Abstract
Background. Cross-sectional analyses of kidney function and physical function have identified profound quality of life impairments in people with advanced kidney dysfunction. No data are currently available, however, on how kidney function decline may be associated with physical function.
Methods. We undertook a study of kidney function decline and physical function in 2544 women participating in the Nurses’ Health Study. Glomerular filtration rates (GFR) were estimated using the four-variable MDRD equation from plasma creatinine measured in blood collected in 1989 and 2000. Physical function was assessed by the Physical Function Sub-Scale (PFS) score of the Short Form 36 (SF-36) in a questionnaire administered in the year 2000. PFS scores have been shown to correlate well with direct measures of physical function.
Results. In the year 2000, the median age was 67 years, median body mass index (BMI) was 25.6 kg/m2, 48.5% had hypertension and 5.8% had diabetes. There were 427 women (16.8%) who experienced an ≥25% decline in eGFR between 1989 and 2000. Median PFS in 2000 for those with an eGFR decline of ≥25% was 80 compared to a PFS score of 85 for those without (P < 0.001). In fully adjusted models, the presence of an eGFR decline of ≥25% was independently associated with a 3.5-point lower PFS score (95% CI −5.4 to −1.5). Also, an eGFR decline of ≥25% was independently associated with an increased odds ratio of being in the lowest quartile of PFS score (OR 1.37; 95% CI 1.04–1.81).
Conclusions. We conclude that an eGFR decline of ≥25% over 11 years is independently associated with lower physical function in women.
Keywords: kidney function, eGFR (estimated glomerular function rate), physical function, PFS of SF-36 (physical function subscale of Short Form 36)
Introduction
Chronic kidney disease (CKD) is increasing in epidemic proportions. An estimated 11% of the total adult population in the United States has CKD as defined as persistent albuminuria or decreased glomerular filitration rate (GFR) [1]. Approximately 6% of this population has an estimated glomerular filtration rate (eGFR) ≤60 ml/min/1.73 m2, which is widely considered to represent moderate or worse kidney dysfunction [2]. An analysis of National Health and Nutrition Examination Survey (NHANES) II and III data estimated that the CKD prevalence increased by 25% between 1978 and 1991 [3].
Physical dysfunction may be an important determinant of morbidity and mortality in people with kidney diseases. Studies have uniformly reported significantly decreased physical function in end-stage renal disease (ESRD) patients [4,5], and this physical dysfunction in ESRD is powerfully associated with mortality. Lower baseline physical component summary scores on the SF-36 are independent predictors of 1-year mortality [6–8] and hospitalization [6,8].
In pre-dialysis populations, a decreased eGFR of <60 ml/min/1.73 m2 has been associated with impaired walking and lifting ability in the NHANES III participants in an unadjusted cross-sectional analysis [9] as well with lower physical functioning sub-scores on the SF-36 that persisted after multivariable adjustment in >10 000 Australians participating in the population-based AusDiab study [10]. In addition, a linear regression analysis of NHANES III data reported that estimated creatinine clearance by Cockcroft-Gault was directly associated with activity level measured by metabolic equivalents (METS) [11]. A Japanese study of 294 patients with advanced chronic kidney failure (mean baseline plasma creatinine of 4.7 mg/dl) also reported faster rates of decline in health-related quality of life measures on the SF-36 including the Physical Function Sub-Scale (PFS) in these patients when compared to the general population [12].
No data are currently available, however, on how kidney function decline may be associated with physical function. We undertook this current investigation to test the hypothesis that kidney function decline is independently associated with lower physical function.
Subjects and methods
Study population
The Nurses’ Health Study (NHS) was established in 1976 when 121 700 female registered nurses aged 30–55 years and residing in 11 large U.S. states completed a mailed questionnaire on their medical history and lifestyle characteristics; participants return follow-up questionnaires every 2 years. The follow-up of participants exceeds 90% in the 30 years since the study's initiation.
The participants in the current study were part of a substudy designed to assess the association between analgesic use and change in renal function [13]. Briefly, we first limited our study sample to the 32 826 participants who provided a blood sample in 1989. Women were excluded from this initial blood collection if they had a history of either cancer (except non-melanoma skin cancer) or cardiovascular disease (myocardial infarction, angina, stroke or transient ischemic attack). The characteristics of the women who provided blood samples were similar to those of the total cohort in terms of the prevalent hypertension, age, weight, diabetes mellitus and hyperlipidemia.
