnostica, Greenwich, CT, USA) for fibrosis; and haem oxygenase 1 (Stressgen, Ann Arbor, MI, USA) for oxidative stress [33]. Immunostaining was carried out with an avidin-biotin-peroxidase complex kit, and counterstained with haematoxilin. The sections were also stained with Masson's trichrome [17].
For immunofluorescence, frozen sections (5 μm) were treated with a goat polyclonal antibody anti-rat nephrin (1:50) (Santa Cruz), followed by a donkey anti-goat IgG-FITC (1/100) (Santa Cruz). Negative controls consisted of histological sections incubated with PBS rather than the primary antibody. Immunostaining was visualized on a Nikon E400 fluorescence microscope equipped with a high-pressure mercury lamp. Images were acquired with a digital camera and processed (Nikon Instrument Group, Melville, NY, USA).
Tissue samples were blindly evaluated by two investigators using an image analyser Image-Pro Plus version 4.5 for windows (Media Cybernetics, L.P. Silver Spring, MD, USA) [17,21,22]. Histomorphometric evaluation of the kidney was assessed on 20 consecutive microscopic fields. Data were averaged and expressed as a percentage/mm2 as indicated in Tables 1 and 2.
Table 1.
Quantitative image analysis of the histological and imnunohistochemical determinations in the kidney of the OZR treated short- and long-term with a high or low dose of pioglitazone
| Rat strain | LZR | OZR | OZR | OZR | OZR | OZR |
|---|---|---|---|---|---|---|
| Pioglitazone dose | – | – | – | High | Low | Low |
| Treatment (months) | – | 2 | 5 | 2 | 2 | 5 |
| Age (months) | 5 | 5 | 7 | 5 | 5 | 7 |
| Body weight (g) | 432 ± 40* | 680 ± 44 | 756 ± 62 | 863 ± 81 | 801 ± 61 | 864 ± 76 |
| Glycaemia (mg/dl) | 139 ± 13 | 263 ± 173** | 244 ± 35** | 130 ± 20** | 171 ± 31† | 320 ± 145 |
| GS (%)/glomerulus | 0.9 ± 0.5* | 19.3 ± 3.9* | 28.7 ± 6.5* | 8.0 ± 1.3** | 12.9 ± 2.0† | 6.9 ± 1.8 |
| IF (%)/cross section | 2.6 ± 1.1* | 12.1 ± 1.8* | 16.9 ± 2.8* | 6.9 ± 1.0** | 9.9 ± 1.8† | 6.8 ± 1.1 |
| TA (%)/cross section | 1.7 ± 0.8* | 7.3 ± 1.5* | 9.2 ± 1.8* | 4.4 ± 1.2** | 6.4 ± 0.9† | 4.5 ± 0.8 |
| αSMA (%)/cross section | 1.3 ± 0.5* | 11.9 ± 1.5* | 14.0 ± 1.2* | 3.8 ± 0.7** | 7.1 ± 1.3† | 4.8 ± 0.9 |
| TGFβ1 (%)/cross section | 1.8 ± 0.4* | 24.3 ± 2.3* | 26.7 ± 3.0* | 8.8 ± 2.4** | 14.8 ± 2.7† | 9.7 ± 1.8 |
| PAI-1 (%)/cross section | 1.3 ± 0.4* | 12.0 ± 2.4* | 20.8 ± 3.0* | 3.5 ± 1.4** | 6.6 ± 1.2† | 2.2 ± 0.4 |
| Col III | 4.6 ± 0.5* | 13.3 ± 1.9* | 17.1 ± 1.8* | 6.7 ± 1.5** | 10.1 ± 1.6† | 7.8 ± 1.5 |
| Col I | 1.1 ± 0.3* | 9.5 ± 1.6* | 12.3 ± 1.4* | 4.4 ± 0.6** | 6.5 ± 0.7† | 5.2 ± 0.5 |
| Col III/col I ratio | 4.8 ± 0.7* | 1.4 ± 0.3 | 1.4 ± 0.1 | 1.5 ± 0.4 | 1.5 ± 0.3 | 1.5 ± 0.2 |
| Nephrin (% glomerulus) | 20.4 ± 2.4* | 7.2 ± 1.5* | 4.5 ± 1.1* | 18.9 ± 1.9** | 12.5 ± 2.0† | 18.1 ± 2.8 |
| Haem ox I (IOD/area) | 12.6 ± 5.0 | – | 136 ± 64* | – | – | 15.7 ± 2.2 |
GS = glomerulosclerosis; IF = interstitial fibrosis; TA = tubular atrophy; ASMA = α-smooth muscle actin; TGFβ1 = transforming growth factor β1; Col: collagen; Haem ox: haem oxygenase 1.
All values expressed as means ± SD.
*Versus all groups P < 0.01.
**Versus LZR; OZR 5 months; OZR 7 months and OZR 5 months, PGT low, P < 0.01.
†Versus OZR, 7 months, PGT low, P < 0.01.
Table 2.
Quantitative image analysis of the histochemical and imnunohistochemical determinations, and other in the kidney of ZDFR treated long-term with a low dose pioglitazone
| Rat strain | LZR | ZDFR | ZDFR |
|---|---|---|---|
| Pioglitazone dose | – | – | Low |
| Treatment (months) | 4 | 4 | 4 |
| Age (months) | 7 | 7 | 7 |
| Body weight (g) | 379 ± 19* | 486 ± 13 | 490 ± 12 |
| Glycaemia (mg/dl) | 105 ± 7* | 455 ± 32** | 243 ± 21 |
| GS (%)/glomerulus | 1.0 ± 0.4* | 27.1 ± 5.1** | 7.6 ± 2.2 |
| IF (%)/cross section | 2.4 ± 1.2* | 15.9 ± 3.0** | 6.0 ± 1.6 |
| TA (%)/cross section | 1.5 ± 0.8* | 8.7 ± 2.2** | 4.2 ± 1.2 |
| αSMA (%)/cross section | 1.2 ± 0.5* | 13.3 ± 1.9** | 4.5 ± 1.2 |
| Nephrin (%)/per glomerulus | 20.4 ± 2.4* | 5.1 ± 1.3** | 16.9 ± 2.3 |
| Haem oxygenase 1 | 10.6 ± 1.8* | 66.2 ± 12.3** | 20.4 ± 3.9 |
| GSH/GSSG ratio | 8.8 ± 0.9* | 3.1 ± 0.6** | 5.9 ± 0.7 |
| Malonyldialdehyde (nmoles/mg protein) | 128 ± 18.3* | 279 ± 18.7** | 195 ± 12.8 |
| ED1 (CD68) | 1.3 ± 0.9* | 15.3 ± 2.2** | 3.4 ± 1.0 |
GS = glomerulosclerosis; IF = interstitial fibrosis; TA = tubular atrophy; ASMA = α-smooth muscle actin; TGFβ1 = transforming growth factor β1.
