Abstract
Background. Short-term studies have demonstrated that rapamycin or everolimus treatment decreases cyst formation and improves renal function in animal models of polycystic kidney disease (PKD). Autosomal dominant polycystic kidney disease (ADPKD) patients would likely require life-long treatment with rapamycin.
Methods. Male Han:SPRD rats with PKD (Cy/+) were treated with rapamycin (0.2 mg/kg/day IP) or vehicle from 1 to 12 months of age. Mean trough levels of rapamycin (ng/mL) were 6.6 ± 0.1 at 8 weeks of age. Twelve-month-old littermates (+/+) were used as normal controls.
Results. Twelve-month-old male Cy/+ rats treated with the vehicle had a more than doubling of kidney volume, severe chronic renal failure, severe hypertension and increased heart weight compared to normal littermate controls (+/+). After rapamycin treatment, 12-month-old Cy/+ rats had markedly improved kidney volume, renal function, blood pressure and heart weight not statistically different from controls. Rapamycin reduced the cyst volume density (CVD) by 72%. Mammalian target of rapamycin (mTOR) activation in the heart, as evidenced by a marked increase in the phospho-S6 protein that was inhibited by rapamycin, was demonstrated in 12-month-old Cy/+ rats.
Conclusion. In conclusion, long-term rapamycin treatment in Cy/+ rats results in a normalization of kidney volume, renal function, blood pressure and heart weight. The novel finding that rapamycin decreases hypertension, heart enlargement and mTOR signalling in the heart in PKD rats is reported. The only side effect of rapamycin treatment was an 11% decrease in body weight.
Keywords: chronic kidney disease, heart, mTOR, polycystic kidney, rapamycin
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common life threatening hereditary disease in the USA. ADPKD accounts for ∼5–10% of end-stage renal failure in the USA requiring dialysis and renal transplantation [1]. While there is no effective treatment for ADPKD, numerous agents e.g. tolvaptan, rapamycin, everolimus, statins, octreotide are currently being tested in clinical trials.
There have been four short-term studies of mammalian target of rapamycin (mTOR) inhibitors in animal models of PKD. In Han:SPRD rats, studies demonstrate that rapamycin or everolimus delays the loss of function and retards cyst development [2,3,4]. In the bpk and orpk rescue mouse models of PKD, rapamycin reduces renal cystogenesis [5].
Studies of mTOR inhibitors in humans with PKD have been initiated. Adults and children with ADPKD will likely require long-term or even life-long therapy with mTOR inhibitors. The effect of long-term rapamycin treatment in animal models of PKD is not known. The effect of rapamycin on blood pressure and heart weight has not previously been studied. In the present study, we treated Han:SPRD rats with rapamycin for 11 months and evaluated side effects, cyst growth, renal function, blood pressure and cardiac size.
Methods
Han:SPRD rats
The study was conducted in heterozygous (Cy/+) and normal littermate control (+/+) Han:SPRD rats. All the normal rats and Cy/+ rats studied were males. A colony of Han:SPRD rats was established in our animal care facility from a litter that was obtained from the Polycystic Kidney Program at the University of Kansas Medical Center. The study protocol was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. The rats had free access to tap water and standard rat chow.
Experimental protocol
Male Cy/+ and +/+ rats were weaned at 3 weeks of age and then treated with rapamycin 0.2 mg/kg/day IP or vehicle (10% ethanol in normal saline) on weekdays from 4 to 52 weeks of age. Rapamycin was obtained from LC Laboratories, Woburn, MA, USA, and a 1 mg/mL stock solution in 100% ethanol was kept at 4°C.
In separate studies, rapamycin levels were drawn on a weekday, just before the next dose of rapamycin. Mean trough levels of rapamycin (ng/mL) were 6.6 ± 0.1 at 8 weeks of age (n = 3) and 6.9 ± 1.2 at 12 weeks of age (n = 3).
At 52 weeks of age, the rats were anaesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body weight). Mean arterial pressure (MAP) was measured via a femoral artery catheter connected to a Transpac IV transducer and monitored continuously using the Windaq Waveform recording software (Dataq instruments).
Cyst volume density (CVD)
Haematoxylin–eosin-stained sections were used to determine the cyst volume density. This was performed by a reviewer, blinded to the identity of the treatment modality, using standard point counting stereology [6]. Briefly, a kidney was viewed using a Nikon Eclipse E400 microscope equipped with a digital camera connected to the Spot Advanced imaging software (version 3.5). Areas of the cortex at 90°, 180° and 270° from the hilum of each section were selected to guard against field selection variation. Kidney areas were viewed under a grid containing 100 crossover points. The number of crossover points in a cyst versus noncystic kidney was quantified. The CVD represents the percentage of kidney cortex that is cystic.