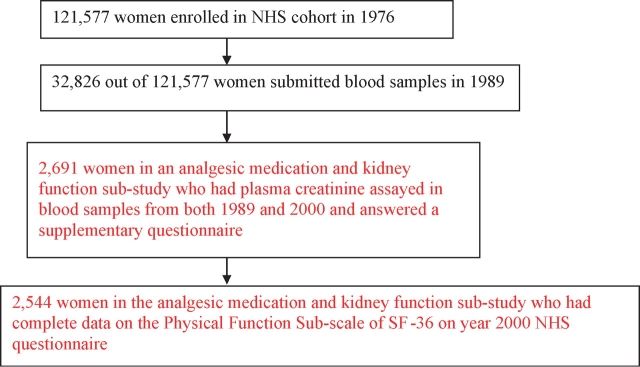
The population was then further limited to 2691 women who answered a supplementary questionnaire about analgesic use and who had serum creatinine measured in both blood samples from 1989 and 2000, and finally to 2544 women who had complete data for the PFS sub-score of the SF-36 from the 2000 questionnaire (Figure 1). Furthermore, these 2544 NHS participants appeared to be representative of all women who returned the year 2000 questionnaire (mean age 66, BMI 26 kg/m2 and similar lifestyle characteristics).
Fig. 1.
Participant selection.
Biological samples collection
Blood was collected from 32 826 NHS participants in 1989, and a second blood sample was obtained from 18 720 of these women in 2000. Participants invited to submit blood were free of cardiovascular disease (myocardial infarction, angina, stroke or transient ischemic attack) and cancer (with the exception of non-melanoma skin cancer). Each interested participant was sent a blood collection kit containing instructions and needed supplies. The participants made arrangements for the blood to be drawn locally and then sent the sample back by overnight courier. A total of 97% of samples arrived in our laboratory within 26 h of being drawn. After centrifugation, the cryotubes were stored in the vapor phase of liquid nitrogen freezers at −130°C.
NHS participants who did and did not return a blood sample in 1989 were generally similar in age and lifestyle characteristics (mean age 49.5 versus 49.4 years, BMI 24.2 versus 24.5 kg/m2, physical activity 14.5 versus 13.7 METS/day and almost identical dietary patterns).
Assessment of kidney function
Estimated GFR (eGFR) decline between 1989 and 2000 was estimated by the by four-variable MDRD equation [14]. A decline of ≥25% was chosen as the primary exposure of interest. Secondary cross-sectional analyses of associations of eGFR <60 ml/min/1.73 m2 or plasma creatinine ≥1.1 mg/dl (which represented the top 5th percentile for this study cohort in 2000) in 2000 with physical function at that time were also examined. Third, we also analyzed the association between eGFR in 1989 and physical function in 2000.
Plasma collected from the study participants was thawed and sent to the laboratory at Children's Hospital, Boston. Plasma creatinine was analyzed using the DuPont CREA method, which is a modification of the kinetic Jaffe reaction. Plasma samples from both the 1989 and 2000 collections were assayed at the same time. The percent coefficient of variation for creatinine in masked quality control samples in the NHS was 10% [13]. In 2007, repeat creatinine assays of 20 NHS plasma samples (with a range of 0.6–1.4 mg/dl) initially measured in the year 2000 revealed a mean recalibration coefficient (new value/original value) of 0.97 and confirmed that plasma creatinine is stable for many years under our storage conditions.
Outcomes
Our primary outcome of interest was the PFS of the SF-36 assessed by questionnaire in the year 2000. The PFS is widely used in clinical and epidemiologic studies as a measure of physical function and is consistent, reliable and predictive of morbidity and mortality in a variety of populations [15,16] as well as in patients with end-stage kidney disease [6–8]. Moreover, this questionnaire has been validated in patients with kidney disease who underwent direct physical performance testing including gait speed, stair climbing and chair rising [17]. The PFS has been used effectively in the NHS cohort to investigate the associations between physical function and interstitial cystitis [18], social networks [19] and weight gain [20].
The PFS comprises 10 questions with the same three response choices for each question; each answer of ‘Yes, limited a lot’ receives one point, an answer of ‘Yes, limited a little’ receives two points and an answer of ‘No, not limited at all’ receives three points. A raw score was calculated for the entire set of 10 questions and can range from a minimum of 10 to a maximum of 30 points. The raw score was transformed to a 100-point scale [21]. A PFS score of 100 is considered a perfect physical function and a score of 80 or less is considered significant physical impairment [21]. We also considered a PFS score ≤65 (representing the lowest quartile) as a dependent variable.