All values expressed as means ± SD.
*Versus all groups P < 0.01.
**Versus ZDFR, 7 months, PGT low, P < 0.01.
Determinations in fresh tissue
Pieces from the middle region of the frozen right kidney were homogenized in a buffer with protease inhibitors, and supernatants (10 000 g, 5 min) were run on 10% polyacrylamide gels and submitted to western blot immunodetection [21,22,26] with a monoclonal ASMA IgG (1:1000; Oncogene) followed by a secondary polyclonal horse anti-mouse IgG linked to horseradish peroxidase (1:2000; BD Transduction Laboratories). Bands were visualized with luminol (Pierce, Rockford IL). For negative controls, the primary antibody was omitted. Band intensities were determined by densitometry and corrected by the respective intensities for glyceraldehyde phosphate dehydrogenase (GAPDH) upon re-probing.
Collagen was estimated as described [21,26,34] from 200 mg aliquots of whole renal tissue that was homogenized in saline, hydrolysed with 2 M NaOH, followed by hydroxyproline assays. Values were expressed as μg of collagen per mg of tissue. Oxidative stress was measured by the reduced glutathione (GSH)/oxidized glutathione (GSSG) ratio in the 10 000 g supernatant from a 0.25 M sucrose homogenate of a kidney aliquot [35]. Another aliquot was homogenized in a 0.05 M sodium phosphate buffer, and pH 7.4 was used for determining malondialdehyde to evaluate lipoperoxidation by thiobarbituric reactive species (TBARS).
Statistical method
Values were expressed as mean ± SD. All statistical analyses were processed through GraphPad Prism, version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). The Gaussian distribution was performed by the Kolmogorov and Smirnov method. For parameters with the Gaussian distribution, comparisons among groups were carried out using one-way analysis of variance (ANOVA) followed by Bonferroni's test, and two-way ANOVA for repeated measures was used as appropriate. For those parameters with non-Gaussian distribution, comparisons were performed by the Kruskal–Wallis test (Nonparametric ANOVA) and Dunnś multiple comparison test. A value of P < 0.05 was considered significant.
Results
Long-term treatment with low-dose pioglitazone in the OZR reduces glomerulosclerosis and tubulointerstitial fibrosis, without affecting glycaemia or body weight
Hyperglycaemia duration was about 2 months in groups 2, 4 and 5, and 5 months in groups 3 and 6. Although high-dose pioglitazone for 2 months normalized glycaemia, a considerable hyperglycaemia remained after treatment for 2 months with a low dose (Table 1). The low-dose effects were transient, since hyperglycaemia was not reduced at all after a more prolonged treatment (5 months). No high-dose treatment was conducted at this period. The considerable increase in body weights, as compared to the untreated OZR by the high-dose short-term pioglitazone, was not seen with the low-dose pioglitazone at either period (Table 1) [21].
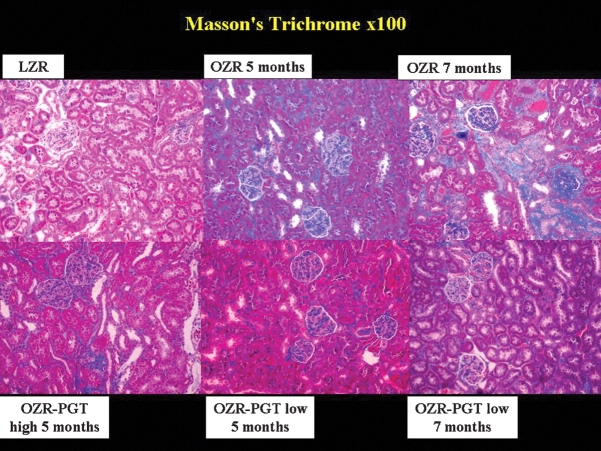
Masson trichrome (Figure 1) revealed that collagen deposition as an indicator of tubulointerstitial fibrosis was much higher in the untreated OZR than in the LZR, and that pioglitazone reduced the staining to the one in the LZR. Quantitative image analysis (Table 1) shows the percentage of the glomerulosclerosis per glomerulus. When these data are expressed in the OZR in reference to the non-diabetic LZR controls (differential glomerulosclerosis), by subtracting the basal value for the LZR, they were reduced by short-term pioglitazone at high and low doses by 59% and 35%, respectively, and the long-term treatment with low dose was even more effective: a 78% reduction.
Fig. 1.
Effect of pioglitazone on glomerulosclerosis and interstitial fibrosis in the kidney of the Zucker fa/fa rat. Representative pictures of tissue sections stained with Masson's trichrome staining. Magnification: ×100. Top panels: PGT, pioglitazone; LZR, lean Zucker rats, untreated. OZR: obese Zucker rats, untreated for the indicated time periods. Bottom panels: OZR-PGT, OZR treated with pioglitazone for the indicated time periods and doses.
Tubular atrophy and interstitial fibrosis were higher in the untreated OZR groups versus the LZR group, as seen in Figure 1 and Table 1. Calculating the differential values as for glomerulosclerosis, the short-term treatment with pioglitazone at the high and low doses reduced tubular atrophy and interstitial fibrosis in the OZR by 52 and 55%, respectively, whereas at low dose the response was lower: only 16 and 23%, respectively. In contrast, long-term, low-dose pioglitazone was more effective, with 63 and 70% reduction, respectively.
