Abstract
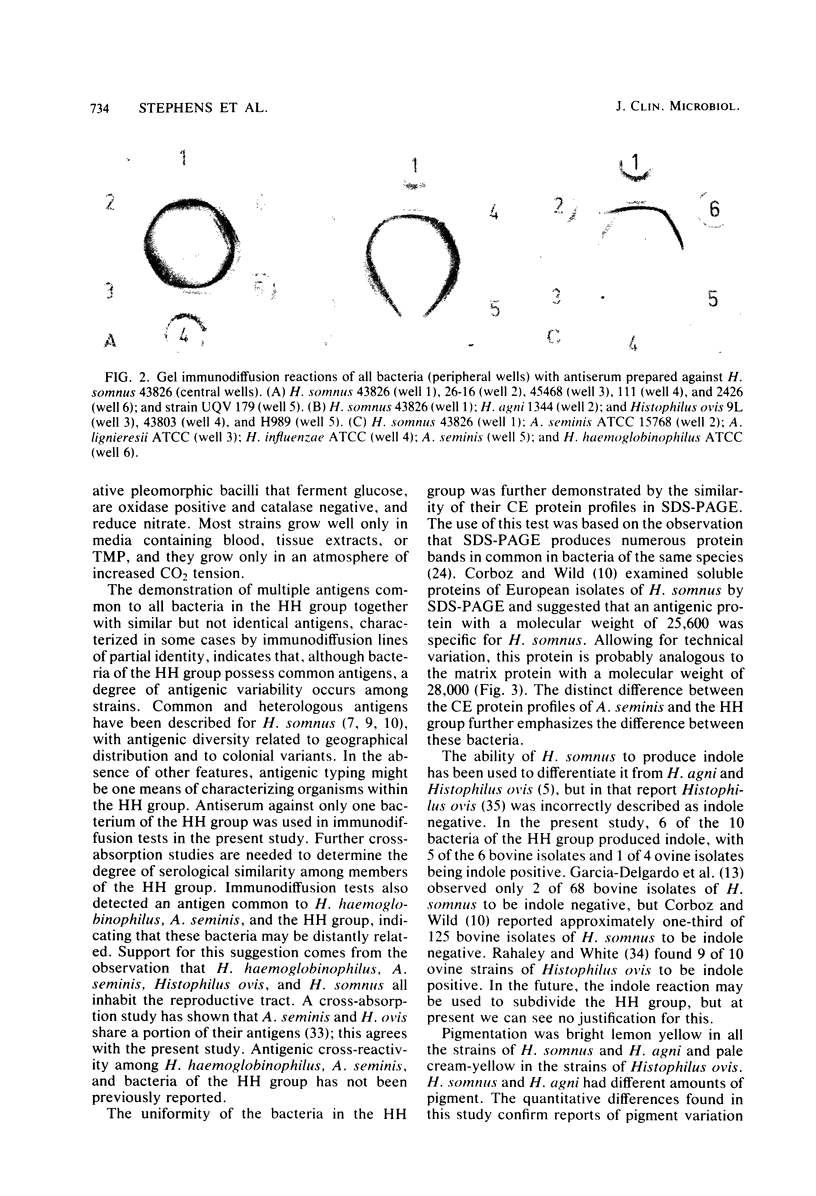
Morphology, biochemical reactions, pigmentation, antigens, and cell envelope proteins were examined in 12 strains of Haemophilus somnus, Haemophilus agni, Histophilus ovis, and Actinobacillus seminis. All of the strains except A. seminis are related and are considered as a single Haemophilus-Histophilus (HH) group. In immunodiffusion tests, HH group bacteria had at least two antigens common to all members of the group, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis showed that they have similar cell envelope protein profiles. A quantitatively variable yellow pigment with absorption maxima of 430 to 435 nm was present in strains of H. somnus and H. agni. The HH group did not produce catalase and grew only in air containing 10% CO2. Of 10 HH group bacteria, 9 required thiamine monophosphate for growth. A. seminis was distinguished from the HH group by its lack of yellow pigment, production of catalase, growth in air, lack of a thiamine monophosphate requirement, and different cell envelope protein profile. In gel immunodiffusion tests, A. seminis antigens produced two lines of partial identity with the HH group when antiserum against H. somnus was used. Reference strains of Haemophilus influenzae, Actinobacillus lignieresii, and Haemophilus haemoglobinophilus were compared with the test strains. In immunodiffusion tests, a single antigen was found to be common to H. haemoglobinophilus, A. seminis, and the HH group. No similarities between any of the test strains and H. influenzae or A. lignieresii were noted. The close relationship of H. somnus, H. agni, and Histophilus ovis suggests that these unofficially named bacteria may belong to a single taxon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asmussen M. D., Baugh C. L. Thiamine pyrophosphate (cocarboxylase) as a growth factor for Haemophilus somnus. J Clin Microbiol. 1981 Aug;14(2):178–183. doi: 10.1128/jcm.14.2.178-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailie W. E., Anthony H. D., Weide K. D. Infectious thromboembolic meningoencephalomyelitis (sleeper syndrome) in feedlot cattle. J Am Vet Med Assoc. 1966 Jan 15;148(2):162–166. [PubMed] [Google Scholar]
- Canto G. J., Biberstein E. L. Serological diversity in Haemophilus somnus. J Clin Microbiol. 1982 Jun;15(6):1009–1015. doi: 10.1128/jcm.15.6.1009-1015.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton P. D., Everett R. E. Recovery of an organism resembling Histophilus ovis from a ram. Aust Vet J. 1966 Dec;42(12):457–458. doi: 10.1111/j.1751-0813.1966.tb14473.x. [DOI] [PubMed] [Google Scholar]