Covariates
Multivariable models examining associations between kidney function and physical function were adjusted for age, BMI, hypertension (HTN), diabetes, a history of past or current cigarette smoking, category of alcohol use (none, 0.1–4.9, 5–14, 15–29, ≥30 g/day), current use of diuretics, calcium channel blocker, beta-blocker, angiotensin converting enzyme (ACE) inhibitor or angiotensin-2 receptor blocker (ARB), other blood pressure medications, ibuprofen, COX-2 inhibitor, acetaminophen, aspirin, a history of cardiovascular disease (myocardial infarction, angina, coronary artery bypass grafting or stroke), congestive heart failure, peripheral vascular disease, emphysema, osteoarthritis and rheumatoid arthritis. We also considered BMI categories (normal weight <25 kg/m2, overweight 25–29.9 kg/m2, obese 30–34.9 kg/m2 and morbidly obese >35 kg/m2) instead of BMI as a continuous covariate in multivariable modeling. These variables are self-reported on biennial questionnaires and many have been validated through chart review in a subset of participants [22–27].
Statistical analysis
The Wilcoxon signed-rank test was used for comparisons of continuous variables and the chi-square test for comparisons of proportions. Linear regression modeling was used to test associations between PFS score (dependent variable) and eGFR decline. Residuals in the linear regression modeling were examined and appeared normally distributed. Logistic regression modeling was used for the dependent variables of PFS score ≤80 or in the bottom quartile (PFS ≤65). With these data, we had 90% power to detect odds of 1.3 and 59% power to detect odds of 1.2. All analyses were performed with SAS software, version 9.1 (SAS Institute, Inc., Cary, NC, USA).
Results
Our study population comprised 2544 women with a median age of 67 years (range 53–80). When stratified by an eGFR decline of at least 25% between 1989 and 2000, those with eGFR decline were older, had higher BMI, higher frequencies of hypertension and diabetes, emphysema and blood pressure medication use (Table 1). An average weight gain of 0.8 kg over the follow-up time was noted in both groups. The prevalence of individual diuretic and blood pressure medication use ranged from 3% for furosemide to 16.6% for thiazides. In the overall cohort in 2000, 44% had a PFS score ≤80 and 25% had a PFS score ≤65 in 2000. Higher proportions of women with an eGFR decline of ≥25% had PFS ≤80 and ≤65 (Table 1). On univariate analyses, an eGFR decline of ≥25% was significantly associated with the presence of hypertension, diabetes, emphysema, ibuprofen use, acetaminophen use, thiazide use, angiotensin-converting enzyme inhibitor medication and any blood pressure medication use (all P < 0.05).
Table 1.
Clinical and laboratory characteristics of 2544 Nurses’ Health Study participants
| Total study population (n = 2544) | eGFR decline <25% (n = 2117) | eGFR decline ≥25% (n = 427) | |
|---|---|---|---|
| Age (years) | 67 (53–80) | 67 (53–79) | 68 (53–80)** |
| African American | 12 (0.5) | 10 (0.5) | 2 (0.5) |
| Body mass index in 1990 (BMI) (kg/m2) | 24.4 (14.1–63.8) | 24.4 (14.1–53.2) | 24.7 (15.1–63.8) |
| Body mass index in 2000 (BMI) (kg/m2) | 25.6 (11.7–64.7) | 25.5 (11.7–54.8) | 25.7 (14.6–64.7)* |
| Change in BMI between 1990 and 2000 (kg/m2) | 0.8 (−13.6–20.2) | 0.8 (−8.6–20.2) | 0.8 (−13.6–16.5) |
| Current cigarette smoking | 145 (5.7) | 125 (5.9) | 20 (4.7) |
| Ever smoked | 1356 (53.3) | 1115 (43.8) | 241 (56.4) |
| Alcohol intake of 30+ g/day | 90 (3.5) | 80 (3.8) | 10 (2.3) |
| Hypertension | 1233 (48.5) | 978 (46.2) | 255 (59.7)*** |
| Diabetes | 148 (5.8) | 106 (5.0) | 42 (9.8)*** |
| CHF | 25 (1.0) | 18 (0.9) | 7 (1.6) |
| Peripheral vascular disease | 86 (3.4) | 73 (3.5) | 13 (3.0) |
| Any CVD (MI, angina, CABG or stroke) | 58 (2.3) | 46 (2.2) | 12 (2.8) |
| Emphysema | 126 (5.0) | 95 (4.5) | 31 (7.3)* |
| Osteoarthritis | 903 (35.5) | 745 (35.6) | 149 (34.9) |
| Any BP medication (yes/no) | 954 (37.5) | 760 (35.9) | 194 (45.4)*** |
| Measured sCr (mg/dl) in 1989 | 0.8 (0.5–2.8) | 0.8 (0.5–2.8) | 0.7 (0.6–1.6)*** |
| Measured sCr (mg/dl) in 2000 | 0.8 (0.5–2.6) | 0.8 (0.5–1.9) | 0.9 (0.6–2.6)*** |
| eGFR (ml/min/1.73 m2) in 1989 | 85 (19–139) | 84 (19–139) | 95 (35–137)*** |
| eGFR (ml/min/1.73 m2) in 2000 | 76 (19–134) | 79 (27–134) | 63 (19–99)*** |
| PFS score in 2000 | 85 (5–100) | 85 (5–100) | 80 (5–100)*** |
| PFS ≤80 in 2000 | 1127 (44.3) | 906 (42.8) | 221 (51.8)*** |
| PFS ≤65 in 2000 | 645 (25.4) | 502 (23.7) | 143 (33.5)*** |
Results are expressed as median (range) or number (%).