Chemistry
Blood urea nitrogen (BUN) and serum creatinine were measured using quantitative colorimetric urea determination (QuantiChrom™ urea assay kit-DIUR-500) (Bioassay Systems, Hayward, CA, USA) and quantitative colorimetric creatinine determination (QuantiChrom™ creatinine assay kit-DICT-500). Rapamycin levels were measured using liquid chromatography/mass spectrometry by the Clinical laboratory at University Hospital.
Immunoblotting
Immunoblot analysis was performed as we have previously described [7]. Renal cortex was homogenized in a lysis buffer (in mM: 5 Na2HPO4, 5 NaH2PO4, 150 NaCl, 1 EDTA, 0.1% Triton X-100, 50 NaF, and 0.2 Na3VO4, and 0.1% β-mercaptoethanol, pH 7.2) plus proteinase inhibitors: 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 15 μM pepstatin A, 14 μM l-trans-epoxysuccinyl-leucylamide-(4-guanido)-butane (E-64), 40 μM bestatin, 22 μM leupeptin, 0.8 μM aprotonin. Hearts were powdered in liquid nitrogen and then homogenized in the lysis buffer as described above. The homogenates were centrifuged (10 000 ×g at 4°C for 10 min) to remove unbroken cells and debris. Supernatants were mixed with a sample buffer containing 50 mM Tris-base (pH 6.8), 0.5% glycerol, 0.01% bromphenol blue and 0.75% sodium dodecyl sulphate (SDS) and heated at 95°C for 5 min. Equal amounts of protein (60 μg/lane) were fractionated by Tris-glycine-SDS-12.5% PAGE. The electrophoretically separated proteins were then transferred to a nitrocellulose membrane (Millipore, Bedford, MA, USA) by wet electroblotting. The membranes were blocked with 5% nonfat dry milk in TBST [50 mM Tris (pH 7.5), 150 mM NaCl and 0.1% Tween buffer at pH 7.5 overnight at 4°C. Immunoblot analyses were performed with the following antibodies: (1) a phosho-S6 ribosomal protein (Ser235/236) antibody (Cell Signalling Technology, Beverly, MA, USA; catalogue number 2211) that detects the ribosomal S6 protein only when phosphorylated at serine 235 and 236. The main in vivo S6 ribosomal protein phosphorylation sites for p70S6 kinase are Ser235/236 and Ser240/244 [8,9]. The phosho-S6 ribosomal protein is recognized as a 32 kDa protein. (2) A S6 ribosomal protein (5G10) antibody (Cell Signaling Technology; Catalogue number 2217) that detects levels of total S6 protein independent of phosphorylation: the total S6 ribosomal protein is recognized as a 32 kDa protein. The membranes were incubated with primary antibodies for 1 h at room temperature, washed in a TBST buffer and further incubated with donkey anti-rabbit IgG coupled to horseradish peroxidase (Amersham) at 1:1000 dilution in the TBST buffer for 1 h at room temperature. Subsequent detection was carried out by enhanced chemiluminescence (Amersham), according to the manufacturer's instructions. Prestained protein markers (Biorad) were used for molecular mass determination. Chemiluminescence was recorded with a film, and results were analysed with the 1D Image Software (Kodak Digital Science).
Statistical analysis
Multiple group comparisons were performed using analysis of variance (ANOVA) with posttest according to the Newman–Keuls procedure. A P-value of <0.05 was considered statistically significant. The values were expressed as means ± SE.
Results
A total of 28 rats were studied. Four of the Cy/+ rats died. Of the remaining 24 rats, 4 +/+ rats received vehicle, 8 Cy/+ rats received vehicle and 12 Cy/+ rats received rapamycin.
Two rats out of a total of eight Cy/+ vehicle-treated rats died. Two Cy/+ rats out of a total of 12 rats treated with rapamycin died. The rats died at 10–11 months into the study. These rats were not noted to be sick prior to death. The cause of death could not be determined on examination of the dead rats.
Rapamycin-treated Cy/+ rats had an 11% decrease in body mass compared to vehicle-treated Cy/+ (Table 1). Despite the loss in body weight, all the rats appeared healthy during the study.
Table 1.