- Corboz L., Wild P. Epidemiologie der Haemophilus somnus-Infektion beim Rind: Vergleich von Stämmen in der Polyacrylamidgel-Elektrophorese (PAGE). Schweiz Arch Tierheilkd. 1981 Feb;123(2):79–88. [PubMed] [Google Scholar]
- Garcia-Delgado G. A., Little P. B., Barnum D. A. A comparison of various Haemophilus somnus strains. Can J Comp Med. 1977 Oct;41(4):380–388. [PMC free article] [PubMed] [Google Scholar]
- Gourlay R. N., Flanagan B. F., Wyld S. G. Streptobacillus actinoides (Bacillus actinoides): isolation from pneumonic lungs of calves and pathogenicity studies in gnotobiotic calves. Res Vet Sci. 1982 Jan;32(1):27–34. [PubMed] [Google Scholar]
- Gumbrell R. C., Smith J. M. Deoxyribonucleic acid base composition of ovine actinobacilli. J Gen Microbiol. 1974 Oct;84(2):399–402. doi: 10.1099/00221287-84-2-399. [DOI] [PubMed] [Google Scholar]
- Higgins R., Godbout-DeLaSalle F., Messier S., Couture Y., Lamothe P. Isolation of Histophilus ovis from vaginal discharge in ewes in Canada. Can Vet J. 1981 Dec;22(12):395–396. [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. D., Little P. B., Barnum D. A., Doig P. A., Stephens L. R., Thorsen J. Occurrence of "Haemophilus somnus" in bovine semen and in the prepuce of bulls and steers. Can J Comp Med. 1982 Apr;46(2):215–217. [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. D., Little P. B., Stephens L. R., Barnum D. A., Doig P. A., Thorsen J. Prevalence and distribution of Haemophilus somnus in the male bovine reproductive tract. Am J Vet Res. 1982 May;43(5):791–795. [PubMed] [Google Scholar]
- KENNEDY P. C., BIBERSTEIN E. L., HOWARTH J. A., FRAZIER L. M., DUNGWORTH D. L. Infectious meningo-encephalitis in cattle, caused by a haemophilus-like organism. Am J Vet Res. 1960 Mar;21:403–409. [PubMed] [Google Scholar]
- KENNEDY P. C., FRAZIER L. M., THEILEN G. H., BIBERSTEIN E. L. A septicemic disease of lambs caused by Hemophilus agni (new species). Am J Vet Res. 1958 Jul;19(72):645–654. [PubMed] [Google Scholar]
- Kersters K., De Ley J. Identification and grouping of bacteria by numerical analysis of their electrophoretic protein patterns. J Gen Microbiol. 1975 Apr;87(2):333–342. doi: 10.1099/00221287-87-2-333. [DOI] [PubMed] [Google Scholar]