*P < 0.05, **P < 0.01, ***P < 0.001 when compared to referent group with the eGFR decline <25%.
eGFR: estimated glomerular filtration rate; PFS: Physical Function Subscale of the SF-36.
After multivariable adjustment, eGFR decline of ≥25% over 11 years was associated with a 3.5 point lower PFS score (95% CI, −5.4 to −1.5) when compared to the group without a ≥25% eGFR decline (Table 2). Covariates including age, BMI, smoking, several chronic medical conditions and blood pressure lowering medications remained significantly associated with a lower PFS in the fully adjusted linear model adjusting for eGFR in 1989, categories of BMI, change in BMI or excluding those with BMI >35 did not alter the findings. As illustrated in Table 3, an eGFR decline of ≥25% was marginally associated with an increased odds of PFS score ≤80 (OR 1.24; 95% CI 0.96–1.60) and significantly associated with PFS score ≤65 (OR 1.37; 95% CI 1.04–1.81) after multivariable adjustment. Further adjustment for baseline eGFR in 1989 in the model did not change these results. The results were also not significantly different if the change in BMI between 1989 and 2000 was included as a covariate in the linear and logistic models nor when those with morbid obesity (BMI >35) were excluded. Interaction terms of an eGFR decline of ≥25% and (a) BMI as either a continuous or categorical variable or (b) BMI change were not significant (all P > 0.30) when tested. Almost all covariates that were significantly associated with worse physical function in linear regression analyses were also directly associated with PFS ≤65 in the multivariable logistic regression model. All results were materially unchanged when analyses were restricted to the 2226 women who were self-reported as Caucasian.
Table 2.
Multivariable linear regression models for association between eGFR decline ≥25% between 1989 and 2000 and PFS in 2000
| β-coefficient (95% CI) for eGFR decline ≥25% | P-value | |
|---|---|---|
| Univariate age-adjusted | −6.4 (−8.7, −4.0) | <0.001 |
| model | ||
| Full MV modela | −3.5 (−5.4, −1.5) | <0.001 |
aFull MV model adjusted for age, BMI, HTN, diabetes, ever smoked, category of alcohol use (none, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/day), thiazide, furosemide, calcium channel blocker, β-blocker, ACE or ARB, other blood pressure medications, CVD (MI, angina, CABG or stroke), CHF, peripheral vascular disease, emphysema, osteoarthritis, rheumatoid arthritis, ibuprofen, Cox-2 inhibitors, acetaminophen and ≥1 aspirin use per week.
Table 3.
Multivariable logistic regression models for association between eGFR decline ≥25% between 1989 and 2000 and measures of physical function in 2000
| Odds ratio (95% CI) for eGFR decline ≥25% | P-value | |
|---|---|---|
| PFS ≤80 (physical dysfunction) | ||
| Age-adjusted univariate | 1.39 (1.12, 1.72) | 0.002 |
| model | ||
| Full MV modela | 1.24 (0.96, 1.60) | 0.09 |
| PFS ≤65 (lowest quartile of physical function) | ||
| Age-adjusted univariate | 1.57 (1.25, 1.98) | <0.0001 |
| model | ||
| Full MV modela | 1.37 (1.04, 1.81) | 0.03 |
aFull MV model adjusted for age, BMI, HTN, diabetes, ever smoked, category of alcohol use (none, 0.1–4.9, 5–14.9, 15–29.9, ≥30 g/day), thiazide, furosemide, calcium channel blocker, β-blocker, ACE or ARB medication use, other blood pressure medications, CVD (MI, angina, CABG or stroke), CHF, peripheral vascular disease, emphysema, osteoarthritis, rheumatoid arthritis, ibuprofen, Cox-2 inhibitors, acetaminophen and ≥1 aspirin use per week.
