Abstract
Vancomycin-resistant Staphylococcus aureus (VRSA) infections, which are always methicillin-resistant, are a rare but serious public health concern. We examined 2 cases in Michigan in 2007. Both patients had underlying illnesses. Isolates were vanA-positive. VRSA was neither transmitted to or from another known VRSA patient nor transmitted from patients to identified contacts.
Keywords: VRSA, MRSA, Staphylococcus aureus, antimicrobial resistance, vancomycin resistance, bacteria, Michigan, USA, dispatch
Vancomycin continues to be used as a first-line antimicrobial agent for the treatment of infection with methicillin-resistant Staphylococcus aureus (MRSA). Because alternative treatments are limited, development of resistance to vancomycin can make treatment of MRSA infections increasingly difficult. Fortunately, only 7 cases of vancomycin-resistant S. aureus (VRSA) infection, which is always methicillin-resistant, have been reported in the United States (Table) (1); 5 of these cases occurred in Michigan. We report 2 additional cases of VRSA that occurred in Michigan in 2007. The Michigan Department of Community Health (MDCH) examined the patients’ records, compared genetic characteristics of isolates, assessed possible transmission to contacts, and assessed infection control practices at facilities providing patient care.
Table. Vancomycin-resistant Staphylococcus aureus isolates detected in the United States, 2002–2006.
| Isolate no. | State | Date isolated |
|---|---|---|
| 1 | Michigan | 2002 Jun |
| 2 | Pennsylvania | 2002 Sep |
| 3 | New York | 2004 Mar |
| 4 | Michigan | 2005 Feb |
| 5 | Michigan | 2005 Oct |
| 6 | Michigan | 2005 Dec |
| 7 | Michigan | 2006 Oct |
The Cases
From each patient’s medical records, we collected information about demographics and concurrent illness, antimicrobial drug history, history of prior MRSA and vancomycin-resistant Enterococcus spp. (VRE) infections, and VRSA site co-infections. Initial isolate identification and antimicrobial drug susceptibility testing were conducted by 2 independent Michigan hospitals. Confirmatory organism identification by conventional biochemical methods and antimicrobial drug susceptibility testing were performed by MDCH’s Bureau of Laboratories (2,3). Vancomycin resistance is defined as MIC >16 µg/mL (4). Isolates were submitted to the Centers for Disease Control and Prevention (CDC) for PCR testing for van genes, which encode vancomycin resistance, and for genetic analysis by pulsed-field gel electrophoresis (PFGE) and plasmid restriction digest to compare with other VRSA isolates (5–7).
By following the CDC guide for investigating and controlling VRSA (8), we defined periods of potential transmissibility. The length of this period is flexible: start date depends on recent culture results, patient care settings, and clinical assessment; end date is determined by 2 negative cultures, which are submitted weekly posttherapy. To develop a list of potential patient contacts, we assessed healthcare visits, community activities, and personal acquaintances from this period. Contacts were then screened for VRSA, starting with persons who had had the most extensive contact (8). Swabs of bilateral anterior nares and open wounds were collected from each contact and spread onto blood agar (TSA with sheep blood) and mannitol salt agar (both from Remel, Lenexa, KS, USA). Plates were incubated for 72 h at 35°C and then for 72 h at room temperature; results were reported as negative when no growth occurred after incubation at these conditions. Serial swabs were collected from contacts who had ongoing exposure. Infection control practices were assessed at all facilities that had provided care to each patient.
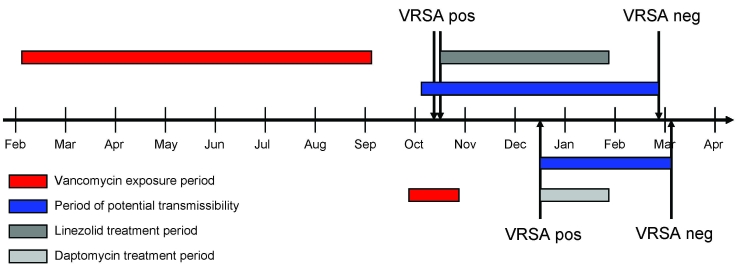
On October 12, 2007, VRSA and MRSA were cultured from a right plantar foot wound of a 48-year-old patient (patient 1) who had a history of insulin-dependent diabetes, chronic foot ulcers, and prior concurrent MRSA and VRE infections. The patient had recently received vancomycin and ceftriaxone for 7 months to treat osteomyelitis of the right metatarsals. The patient’s VRSA infection was treated with linezolid and meropenem for 15 weeks. Final VRSA-negative posttreatment swabs were collected on February 26, 2008. The investigation was closed 134 days after initial VRSA-positive culture (Figure 1).
Figure 1.
Vancomycin-resistant Staphylococcus aureus (VRSA) culture, treatment, and period of potential transmissibility timelines, 2 patients, February 2007–April 2008. Top, patient 1; bottom, patient 2; pos, positive; neg, negative.
On December 13, 2007, VRSA, VRE, and Citrobacter youngae were cultured from a left plantar foot wound of a 54-year-old patient (patient 2) who had inadequately controlled insulin-dependent diabetes. This patient had no documented history of MRSA infection and had recently received vancomycin and levofloxacin for 4 weeks to treat osteomyelitis of the left metatarsals. The patient’s VRSA infection was treated with daptomycin for 6 weeks. Final VRSA-negative posttreatment swabs were collected on March 4, 2008. The investigation was closed 81 days after initial VRSA-positive culture (Figure 1).