The drug reduced the content of myofibroblasts that characterize fibrosis [4,6,21] (Figure 2) as seen from the high immunostaining for αSMA in the untreated OZR in renal interstitium and periglomerular area, in comparison with the LZR. Pioglitazone treatment at low and high doses showed a lower immunostaining. The quantitative assessment in Table 1 shows a good agreement with the images, with long-term pioglitazone at low dose being the most efficacious treatment by reducing αSMA in the OZR by 72%.
Fig. 2.
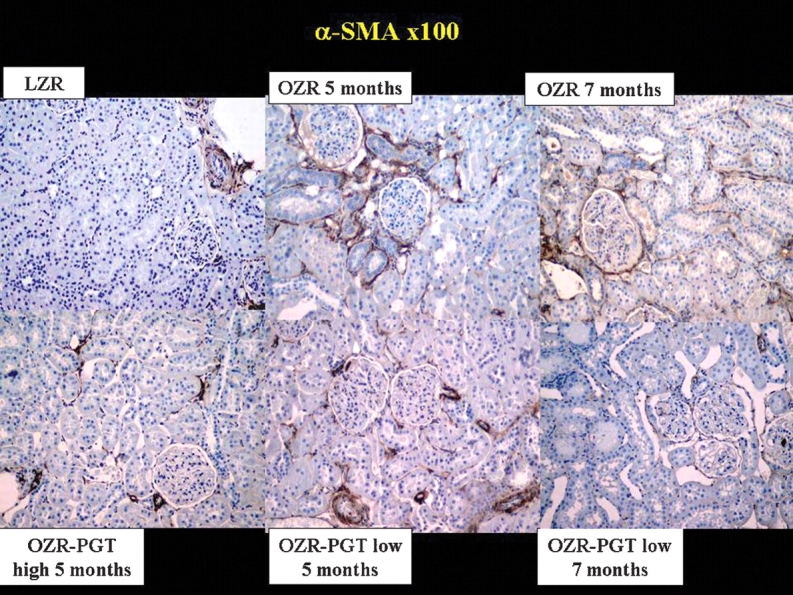
Effect of pioglitazone on the content of myofibroblasts in the kidney of the Zucker fa/fa rat. Representative pictures of tissue sections immunostained for ASMA. Magnification: ×100. Top panels: PGT, pioglitazone; LZR, lean Zucker rats, untreated. OZR: obese Zucker rats, untreated for the indicated time periods. Bottom panels: OZR-PGT, OZR treated with pioglitazone for the indicated time periods and doses.
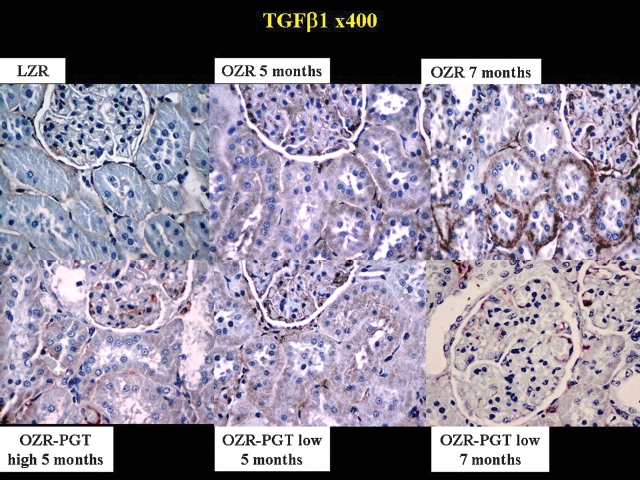
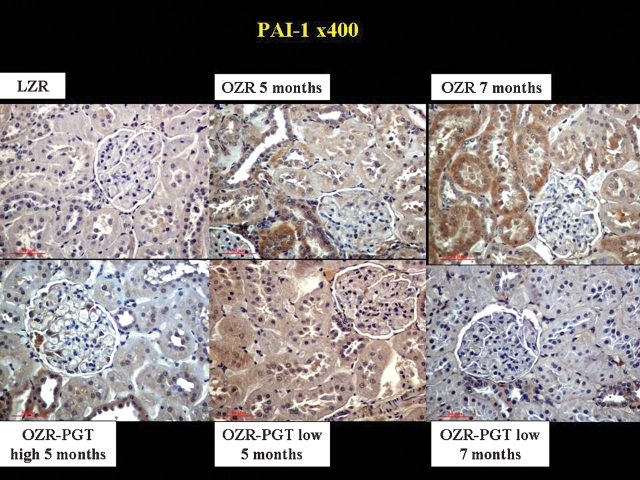
Pioglitazone also reduced TGFβ1 and PAI-1, key profibrotic factors particularly in diabetic nephropathy [12,34]. Figure 3 shows higher levels of TGFβ1 in glomeruli, tubular cells and renal interstitium in the untreated OZR relative to the LZR. Pioglitazone at low and high doses reduced TGFβ1, and the differential expression in the OZR referred to the LZR was decreased by the low-dose long-term treatment by 68% (Table 1). PAI-1 immunostaining (Figure 4) revealed a similar pattern as for TGFβ1, and the differential expression in the OZR referred to the LZR in Table 1 was reduced by 95% by long-term low-dose pioglitazone.
Fig. 3.
Effect of pioglitazone on the expression of the fibrotic factor TGFβ1 in the kidney of the Zucker fa/fa rat. Representative pictures of tissue sections immunostained for TGFβ1. Magnification: ×400. Top panels: PGT, pioglitazone; LZ: lean Zucker rats, untreated. OZR: obese Zucker rats, untreated for the indicated time periods. Bottom panels: OZR-PGT, OZR treated with pioglitazone for the indicated time periods and doses.
Fig. 4.
Effect of pioglitazone on the expression of the fibrotic factor PAI-1 in the kidney of the Zucker fa/fa rat. Representative pictures of tissue sections immunostained for PAI-1. Magnification: ×400. Top panels: PGT, pioglitazone; LZR, lean Zucker rats, untreated. OZR: obese Zucker rats, untreated for the indicated time periods. Bottom panels: OZR-PGT, OZR treated with pioglitazone for the indicated time periods and doses.