Removing those participants with chronic comorbidities (diabetes, CHF, vascular disease, any CVD or emphysema) resulted in a sample size of 2162 and the associations between eGFR decline and dichotomized PFS scores were attenuated (OR 1.18; 95% CI 0.90–1.56 for PFS score <80 and OR 1.21; 95% CI 0.88–1.67, for PFS ≤65). All interaction terms of an eGFR decline of ≥25% and each of these comorbidities, however, were non-significant (all P-values >0.20).
In secondary analyses, we first examined for univariate associations between eGFR categories of ≥90 (n = 556), 75–89 (n = 801), 60–74 (n = 819) and <60 (n = 368) ml/min/1.73 m2 and PFS score in the year 2000 and found that only eGFR <60 was significantly associated with a 4.7 point lower PFS score (95% CI −7.7 to −1.7). Regression models examining the cross-sectional associations between the exposure of eGFR <60 ml/min/1.73 m2 in 2000 and PFS score as a continuous dependent variable were also constructed. Although the age-adjusted univariate model demonstrated a statistically significant lower PFS score by 3.6 points (95% CI −6.2 to −1.1) in those with eGFR <60 ml/min/1.73 m2, this association was markedly attenuated and no longer significant when BMI was included in the model (PFS difference of 1.9 points, 95% CI −4.2–0.4).
Similar to the results of linear regression analysis, logistic regression showed that age-adjusted eGFR <60 ml/min/1.73 m2 was significantly associated with PFS score ≤80 (OR 1.32; 95% CI 1.04–1.69), but this was no longer significant when BMI added to model (OR 1.15; 95% CI 0.90–1.47, P = 0.27). All cross-sectional linear and logistic regression model results were very similar when plasma creatinine was ≥1.1 mg/dl in 2000, which corresponded to a median eGFR of 47 ml/min/1.73 m2 and a mean eGFR of 44 ml/min/1.73 m2 for the group meeting this cutpoint, was used as the definition of kidney dysfunction (data not shown). Moreover, in examining the association between eGFR <60 ml/min/1.73 m2 in 1989 and physical function in 2000, we again observed that eGFR was not significantly associated with PFS score once BMI was included in all multivariable linear and logistic models (data not shown).
Discussion
We report new findings that an eGFR decline of ≥25% over 11 years is associated with an increased risk of lower physical function in middle-aged and older women. We adjusted for several independent predictors of CKD identified in population-based studies including diabetes [2,28], hypertension [2,28], higher BMI [28,29] and cigarette smoking [28], which were also inversely associated with PFS in our cohort. The 3.5-point difference in PFS score in the presence of a ≥25% eGFR decline is similar to reported magnitudes of adjusted PFS scores associated with chronic conditions such as diabetes mellitus (−2.9 points), hypertension (−2.6 points), musculoskeletal disease (−3.8 points) and heart disease (−5.0 points) [30]. This association between eGFR decline and physical function appeared to be most robust for those women in the lowest quartile of physical function (PFS ≤65) (OR 1.37; 95% CI 1.04–1.81).
Progressively decreasing clearance of toxins could be a potential etiology of the underlying pathophysiology of our findings. Because of the sparse data on physical function in those with CKD, this hypothesis is derived from proposed mechanisms contributing to decreased physical function in ESRD including uremic myopathy and neural and oxidative abnormalities. For example, impairment in musculoskeletal strength, balance and speed of muscle recruitment has been noted in dialysis-dependent patients who underwent direct testing of quadriceps torque, walking speed and balance [31]. Dysfunction in peripheral and central neural activation as well as decreased oxidative potential in the muscles may also contribute to the excessive muscular fatigue seen in ESRD patients [32].