Effect of rapamycin in 52-week-old Han:SPRD (Cy/+) rats
| +/+ Vehicle | Cy/+ Vehicle | Cy/+ Rapamycin | |
|---|---|---|---|
| (n = 4) | (n = 8) | (n = 12) | |
| Body weight (g) | 494 ± 10 | 467 ± 15 | 415 ± 9+ |
| 2K/TBW (%) | 0.6 ± 0.004 | 1.4 ± 0.08*** | 0.6 ± 0.02### |
| Cyst volume density | 1 ± 0.5 | 46 ± 7** | 13 ± 1.6## |
| (CVD) (%) | |||
| BUN (mg/dL) | 19 ± 3 | 176 ± 24*** | 39 ± 2### |
| SCr (mg/dL) | 0.6 ± 0.1 | 3.2 ± 1.5** | 0.7 ± 0.6## |
| MAP (mmHg) | 81 ± 11 | 116 ± 6* | 96 ± 4# |
| Heart weight (g) | 1.6 ± 0.1 | 2.3 ± 0.2* | 1.7 ± 0.8## |
*P < 0.05 versus +/+; **P < 0.01 versus +/+; ***P < 0.001 versus +/+.
#P < 0.05 versus Cy/+, NS versus +/+; ##P < 0.01 versus Cy/+, NS versus +/+; ###P < 0.001 versus Cy/+, NS versus +/+.
+P < 0.01 versus Cy/+ and +/+.
The two-kidney/total body weight ratio (2K/TBW) was determined to correct for the lower body mass caused by rapamycin. Cy/+ rats had a more than doubling of the kidney size compared to +/+ controls. Rapamycin reduced the kidney enlargement by 100% (Table 1). Rapamycin reduced the CVD by 72% (Table 1). Rapamycin completely prevented the increase in BUN and serum creatinine levels in the Cy/+ rats (Table 1). Cy/+ rats treated with vehicle, which had massive renal cyst enlargement, had an increased MAP and heart weight compared to Cy/+ rats treated with rapamycin. MAP and heart weight were significantly decreased by rapamycin. MAP and heart weight were not statistically different between rapamycin-treated Cy/+ kidneys and +/+ kidneys (Table 1).
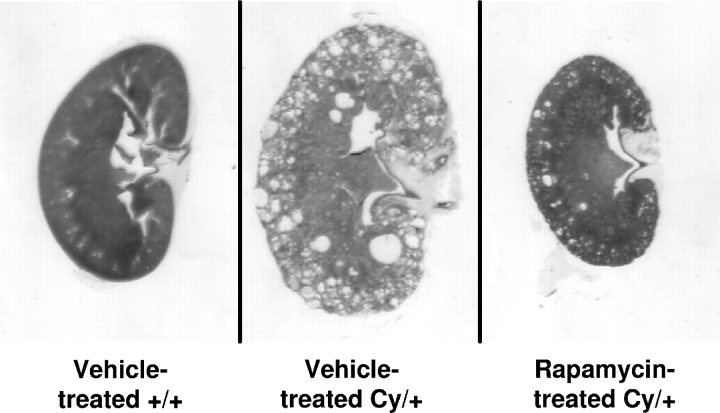
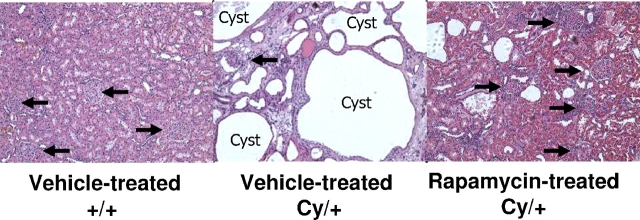
Representative kidney sections of vehicle-treated +/+, vehicle-treated Cy/+ and rapamycin-treated Cy/+ rats, at the same magnification are demonstrated in Figure 1. Representative pictures of the kidney histology are shown in Figure 2.
Fig. 1.
Effect of rapamycin on PKD in 52-week-old Cy/+ rats: representative kidney sections at the same magnification of vehicle-treated normal littermate controls (+/+), vehicle-treated Cy/+ and rapamycin-treated Cy/+ rats. Vehicle-treated Cy/+ kidneys were massively cystic. Rapamycin reduced kidney enlargement as assessed by the 2K/TBW ratio by 100% as demonstrated in rapamycin-treated Cy/+ kidneys. Rapamycin-treated Cy/+ rats kidneys were not different in size from vehicle-treated normal littermate control kidneys.