Collagens III and I were evaluated by immunostaining to corroborate the Masson trichrome data. Collagen I (Figure 5) was increased in the untreated OZR as compared to the LZR, and reduced by pioglitazone. Similar results were obtained for collagen III (not shown). The differential expression values of collagens III and I in the OZR when referred to the LZR are 75 and 61%, respectively (Table 1). The collagen III/I ratio was lower in the kidney of the untreated OZR as compared to the LZR, but none of the pioglitazone treatments affected this ratio.
Fig. 5.
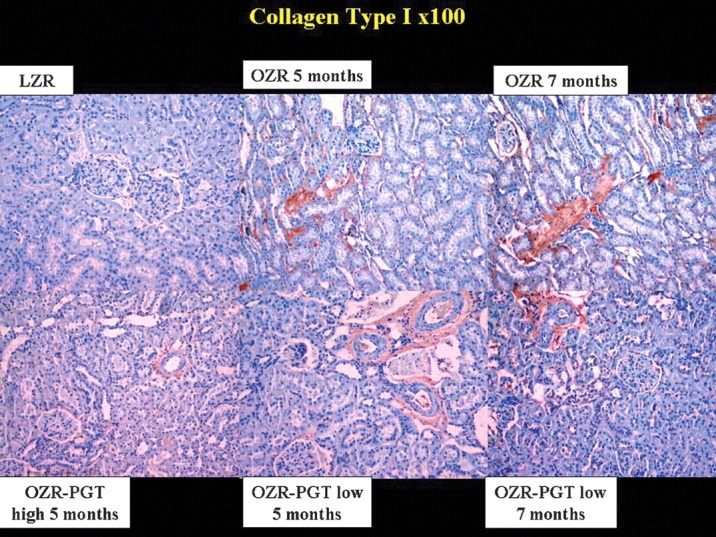
Effect of pioglitazone on collagen I deposition in the kidney of the Zucker fa/fa rat. Representative pictures of tissue sections immunostained for collagen I after antigen retrieval. Magnification: ×100. Top panels: PGT, pioglitazone; LZR, lean Zucker rats, untreated. OZR: obese Zucker rats, untreated for the indicated time periods. Bottom panels: OZR-PGT, OZR treated with pioglitazone for the indicated time periods and doses.
Collagen content in hydrolyzates of kidney tissue of the untreated OZR (3.68 ± 0.17 μg/mg tissue) was reduced by the long-term low-dose pioglitazone OZR (3.31 ± 0.14), or a 12% decrease, probably due to the tubulointerstitial fibrosis affecting a small area of the total kidney tissue, and hence a small fraction of the hydrolyzate. The respective values of band intensities for ASMA corrected by GAPDH in homogenates of kidney tissue estimated by quantitative western blot (not shown) were 0.61 ± 0.08 versus 0.54 ± 0.10, also a 12% reduction by pioglitazone. The contribution of ASMA originated from smooth muscle cells in the kidney vasculature dilutes out the ASMA from myofibroblasts, that in tissue sections can be recognized by their location.
Long-term treatment with low-dose pioglitazone normalizes kidney function in the ZDFR, while correcting the underlying histopathology to the same extent as in the OZR
The striking hyperglycaemia observed in the ZDFR from the beginning of the study nearly doubled the level in the OZR at killing (Figure 6, Table 2). The long-term low-dose pioglitazone treatment reduced glycaemia in the ZDFR, but only to the high values in the untreated OZR or the OZR receiving low-dose pioglitazone; therefore, the rats remained highly hyperglycaemic. This treatment, however, counteracted the proteinuria in the ZDFR and improved creatinine clearance, even after correcting by body weights that were not affected by treatment (Table 2). The systolic and diastolic hypertension that develops after 6–8 weeks in the untreated ZDFR, and that peaks at ∼160 and 95 mmHg, as compared to 125 and 72 mmHg in the LZR, respectively, was nearly normalized by this treatment paradigm (Figure 7).
Fig. 6.
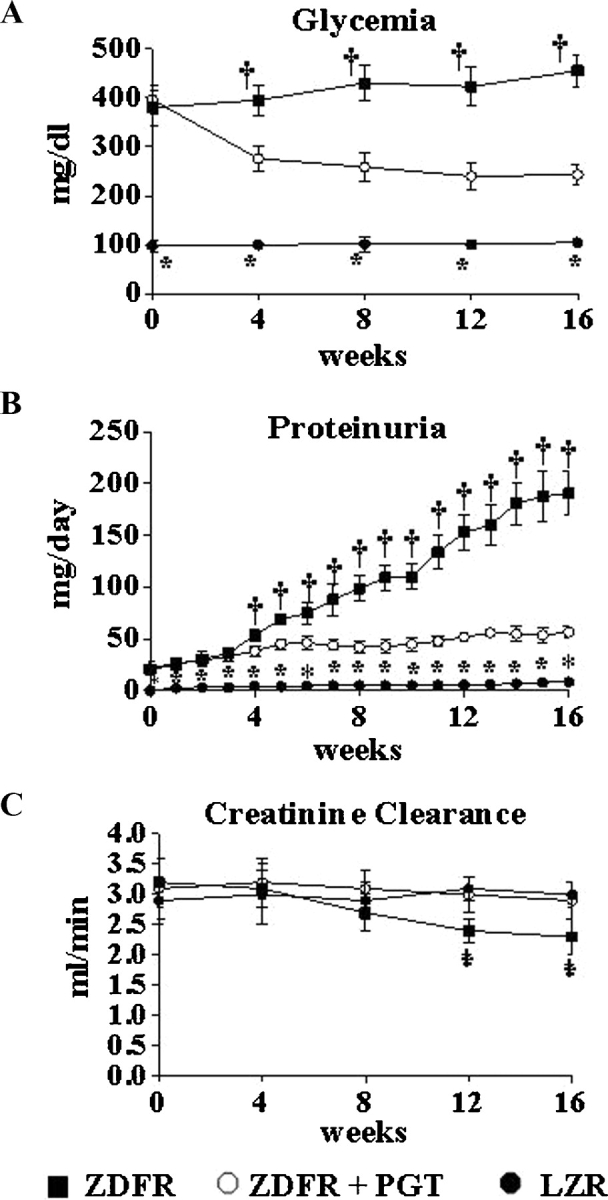
Effect of pioglitazone on blood glucose levels, creatinine clearance and proteinuria, throughout treatment in the ZDF fa/fa rat. Values were obtained at the indicated periods. Solid circles: LZR, untreated; solid squares: ZDFR, untreated; blank circles: ZDFR treated with pioglitazone (low dose, long-term). *P < 0.05 for LZR versus other groups; †P < 0.05 for untreated ZDFR versus other groups.