After multivariable adjustment for BMI, comorbid medical conditions and medications, we found no association between eGFR <60 and PFS score in cross-sectional analyses. BMI appears to be one of the most important confounders between the cross-sectional association of eGFR <60 and physical function. Higher BMI has been associated with lower levels of physical function by the PFS of the SF-36 in studies of community-dwelling women [33,34]. Higher BMI is also an independent risk factor for kidney dysfunction [29]. These results are consistent with the recent cross-sectional analysis of NHANES III data by Finkelstein et al. that reported all associations between estimated creatinine clearance and measures of physical function were null in adults ≥56 years old after multivariable adjustment [11]. Another study of ∼3000 people aged 70–79 years in the Health, Aging, and Body Composition study also reported that univariate associations between MDRD eGFR <60 and measures of physical function became null in multivariable models that included BMI [35]. Future investigations of associations between eGFR with physical function should therefore include BMI, comorbidities and medication use in adjusted models.
One possible explanation for the absence of an association between eGFR <60 ml/min/1.73 m2 and PFS in adjusted models is that participants were being misclassified with eGFR <60 because of the bias of the estimating equation, which has been shown to underestimate directly measured eGFR by 20–30 ml/min/1.73 m2 in those with plasma creatinine in the normal range [36,37]. In contrast, perhaps the eGFR decline ≥25% is a more accurate assessment of the presence and progression of CKD. We do not think that the results can be attributed primarily to misclassification, however, because the eGFR <60 ml/min/ 1.73 m2 in both 1989 and 2000 were significantly associated with lower PFS on univariate analysis, consistent with previous univariate cross-sectional reports of eGFR <60 and measures of physical function.
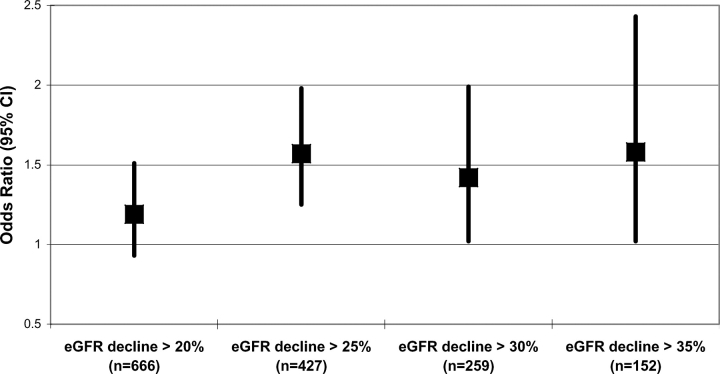
The choice of an eGFR decline of ≥25% over 11 years as the primary exposure of interest was determined a priori and has been used in previous analyses of renal function decline in NHS participants [13,38]. When we examined different cutpoints of eGFR decline in our study population, a decline in eGFR of ≥20% resulted in an attenuated and non-significant association with PFS in linear and logistic regression analyses (Figure 2). In contrast, using an eGFR decline of ≥30% or ≥35%, which should further minimize false positive classification of those with kidney function decline, resulted in similar odds ratios observed with the ≥25% threshold although the 95% confidence intervals were wider because of the smaller numbers of women at these higher cutpoints of eGFR decline (Figure 2). We hypothesize that an eGFR ≥20% may be subject to random misclassification because of the errors in using estimation equations whereas an eGFR decline of ≥25% appears to provide a more robust and meaningful exposure cutpoint while providing a reasonable percentage of participants with this exposure (16.8%).
Fig. 2.
Odds ratios and 95% confidence intervals for different levels of eGFR decline and PFS ≤65.
Investigators have reported an estimated creatinine clearance decline of 1 ml/min/1.73 m2 per year in adults over the age of 40 [39]. We could therefore potentially attribute an eGFR decline of 11 ml/min/1.73 m2 over the 11-year study period to aging alone; however, to meet the definition of a ≥25% eGFR decline attributable only to aging would require a baseline eGFR of 44 ml/min/1.73 m2 or less. Only 10 women in our study had an eGFR of 44 ml/min/ 1.73 m2 or lower at baseline, and of these, only 2 women experienced an ≥25% eGFR decline over 11 years. Therefore, in our cohort, an eGFR decline of ≥25% over 11 years occurred almost exclusively in women with higher renal function at baseline and exceeded the rate of eGFR decline expected with aging alone.