Fig. 2.
Effect of rapamycin on kidney histology of 52-week-old Cy/+ rats: no cysts are seen in vehicle-treated normal littermate control (+/+) kidneys. Multiple large cysts (C) are seen in vehicle-treated Cy/+ kidneys. Glomeruli (arrows) are sparse and abnormal in vehicle-treated Cy/+ kidneys compared to a normal number of glomeruli in rapamycin-treated Cy/+ kidneys. A few small scattered cysts are seen in rapamycin-treated Cy/+ kidneys. Rapamycin reduced the cyst volume density by 72%.
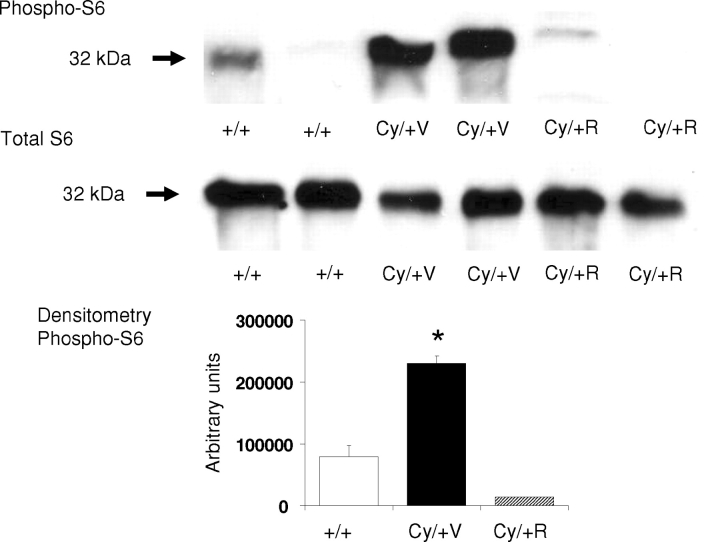
On immunoblotting, there was an increase in the phospho-S6 ribosomal protein in vehicle-treated Cy/+ hearts compared to hearts from normal littermate controls (+/+). The increase in the phospho-S6 ribosomal protein in Cy/+ hearts was inhibited by rapamycin. The total S6 ribosomal protein, used as a control, was not different in vehicle-treated controls (+/+), vehicle-treated Cy/+ and rapamycin-treated Cy/+ hearts. Densitometry analysis demonstrated a significant increase in the phospho-S6 protein in Cy/+ hearts that was inhibited by rapamycin. Representative immunoblots and densitometry of four separate experiments are demonstrated in Figure 3.
Fig. 3.
Increase in the phospho-S6 protein in hearts from 52-week-old (Cy/+) rats. On immunoblotting, there was an increase in the phospho-S6 ribosomal protein in vehicle-treated Cy/+ hearts (Cy/+V) compared to normal littermate control hearts (+/+). The increase in the phospho-S6 ribosomal protein in Cy/+V hearts was inhibited by rapamycin as demonstrated in rapamycin-treated Cy/+ hearts (Cy/+R). Total S6 ribosomal protein, used as a control, was not different in +/+, Cy/+V and Cy/+R hearts. Densitometry analysis demonstrated a significant increase in the phospho-S6 protein in Cy/+V hearts compared to +/+ and Cy/+R kidneys. *P < 0.001 versus +/+ and Cy/R hearts. Representative immunoblots and densitometry of four separate experiments.
Discussion
Abnormal proliferation in tubular epithelial cells plays a crucial role in cyst formation and/or growth in PKD [10–12]. There is also experimental evidence for activation of the mTOR signalling pathway in the kidney in PKD [5]. Activation of the mTOR signalling pathway results in increased proliferation and decreased apoptosis [13]. Rapamycin is a Federal Drug Administration (FDA)-approved immunosuppressive drug and is a powerful anti-proliferative drug via inhibition of mTOR signalling [13]. Thus, there is sound rationale for the use of mTOR inhibitors, which are potent anti-proliferative agents, in PKD.
There have been four short-term studies of mTOR inhibitors in animal models of PKD. Rapamycin (0.2 mg/ kg/day) for 5 weeks from 4 to 8 weeks of age slows disease progression in Han:SPRD rats [2]. Rapamycin (2 mg/kg/day) orally for 3 months delays the loss of function and retards cyst development in the Han:SPRD rat [3]. Everolimus (3 mg/kg/day) by mouth for 5 weeks inhibits cystogenesis in Han:SPRD rats [4]. In two independent mouse models of PKD, the bpk and orpk rescue mice, daily i.p. injections of 1.67 or 5 mg/kg rapamycin for 28 or 14 days, respectively, reduced renal cystogenesis [5]. Our study establishes the long-term efficacy of rapamycin in the treatment of PKD.