Fig. 7.
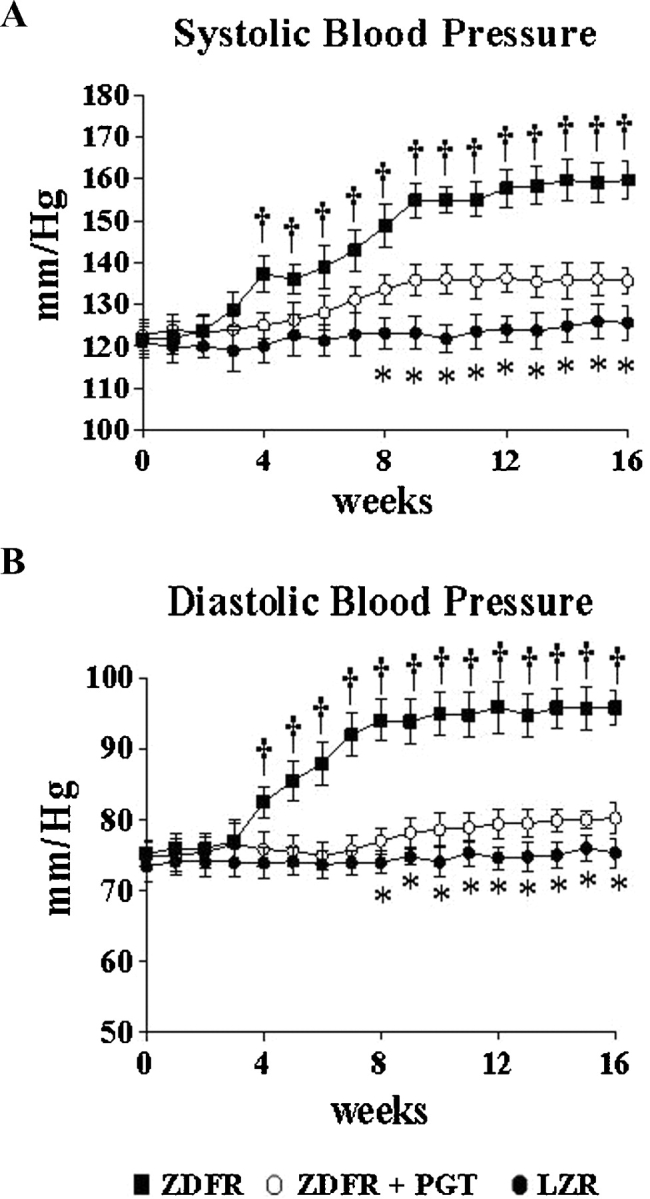
Effect of pioglitazone on blood pressure throughout treatment in the ZDF fa/fa rat. Values were obtained at the indicated periods. Solid circles: LZR, untreated; solid squares: ZDFR, untreated; blank circles: ZDFR treated with pioglitazone (low dose, long-term). *P < 0.05 for LZR versus other groups; †P < 0.05 for untreated ZDFR versus other groups.
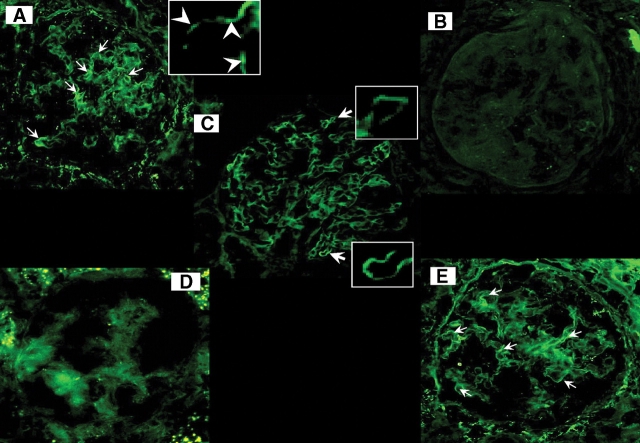
The underlying histopathology and its response to pioglitazone in the ZDFR were similar to the OZR as shown by Masson trichrome staining and ASMA immunostaining, namely an increase in collagen in the diabetic rats and a reduction by the long-term low-dose treatment (not shown). Immunofluorescence detection showed that nephrin was reduced in glomeruli from the untreated ZDFR when compared with the LZR, whereas the ZDFR treated with pioglitazone showed a substantial increase in immunostaining, similar to what was found in the OZR (Figure 8). Quantitative estimations (Table 2) show that the percentages of glomerulosclerosis, tubular atrophy, interstitial fibrosis and myofibroblast content are remarkably similar to the ones for the equivalent groups in the OZR in Table 1. The long-term, low-dose treatment with pioglitazone increased the differential expression of nephrin by over 3-fold in both strains, thus suggesting a protective role of the drug on podocytes.
Fig. 8.
Effect of pioglitazone on nephrin expression in the kidney glomeruli in the obese Zucker and ZDF fa/fa rats. Representative pictures of tissue sections subjected to immuno-fluorescence detection of nephrin with a IgG-FITC-labelled secondary antibody and focusing on the glomeruli (magnification: ×400). (A) LZR, lineal immuno-fluorescence pattern indicated by arrows; amplification denotes irregular lineal distribution of nephrin (arrowheads) on the glomerular capillary loop. (B) Untreated OZR; (C) OZR treated for 4 months with pioglitazone at low dose; (D) untreated ZDFR; (E) ZDFR treated for 4 months with pioglitazone at low dose. LZR: lean Zucker rats, ZDFR: Zucker diabetic fatty rat, PGT: pioglitazone.