Limitations of this investigation include the absence of data about albuminuria, which is an additional marker of progressive CKD. Second, plasma creatinine was not calibrated to the Cleveland Clinic laboratory, but studies that have attempted this type of calibration have reported a persistence of low accuracy (only 64% of eGFR values are within 30% of directly measured GFR) [40] or even a decrease in precision [41]. Moreover, the cutpoint of eGFR <60 ml/min/1.73 m2 appears to be minimally influenced by calibration differences of plasma creatinine across labs [42] and a bias in estimating eGFR should not result in a systematic bias in calculating the rate of decline of eGFR over time in the same person. Low power for an odds ratio of 1.2 seen in the fully adjusted model for PFS ≤80 is another limitation although all analyses of kidney function decline and PFS consistently showed an inverse association. After the 382 women with any chronic comorbidities were removed from the analysis, we still observed elevated odds ratios between an eGFR >25% decline and PFS <80 (OR = 1.18) and PFS <65 (OR = 1.21). The associations were no longer statistically significant, however, likely because of decreased power from a smaller number of outcomes. This study was performed in a cohort of primarily Caucasian women and the findings may not be necessarily generalizable to men or to other ethnic groups although there is no strong reason to believe there would be a biological difference in other populations. Lastly, the possibility of residual confounding in these multivariable models is also present, as in any observational epidemiological study.
A major strength of the current study, however, is the large cohort of well-educated women with a validated instrument for measuring physical function. In addition, we included important potential confounders in our multivariable models and tested for interaction terms. The women included in this analysis also appeared to be representative of the entire NHS cohort. Although there is a potential survivor bias introduced by women who may have experienced faster GFR and physical function decline and who died before the year 2000, this bias would be expected to reduce the magnitude of the association between faster GFR decline and lower PFS as they are each risk factors for mortality.
In conclusion, we found that an eGFR decline of ≥25% over 11 years is independently associated with lower physical function in our cohort of middle-aged and older women. This association may represent an important public health issue in the current epidemic of CKD. Further investigations should include examination of how baseline eGFR and eGFR decline are associated with subsequent change in physical function.
Acknowledgments
This work was supported by NIH grants K08 DK066246 (J.L.), R01DK066574 (G.C.C.) and P01CA055075.
Conflict of interest statement. None declared.
References
- 1.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.Garg AX, Kiberd BA, Clark WF, et al. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, Vittinghoff E, Lin F, et al. The incidence of end-stage renal disease is increasing faster than the prevalence of chronic renal insufficiency. Ann Intern Med. 2004;141:95–101. doi: 10.7326/0003-4819-141-2-200407200-00007. [DOI] [PubMed] [Google Scholar]
- 4.Wight JP, Edwards L, Brazier J, et al. The SF36 as an outcome measure of services for end stage renal failure. Qual Health Care. 1998;7:209–221. doi: 10.1136/qshc.7.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake C, Codd MB, Cassidy A, et al. Physical function, employment and quality of life in end-stage renal disease. J Nephrol. 2000;13:142–149. [PubMed] [Google Scholar]
- 6.Lowrie EG, Curtin RB, LePain N, et al. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 7.Knight EL, Ofsthun N, Teng M, et al. The association between mental health, physical function, and hemodialysis mortality. Kidney Int. 2003;63:1843–1851. doi: 10.1046/j.1523-1755.2003.00931.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kopple JD, Block G, et al. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12:2797–2806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 9.Part 6. Association of level of GFR with complications in adults. Am J Kidney Dis. 2002;39:S111–S169. [Google Scholar]
- 10.Chow FY, Briganti EM, Kerr PG, et al. Health-related quality of life in Australian adults with renal insufficiency: a population-based study. Am J Kidney Dis. 2003;41:596–604. doi: 10.1053/ajkd.2003.50121. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein J, Joshi A, Hise MK. Association of physical activity and renal function in subjects with and without metabolic syndrome: a review of the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;48:372–382. doi: 10.1053/j.ajkd.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Fukuhara S, Yamazaki S, Marumo F, et al. Health-related quality of life of predialysis patients with chronic renal failure. Nephron Clin Pract. 2007;105:c1–c8. doi: 10.1159/000096802. [DOI] [PubMed] [Google Scholar]