Adults and children with ADPKD will likely require long-term or even life-long therapy. The effect of long-term rapamycin treatment in animal models of PKD is not known. In the present study, we treated Han:SPRD rats with rapamycin from 1 to 12 months of age and evaluated side effects, cyst growth, renal function, blood pressure and cardiac size. The only side effect of treatment was weight loss. The weight loss of 11% in the present study of long-term treatment was less than the 22% we previously reported with short-term treatment [2]. Food intake was monitored in vehicle- and rapamycin-treated rats. The weight loss occurred without any apparent decrease in food intake.
Side effects of rapamycin in humans include (in order of frequency) hyperlipidaemia, hypertension, diarrhoea, anaemia, nausea or vomiting, thrombocytopenia, skin rash, stomatitis and malignancies (lymphoma and skin cancers). Serum lipids, haemoglobin and platelets were not measured in the present study. Both the vehicle- and rapamycin-treated rats were carefully examined during the study, and at sacrifice they did not have diarrhoea, skin rash, stomatits or malignancies.
Survival was not an end-point of the study. However, the rapamycin-treated PKD rats were very much healthier than the vehicle-treated rats at 1 year of age. The vehicle-treated PKD rats at 1 year of age had hypertension, chronic kidney disease (CKD) (mean BUN of 176 mg/dL and serum creatinine of 3.2 mg/dL) and looked extremely sick as judged by much decreased activity and abdominal swelling, which on sacrifice revealed ascites. The rapamycin-treated PKD rats did not show signs of any side effects of treatment, did not have CKD and were much healthier than the vehicle-treated PKD rats at 1 year of age.
Increasing cyst enlargement in PKD is known to cause hypertension mediated by the renin–angiotensin–aldosterone system [14]. Hypertension occurs early in the course of PKD and is an important factor in the mortality and morbidity of PKD [15]. Treatment of the hypertension with enalapril in patients with PKD has been shown to decrease left ventricular hypertrophy [16]. Hypertension is a feature of PKD in Han:SPRD rats [17,18]. The present study demonstrates for the first time a significant decrease in hypertension and heart enlargement due to rapamycin treatment in PKD. The decrease in hypertension may be related to the decrease in cyst volume as increased cyst volume has been associated with hypertension in PKD [19]. The decrease in heart enlargement is likely due to the decrease in blood pressure.
Increased mTOR signalling has been described in the heart due to certain hypertrophic agents [20,21], and inhibition of mTOR signalling with rapamycin reduces the myocyte cell size and attenuates load-induced cardiac hypertrophy in mice [20]. p70S6 Kinase (p70S6K) is the best characterized downstream target of mTOR. p70S6K is phosphorylated by mTOR. Phospho-p70S6K in turn phosphorylates the S6 ribosomal protein. An increase in the phospho-S6 protein that is inhibited by rapamycin is a measure of increased mTOR signalling. [13]. Thus, we measured the level of the phospho-S6 protein as an index of mTOR signalling in the heart. We describe the novel finding of the increased phospho-S6 protein in the heart in PKD that is inhibited by rapamycin, indicative of increased mTOR signalling. In kidney transplant patients, rapamycin has been shown to decrease left ventricular hypertrophy, independent of blood pressure [22]. Thus, rapamycin may reduce left ventricular hypertrophy independent of blood pressure reduction in PKD rats. A future study of the effect of hypertension on mTOR signalling in the heart of PKD rats is an exciting prospect.
The male Cy/+ Han:SPRD rat develops slowly progressive renal cystic disease with interstitial fibrosis and azotaemia seen by 6 months of age and dies at approximately 17 months of age from end-stage kidney disease [23,24]. The present study demonstrates that rapamycin treatment prevented the onset of CKD as judged by BUN and serum creatinine levels that were not different from wild-type rats.
In summary, long-term treatment with rapamycin in PKD results in a marked decrease in CKD, hypertension and heart enlargement with minimal side effects. The novel finding that rapamycin decreases hypertension, heart enlargement and mTOR signalling in the heart in PKD rats is reported. Our study supports the feasibility of long-term rapamycin treatment in PKD.
Acknowledgments
This work was supported by NIH grant DK074835 to C.L.E. and a bridging grant from the PKD Foundation.