- Kilian M. A rapid method for the differentiation of Haemophilus strains. The porphyrin test;. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):835–842. doi: 10.1111/j.1699-0463.1974.tb02381.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mannheim W., Pohl S., Holländer R. Zur Systematik von Actinobacillus, Haemophilus and Pasteurella: Basenzusammensetzung der DNS, Atmungschinone und kulturell-biochemische Eigenschaften repräsentativer Sammlungsstämme. Zentralbl Bakteriol A. 1980;246(4):512–540. [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Davis D. G., Johnson J. A., Curtis R. A. Factors associated with morbidity and mortality in feedlot calves: the Bruce County beef project, year two. Can J Comp Med. 1981 Apr;45(2):103–112. [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. G., Macleod N. S. The isolation of Haemophilus somnus following sudden deaths in suckler calves in Scotland. Vet Rec. 1977 Feb 12;100(7):126–127. doi: 10.1136/vr.100.7.126. [DOI] [PubMed] [Google Scholar]
- Rahaley R. S. Pathology of experimental Histophilus ovis infection in sheep. I. Lambs. Vet Pathol. 1978 Sep;15(5):631–637. doi: 10.1177/030098587801500506. [DOI] [PubMed] [Google Scholar]
- Rahaley R. S. Serological comparison between Histophilus ovis, Actinobacillus seminis and Brucella ovis. Aust Vet J. 1978 Sep;54(9):423–425. doi: 10.1111/j.1751-0813.1978.tb05567.x. [DOI] [PubMed] [Google Scholar]
- Rahaley R. S., White W. E. Histophilus ovis infection in sheep in Western Victoria. Aust Vet J. 1977 Mar;53(3):124–127. doi: 10.1111/j.1751-0813.1977.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Sauders J. R., Saunders J. R., Osborne A. D. Prevalence of haemophilus somnus in the semen of bulls in Saskatchewan. Can Vet J. 1981 Nov;22(11):361–362. [PMC free article] [PubMed] [Google Scholar]
- Shigidi M. A., Hoerlein A. B. Characterization of the Haemophilus-like organism of infectious thromboembolic meningoencephalitis of cattle. Am J Vet Res. 1970 Jun;31(6):1017–1022. [PubMed] [Google Scholar]
- Stephens L. R., Little P. B., Wilkie B. N., Barnum D. A. Humoral immunity in experimental thromboembolic meningoencephalitis in cattle caused by Haemophilus somnus. Am J Vet Res. 1981 Mar;42(3):468–473. [PubMed] [Google Scholar]
- Stephens L. R., Little P. B., Wilkie B. N., Barnum D. A. Infectious thromboembolic meningoencephalitis in cattle: a review. J Am Vet Med Assoc. 1981 Feb 15;178(4):378–384. [PubMed] [Google Scholar]
- Watt D. A., Bamford V., Nairn M. E. Actinobacillus seminis as a cause of polyarthritis and posthitis in sheep. Aust Vet J. 1970 Oct;46(10):515–515. doi: 10.1111/j.1751-0813.1970.tb09190.x. [DOI] [PubMed] [Google Scholar]
- van Tonder E. M. Actinobacillus seminis infection in sheep in the Republic of South Africa. III. Growth and cultural characteristics of A. seminis. Onderstepoort J Vet Res. 1979 Sep;46(3):141–148. [PubMed] [Google Scholar]