- 13.Curhan GC, Knight EL, Rosner B, et al. Lifetime nonnarcotic analgesic use and decline in renal function in women. Arch Intern Med. 2004;164:1519–1524. doi: 10.1001/archinte.164.14.1519. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–164. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Johansen KL, Painter P, Kent-Braun JA, et al. Validation of questionnaires to estimate physical activity and functioning in end-stage renal disease. Kidney Int. 2001;59:1121–1127. doi: 10.1046/j.1523-1755.2001.0590031121.x. [DOI] [PubMed] [Google Scholar]
- 18.Michael YL, Kawachi I, Stampfer MJ, et al. Quality of life among women with interstitial cystitis. J Urol. 2000;164:423–427. [PubMed] [Google Scholar]
- 19.Michael YL, Colditz GA, Coakley E, et al. Health behaviors, social networks, and healthy aging: cross-sectional evidence from the Nurses’ Health Study. Qual Life Res. 1999;8:711–722. doi: 10.1023/a:1008949428041. [DOI] [PubMed] [Google Scholar]
- 20.Fine JT, Colditz GA, Coakley EH, et al. A prospective study of weight change and health-related quality of life in women. Jama. 1999;282:2136–2142. doi: 10.1001/jama.282.22.2136. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE. SF-36 Health Survey: Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 22.Hu FB, Willett WC, Colditz GA, et al. Prospective study of snoring and risk of hypertension in women. Am J Epidemiol. 1999;150:806–816. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 23.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 24.Tanasescu M, Leitzmann MF, Rimm EB, et al. Physical activity in relation to cardiovascular disease and total mortality among men with type 2 diabetes. Circulation. 2003;107:2435–2439. doi: 10.1161/01.CIR.0000066906.11109.1F. [DOI] [PubMed] [Google Scholar]
- 25.Cho E, Manson JE, Stampfer MJ, et al. A prospective study of obesity and risk of coronary heart disease among diabetic women. Diabetes Care. 2002;25:1142–1148. doi: 10.2337/diacare.25.7.1142. [DOI] [PubMed] [Google Scholar]
- 26.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med. 1991;151:1141–1147. [PubMed] [Google Scholar]
- 27.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 28.Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 29.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 30.Wee HL, Cheung YB, Li SC, et al. The impact of diabetes mellitus and other chronic medical conditions on health-related quality of life: is the whole greater than the sum of its parts? Health Qual Life Outcomes. 2005;3:2. doi: 10.1186/1477-7525-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blake C, O’Meara YM. Subjective and objective physical limitations in high-functioning renal dialysis patients. Nephrol Dial Transplant. 2004;19:3124–3129. doi: 10.1093/ndt/gfh538. [DOI] [PubMed] [Google Scholar]
- 32.Johansen KL, Doyle J, Sakkas GK, et al. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am J Physiol Regul Integr Comp Physiol. 2005;289:R805–R813. doi: 10.1152/ajpregu.00187.2005. [DOI] [PubMed] [Google Scholar]
- 33.Bohannon RW, Brennan PJ, Pescatello LS, et al. Adiposity of elderly women and its relationship with self-reported and observed physical performance. J Geriatr Phys Ther. 2005;28:10–13. doi: 10.1519/00139143-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Leon-Munoz LM, Guallar-Castillon P, Banegas JR, et al. Changes in body weight and health-related quality-of-life in the older adult population. Int J Obes (Lond) 2005;29:1385–1391. doi: 10.1038/sj.ijo.0803049. [DOI] [PubMed] [Google Scholar]
- 35.Odden MC, Chertow GM, Fried LF, et al. Cystatin C and measures of physical function in elderly adults: the Health, Aging, and Body Composition (HABC) study. Am J Epidemiol. 2006;164:1180–1189. doi: 10.1093/aje/kwj333. [DOI] [PubMed] [Google Scholar]
- 36.Lin J, Knight EL, Hogan ML, et al. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 37.Rule AD, Gussak HM, Pond GR, et al. Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis. 2004;43:112–119. doi: 10.1053/j.ajkd.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Knight EL, Stampfer MJ, Rimm EB, et al. Moderate alcohol intake and renal function decline in women: a prospective study. Nephrol Dial Transplant. 2003;18:1549–1554. doi: 10.1093/ndt/gfg228. [DOI] [PubMed] [Google Scholar]
- 39.Rowe JW, Andres R, Tobin JD, et al. The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol. 1976;31:155–163. doi: 10.1093/geronj/31.2.155. [DOI] [PubMed] [Google Scholar]
- 40.Hallan S, Asberg A, Lindberg M, et al. Validation of the modification of diet in renal disease formula for estimating GFR with special emphasis on calibration of the serum creatinine assay. Am J Kidney Dis. 2004;44:84–93. doi: 10.1053/j.ajkd.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 41.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 42.Murthy K, Stevens LA, Stark PC, et al. Variation in the serum creatinine assay calibration: a practical application to glomerular filtration rate estimation. Kidney Int. 2005;68:1884–1887. doi: 10.1111/j.1523-1755.2005.00608.x. [DOI] [PubMed] [Google Scholar]