Conflict of interest statement. None declared.
References
- 1.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 2.Tao Y, Kim J, Schrier RW, et al. Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease (PKD) J Am Soc Nephrol. 2005;16:46–51. doi: 10.1681/ASN.2004080660. [DOI] [PubMed] [Google Scholar]
- 3.Wahl PR, Serra AL, Le Hir M, et al. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD) Nephrol Dial Transplant. 2006;21:598–604. doi: 10.1093/ndt/gfi181. [DOI] [PubMed] [Google Scholar]
- 4.Wu M, Wahl PR, Le Hir M, et al. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney Blood Press Res. 2007;30:253–259. doi: 10.1159/000104818. [DOI] [PubMed] [Google Scholar]
- 5.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowley BD, Jr, Rupp JC, Muessel MJ, et al. Gender and the effect of gonadal hormones on the progression of inherited polycystic kidney disease in rats. Am J Kidney Dis. 1997;29:265–272. doi: 10.1016/s0272-6386(97)90039-1. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y, Melnikov VY, Schrier RW, et al. Downregulation of the calpain inhibitor protein calpastatin by caspases during renal ischemia-reperfusion. Am J Physiol Renal Physiol. 2000;279:F509–F517. doi: 10.1152/ajprenal.2000.279.3.F509. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari S, Bandi HR, Hofsteenge J, et al. Mitogen-activated 70K S6 kinase. Identification of in vitro 40 S ribosomal S6 phosphorylation sites. J Biol Chem. 1991;266:22770–22775. [PubMed] [Google Scholar]
- 9.Flotow H, Thomas G. Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J Biol Chem. 1992;267:3074–3078. [PubMed] [Google Scholar]
- 10.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 11.Trudel M, D’Agati V, Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 12.Schaffner DL, Barrios R, Massey C, et al. Targeting of the rasT24 oncogene to the proximal convoluted tubules in transgenic mice results in hyperplasia and polycystic kidneys. Am J Pathol. 1993;142:1051–1060. [PMC free article] [PubMed] [Google Scholar]
- 13.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 14.Chapman AB, Johnson A, Gabow PA, et al. The renin—angiotensin–aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 15.Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. [Review] [65 refs] J Am Soc Nephrol. 2001;12:194–200. doi: 10.1681/ASN.V121194. [DOI] [PubMed] [Google Scholar]
- 16.Ecder T, Edelstein CL, Chapman AB, et al. Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 1999;14:1113–1116. doi: 10.1093/ndt/14.5.1113. [DOI] [PubMed] [Google Scholar]
- 17.Kennefick TM, Al-Nimri MA, Oyama TT, et al. Hypertension and renal injury in experimental polycystic kidney disease. Kidney Int. 1999;56:2181–2190. doi: 10.1046/j.1523-1755.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 18.Zafar I, Tao Y, Falk S, et al. Effect of statin and angiotensin-converting enzyme inhibition on structural and hemodynamic alterations in autosomal dominant polycystic kidney disease model. Am J Physiol Renal Physiol. 2007;293:F854–F859. doi: 10.1152/ajprenal.00059.2007. [DOI] [PubMed] [Google Scholar]
- 19.Fick-Brosnahan GM, Belz MM, McFann KK, et al. Relationship between renal volume growth and renal function in autosomal dominant polycystic kidney disease: a longitudinal study. Am J Kidney Dis. 2002;39:1127–1134. doi: 10.1053/ajkd.2002.33379. [DOI] [PubMed] [Google Scholar]
- 20.Shioi T, McMullen JR, Tarnavski O, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 21.Proud CG. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63:403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Paoletti E, Amidone M, Cassottana P, et al. Effect of sirolimus on left ventricular hypertrophy in kidney transplant recipients: a 1-year nonrandomized controlled trial. Am J Kidney Dis. 2008;52:324–330. doi: 10.1053/j.ajkd.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 23.Cowley BD, Jr, Gudapaty S, Kraybill AL, et al. Autosomal-dominant polycystic kidney disease in the rat. Kidney Int. 1993;43:522–534. doi: 10.1038/ki.1993.79. [DOI] [PubMed] [Google Scholar]
- 24.Schafer K, Gretz N, Bader M, et al. Characterization of the Han:SPRD rat model for hereditary polycystic kidney disease. Kidney Int. 1994;46:134–152. doi: 10.1038/ki.1994.253. [DOI] [PubMed] [Google Scholar]