The protective effects of pioglitazone on the kidney appear to be mediated, at least in part, by a reduction of oxidative stress and inflammation
The long-term, low-dose treatment with pioglitazone virtually normalized the 11-fold higher expression of haemoxygenase 1, as a secondary indicator of oxidative stress, seen in the OZR kidney as compared to the LZR, by immunohistochemistry (not shown) and quantitative image analysis (Table 1). Identical results were found in the ZDFR, although oxidative stress was less marked in the ZDFR (6-fold) as compared to the OZR (Table 2). The antioxidant effect of pioglitazone was confirmed in the ZDFR by primary markers: the reduction of malonyldialdehyde production and the increase of the GSH/GSSG ratio to levels close to those found in the LZR (Table 2). This treatment also prevented macrophage infiltration, an indicator of inflammation (Table 2).
Discussion
To our knowledge, this is the first report on the prevention or amelioration of diabetic nephropathy, and specifically of glomerulosclerosis and tubulointerstitial fibrosis underlying hypertension and renal dysfunction, by the long-term administration of a low dose of any thiazolidinedione that does not reduce hyperglycaemia below a rather high level, indicative of non-compensated diabetes. These effects appear to result, at least in part, from the anti-oxidative and anti-inflammatory effects that have been described for thiazolidinediones at doses that exert total glycaemic control [23–25]. We are not aware either of previous studies providing an equally comprehensive integration of a series of key markers of podocyte damage, renal fibrosis, inflammation, oxidative stress and dysfunction in a single animal model of type 2 diabetes for any thiazolidinedione. The remarkable agreement among assays for these processes validates the improvement of the renal histopathology exerted by this treatment and suggests a protective effect independent of glycaemic or obesity control. This confirms our previous work, where a similar paradigm prevented or ameliorated smooth muscle fibrosis in the penile corpora cavernosa of the OZR, and the resulting erectile dysfunction [21]. The 0.6 mg/kg/day of pioglitazone used in the current work would roughly translate into ∼6–8 mg daily doses in the human, considering the correction factor used for extrapolating dosages between both species [36] or ∼1/4 of the daily dose usually applied in the clinic [23,29,31,37–40].
The use of the two variants of a widely accepted rat model of type 2 diabetes and metabolic syndrome that differ in hyperglycaemia, insulinaemia and obesity, and that yielded similar results, validates some speculation on clinical implications. Our results may be representative of the differential impact of each condition within the metabolic syndrome in diabetic patients [3], even with the caveat that the pathogenesis of nephropathy in a rat with leptin receptor mutations may not mimic the one in humans. Fundamental questions are why a severe hyperglycaemia in the ZDFR does not lead to a more intense glomerulosclerosis and tubulointerstitial fibrosis than in the OZR with a milder hyperglycaemia, and why a treatment that does not reduce hyperglycaemia below this level can improve these conditions in both diabetic rat variants to the same extent. Obesity does not seem to be a significant factor in this diabetic nephropathy, since the severity of the latter condition was similar in both variants irrespective that the OZR has nearly 80% more body weight than the ZDFR, and that the low dose of pioglitazone did not affect body weight. This may suggest that hyperglycaemia, through advanced glycation end-products (AGE) formation and lipodegeneration of the cortical tissue, plays a lesser role in kidney fibrosis than in the vascular complications of diabetes.
The single report on the preventive renoprotective effects of pioglitazone in the Zucker strains [27], specifically the OZR, is difficult to compare with our study, since it combined pioglitazone at 4-fold our dose with the ARIIR1 blocker candesartan, without a pioglitazone alone arm. Pioglitazone augmented the antihypertensive, antiproteinuric and renal antifibrotic effects of candesartan. A prior study in the ZDFR used another thiazolidenedione, rosiglitazone, at 5-fold our dose, alone or in combination with an ACE inhibitor, and reduced the number of damaged glomeruli and proteinuria [30]. This confirmed previous results with rosiglitazone in the OZR where hypertension, proteinuria, glomerulosclerosis and interstitial nephritis were ameliorated after a 9-month preventive or a 4-month interventional treatment [41]. Renal glomerular vascular changes were also mitigated in a diet-induced diabetes in the mouse that received 5-fold our dose of pioglitazone [28]. However, all these studies were based on doses exerting glycaemic control, and they addressed only a few of the renal histopathology and dysfunction outcomes.
The mechanism of the antifibrotic effects of a low dose of pioglitazone, unable to normalize glycaemia or modify body weight, is unknown. It may be speculated that it acts, at least in part, by counteracting chronic inflammation and oxidative stress as factors that may precede and trigger tissue fibrosis [5,11,14–16,31], and this view is supported by our current results. In vitro, pioglitazone reduces glucose-induced TGFβ1, fibronectin and collagen IV, all fibrotic markers, in human proximal tubular cells [42,43]. The drug also decreased type IV collagen, fibronectin, TIMP-1 and -2 and proline incorporation as marker of collagen synthesis, at both physiological and supra-physiological glucose concentrations in human cortical fibroblasts [44]. The reduction of blood pressure in our study may also have contributed to the renal protection effects of pioglitazone, although the converse process may also occur, e.g. an improvement of blood pressure due to pioglitazone beneficial effects on the kidney. Finally, since thiazolidinediones modulate stem cell differentiation [45], they may act on the kidney also by stimulating renal endogenous stem cells [46,47] to repair damaged tissue.
In contrast to these experimental results, and despite the fact that thiazolidinediones are widely used for type 2 diabetes in doses higher than the one proposed here, the incidence of chronic kidney disease has not diminished [48]. This discrepancy may be due to the limited number of clinical studies on the progression of diabetic nephropathy per se under pioglitazone treatment. An earlier study in normotensive patients with microalbuminuria showed that the excretion of albumin and podocytes in the urine was reduced [37], although no significant effects on proteinuria were seen in patients with advanced diabetic nephropathy [38]. More recently, pioglitazone normalized urinary albumin expression [39,40], and blood pressure, urinary TGFβ and collagen IV were decreased to the extent achieved with ATIIR-1 blockers or ACE inhibitors [39]. Blood pressure was reduced by pioglitazone at the usual dosage (30 mg, daily) in type 2 diabetic patients undergoing haemodialysis [29]. In addition, low-dose pioglitazone may be effective in non-diabetic nephropathy, such as primary or secondary glomerulopathies, chronic tubulointerstitial renal disease or arterial hypertension, where effects on glycaemia are irrelevant.
Acknowledgments
This work was supported by an investigator-initiated grant from Takeda Pharmaceuticals North America, Inc., and (in part) American Diabetes Association grant #7-05-RA-44 ADA, and NIHR01 DK53069 to N.G.C.
Conflict of interest statement. None declared.
References
- 1.Schernthaner G. Kidney disease in diabetology: lessons from 2007. Nephrol Dial Transplant. 2008;23:1112–1115. doi: 10.1093/ndt/gfn060. [DOI] [PubMed] [Google Scholar]
- 2.Biesenbach G. Highest mortality during the last year before and the first year after start of dialysis treatment in type 2 diabetic patients with nephropathy. Curr Diabetes Rev. 2007;3:123–126. doi: 10.2174/157339907780598234. [DOI] [PubMed] [Google Scholar]
- 3.Sarafidis PA. Obesity, insulin resistance and kidney disease risk: insights into the relationship. Curr Opin Nephrol Hypertens. 2008;17:450–456. doi: 10.1097/MNH.0b013e328305b994. [DOI] [PubMed] [Google Scholar]
- 4.Qian Y, Feldman E, Pennathur S, et al. From fibrosis to sclerosis: mechanisms of glomerulosclerosis in diabetic nephropathy. Diabetes. 2008;57:1439–1445. doi: 10.2337/db08-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swaminathan S, Shah SV. Novel approaches targeted toward oxidative stress for the treatment of chronic kidney disease. Curr Opin Nephrol Hypertens. 2008;17:143–148. doi: 10.1097/MNH.0b013e3282f4e539. [DOI] [PubMed] [Google Scholar]
- 6.Simonson MS. Phenotypic transitions and fibrosis in diabetic nephropathy. Kidney Int. 2007;71:846–854. doi: 10.1038/sj.ki.5002180. [DOI] [PubMed] [Google Scholar]
- 7.Lee HB, Ha H. Plasminogen activator inhibitor-1 and diabetic nephropathy. Nephrology (Carlton) 2005;10(Suppl):S11–13. doi: 10.1111/j.1440-1797.2005.00449.x. [DOI] [PubMed] [Google Scholar]
- 8.Clark J, Palmer CJ, Shaw WN, et al. The diabetic Zucker fatty rat. Proc Soc Exp Biol Med. 1983;173:68–75. doi: 10.3181/00379727-173-41611. [DOI] [PubMed] [Google Scholar]
- 9.Peterson RG, Shaw WN, Neel MA, et al. Zucker diabetic fatty as a model for non-insulin dependent diabetes mellitus. ILAR News. 1990;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Qi Y, Kim MS, et al. Increased renal collagen cross-linking and lipid accumulation in nephropathy of Zucker diabetic fatty rats. Diabetes Metab Res Rev. 2008;24:498–506. doi: 10.1002/dmrr.874. [DOI] [PubMed] [Google Scholar]
- 11.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int J Biol Sci. 2006;3:40–46. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez JH, Wu P, Hawes JW, et al. Renal injury: similarities and differences in male and female rats with the metabolic syndrome. Kidney Int. 2006;69:1969–1976. doi: 10.1038/sj.ki.5000406. [DOI] [PubMed] [Google Scholar]
- 13.Hoshi S, Shu Y, Yoshida F, et al. Podocyte injury promotes progressive nephropathy in zucker diabetic fatty rats. Lab Invest. 2002;82:25–35. doi: 10.1038/labinvest.3780392. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez J, Wu P, Packer CS, et al. Lipotoxic and inflammatory phenotypes in rats with uncontrolled metabolic syndrome and nephropathy. Am J Physiol Renal Physiol. 2007;293:F670–F679. doi: 10.1152/ajprenal.00021.2007. [DOI] [PubMed] [Google Scholar]
- 15.Poirier B, Lannaud-Bournoville M, Conti M, et al. Oxidative stress occurs in absence of hyperglycaemia and inflammation in the onset of kidney lesions in normotensive obese rats. Nephrol Dial Transplant. 2000;15:467–476. doi: 10.1093/ndt/15.4.467. [DOI] [PubMed] [Google Scholar]
- 16.Lavaud S, Poirier B, Mandet C, et al. Inflammation is probably not a prerequisite for renal interstitial fibrosis in normoglycemic obese rats. Am J Physiol Renal Physiol. 2001;280:F683–F694. doi: 10.1152/ajprenal.2001.280.4.F683. [DOI] [PubMed] [Google Scholar]
- 17.Toblli JE, DeRosa G, Cao G, et al. ACE inhibitor and angiotensin type I receptor antagonist in combination reduce renal damage in obese Zucker rats. Kidney Int. 2004;65:2343–2359. doi: 10.1111/j.1523-1755.2004.00661.x. [DOI] [PubMed] [Google Scholar]
- 18.Toblli JE, Muñoz MC, Cao G, et al. ACE inhibition and AT1 receptor blockade prevent fatty liver and fibrosis in obese Zucker rats. Obesity (Silver Spring) 2008;16:770–776. doi: 10.1038/oby.2007.114. [DOI] [PubMed] [Google Scholar]
- 19.Toblli JE, Cao G, Rivas C, et al. Angiotensin-converting enzyme inhibition reduces lipid deposits in myocardium and improves left ventricular function of obese zucker rats. Obesity (Silver Spring) 2006;14:1586–1595. doi: 10.1038/oby.2006.183. [DOI] [PubMed] [Google Scholar]
- 20.Toblli JE, Cao G, DeRosa G, et al. Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor beta1 in obese Zucker rats by perindopril. Heart. 2005;91:80–86. doi: 10.1136/hrt.2003.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kovanecz I, Ferrini MG, Vernet D, et al. Pioglitazone prevents corporal veno-occlusive dysfunction (CVOD) in a rat model of type 2 diabetes mellitus. BJU Int. 2006;98:116–124. doi: 10.1111/j.1464-410X.2006.06268.x. [DOI] [PubMed] [Google Scholar]
- 22.Kovanecz I, Nolazco G, Ferrini MG, et al. Early onset of fibrosis within the arterial media in a rat model of type 2 diabetes mellitus exhibiting erectile dysfunction. BJU Int. 2009 doi: 10.1111/j.1464-410X.2008.08251.x. Jan 9 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Buckingham RE. Thiazolidinediones: pleiotropic drugs with potent anti-inflammatory properties for tissue protection. Hepatol Res. 2005;33:167–170. doi: 10.1016/j.hepres.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Panchapakesan U, Chen XM, Pollock CA. Drug insight: thiazolidinediones and diabetic nephropathy—relevance to renoprotection. Nat Clin Pract Nephrol. 2005;1:33–43. doi: 10.1038/ncpneph0029. [DOI] [PubMed] [Google Scholar]
- 25.Becker J, Delayre-Orthez C, Frossard N, et al. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol. 2006;20:429–447. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 26.Kovanecz I, Ferrini MG, Davila HH, et al. Pioglitazone ameliorates penile corpora veno-occlusive dysfunction (CVOD) in the aged rat. BJU Int. 2007;100:867–874. doi: 10.1111/j.1464-410X.2007.07070.x. [DOI] [PubMed] [Google Scholar]
- 27.Namikoshi T, Tomita N, Satoh M, et al. Pioglitazone enhances the antihypertensive and renoprotective effects of candesartan in Zucker obese rats fed a high-protein diet. Hypertens Res. 2008;31:745–1755. doi: 10.1291/hypres.31.745. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez WE, Tyagi N, Joshua IG, et al. Pioglitazone mitigates renal glomerular vascular changes in high-fat, high-calorie-induced type 2 diabetes mellitus. Am J Physiol Renal Physiol. 2006;291:F694–F701. doi: 10.1152/ajprenal.00398.2005. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy KJ, Routh RE, Shaw W, et al. Troglitazone halts diabetic glomerulosclerosis by blockade of mesangial expansion. Kidney Int. 2000;58:2341–2350. doi: 10.1046/j.1523-1755.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- 30.Buckingham RE, Al-Barazanji KA, Toseland CD, et al. Peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, protects against nephropathy and pancreatic islet abnormalities in Zucker fatty rats. Diabetes. 1998;47:1326–1334. doi: 10.2337/diab.47.8.1326. [DOI] [PubMed] [Google Scholar]
- 31.Rizos CV, Liberopoulos EN, Mikhailidis DP, et al. Pleiotropic effects of thiazolidinediones. Expert Opin Pharmacother. 2008;9:1087–1108. doi: 10.1517/14656566.9.7.1087. [DOI] [PubMed] [Google Scholar]
- 32.Abe M, Okada K, Kikuchi F, et al. Clinical investigation of the effects of pioglitazone on the improvement of insulin resistance and blood pressure in type 2-diabetic patients undergoing hemodialysis. Clin Nephrol. 2008;70:220–228. doi: 10.5414/cnp70220. [DOI] [PubMed] [Google Scholar]
- 33.Ferrini MG, Davila HH, Valente EG, et al. Aging-related induction of inducible nitric oxide synthase is vasculo-protective to the arterial media. Cardiovasc Res. 2004;61:796–805. doi: 10.1016/j.cardiores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Ferrini MG, Kovanecz I, Sanchez S, et al. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–428. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi R, Cardaioli E, Scaloni A, et al. Thiol groups in proteins as endogenous reductants to determine glutathione-protein mixed disulphide in biological systems. Biochim Biophys Acta. 1995;1243:230–238. doi: 10.1016/0304-4165(94)00133-i. [DOI] [PubMed] [Google Scholar]
- 36.Valente EG, Vernet D, Ferrini MG, et al. l-arginine and PDE inhibitors counteract fibrosis in Peyronie's plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–244. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Ushiyama C, Osada S, et al. Pioglitazone reduces urinary podocyte excretion in type 2 diabetes patients with microalbuminuria. Metabolism. 2001;50:1193–1196. doi: 10.1053/meta.2001.26703. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Saha C, Battiwala M, et al. A pilot randomized controlled trial of renal protection with pioglitazone in diabetic nephropathy. Kidney Int. 2005;68:285–292. doi: 10.1111/j.1523-1755.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 39.Katavetin P, Eiam-Ong S, Suwanwalaikorn S. Pioglitazone reduces urinary protein and urinary transforming growth factor-beta excretion in patients with type 2 diabetes and overt nephropathy. J Med Assoc Thai. 2006;89:170–177. [PubMed] [Google Scholar]
- 40.Nakamura T, Sugaya T, Kawagoe Y, et al. Effect of pioglitazone on urinary liver-type fatty acid-binding protein concentrations in diabetes patients with micro-albuminuria. Diabetes Metab Res Rev. 2006;22:385–389. doi: 10.1002/dmrr.633. [DOI] [PubMed] [Google Scholar]
- 41.Baylis C, Atzpodien EA, Freshour G, et al. Peroxisome proliferator-activated receptor [gamma] agonist provides superior renal protection versus angiotensin-converting enzyme inhibition in a rat model of type 2 diabetes with obesity. J Pharmacol Exp Ther. 2003;307:854–860. doi: 10.1124/jpet.103.055616. [DOI] [PubMed] [Google Scholar]
- 42.Panchapakesan U, Sumual S, Pollock CA, et al. PPARgamma agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol. 2005;289:F1153–F1158. doi: 10.1152/ajprenal.00097.2005. [DOI] [PubMed] [Google Scholar]
- 43.Zafiriou S, Stanners SR, Polhill TS, et al. Pioglitazone increases renal tubular cell albumin uptake but limits proinflammatory and fibrotic responses. Kidney Int. 2004;65:1647–1653. doi: 10.1111/j.1523-1755.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 44.Zafiriou S, Stanners SR, Saad S, et al. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16:638–645. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S, Rosenberg ME. Do stem cells exist in the adult kidney? Am J Nephrol. 2008;28:607–613. doi: 10.1159/000117311. [DOI] [PubMed] [Google Scholar]
- 46.Werner C, Kamani CH, Gensch C, et al. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 47.Wada K, Nakajima A, Katayama K, et al. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J Biol Chem. 2006;281:12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- 48.Glassock RJ, Winearls C. The global burden of chronic kidney disease: how valid are the estimates? Nephron Clin Pract. 2008;110:c39–c47. doi: 10.1159/000151244. [DOI] [PubMed] [Google Scholar]