The VRSA isolates from each patient were highly resistant to vancomycin (each MIC 1,024 µg/mL) but susceptible to daptomycin, linezolid, quinupristin/dalfopristin, rifampin, tetracycline, and tigecycline. The isolate from patient 1 was additionally susceptible to chloramphenicol. Isolates from both patients were resistant to trimethoprim/sulfamethoxazole, whereas the 7 VRSA isolates tested previously had been susceptible. VRSA isolates from the 2 patients were PCR positive for the vanA gene, 1 of the 7 van genes that encode vancomycin resistance. PFGE results for both isolates differed from all other US VRSA isolates (data not shown). VRSA isolates from the 2 patients reported here had distinct plasmids (Figure 2), and plasmid restriction patterns of these isolates differed from the other 7 US VRSA isolates (7).
Figure 2.
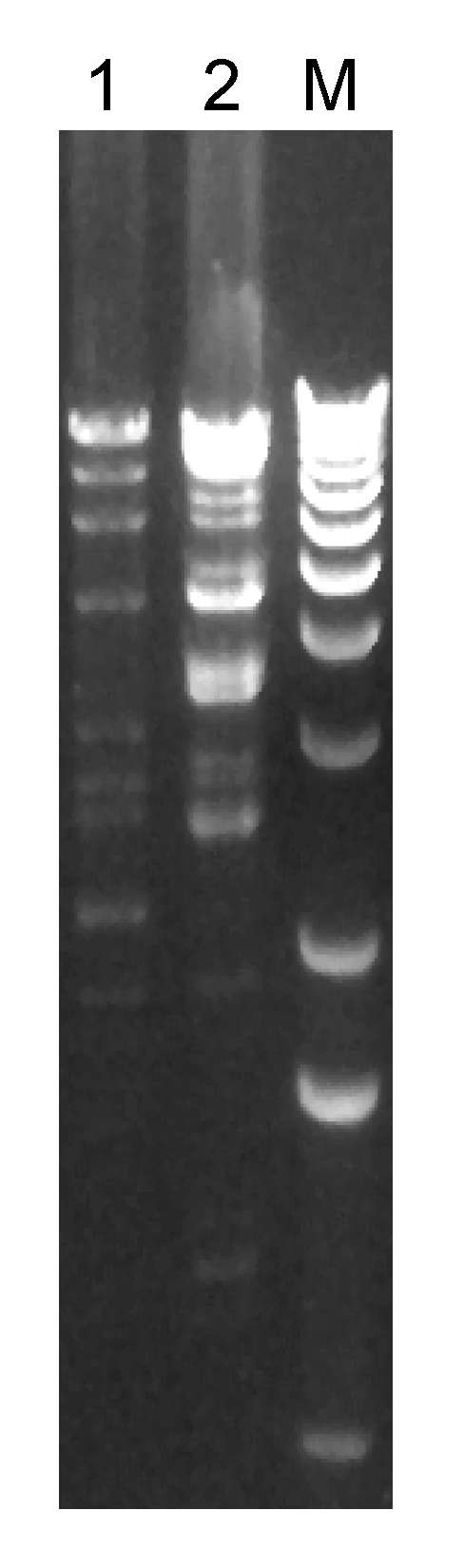
Restriction enzyme (HindIII) digest of plasmids prepared from vancomycin-resistant Staphylococcus aureus (VRSA) isolates from 2 patients in Michigan, USA, 2007. Each lane is labeled with the VRSA isolate number; lane M, 1-kb molecular marker.
The period of potential transmissibility for patient 1 began October 5, a total of 7 days before the date of positive culture, because of possible exposures during a recent hospitalization; the period ended February 26. The period of potential transmissibility for patient 2 began December 13, the date of positive culture, and ended March 4 (Figure 1). Contacts for patient 1 were evaluated at 7 locations and for patient 2 at 5 locations. For patient 1, a total of 111 swabs were collected from 75 (99%) of 76 identified contacts; 19 (25%) contacts were positive for S. aureus; 5 (7%) were positive for MRSA. For patient 2, a total of 140 swabs were collected from 126 (98%) of 128 identified contacts; 40 (32%) contacts were positive for S. aureus, 13 (10%) were positive for MRSA. No contacts of either patient were positive for VRSA. No infection control breaches were identified.
Conclusions
These 2 recent cases are consistent with cases reported in the review by Sievert et al. (1): each patient had substantial underlying concurrent conditions that contributed to the illnesses, genetic analysis of these isolates indicates that VRSA was not transmitted to or from another known VRSA patient, and no identified transmission occurred from patients to contacts. Also consistent with most previous cases, each patient reported here had a history of VRE and of vancomycin use <3 months before VRSA infection. However, patient 2 did not have a documented history of MRSA infection or colonization. Given the patient’s history of diabetes and chronic foot wounds, MRSA might have been present but undiagnosed.
Data from the other 7 US cases support the hypothesis that patients at risk for VRSA are co-infected or co-colonized with VRE and MRSA, which enables transfer of the vanA gene from VRE to MRSA in a biofilm environment, resulting in a VRSA strain. Despite attempts, only 1 laboratory has reported in vitro transfer of vancomycin resistance from VRE to S. aureus, demonstrating that interspecies transfer is not frequent (9). However, in vitro transfer of vancomycin resistance from VRSA to S. aureus has been demonstrated, reinforcing concerns about potential intraspecies transfer of vancomycin resistance among staphylococci (10).
Although VRSA infection continues to be rare and no transmission has been identified, it remains a serious public health concern, especially in Michigan where 7 of the 9 US cases have occurred. MDCH continues to educate healthcare providers about correct infection control strategies (11) and prudent antimicrobial drug use. MDCH's Bureau of Laboratories provides guidance to hospitals on methods of VRSA detection. MDCH field staff educate patients and their household contacts about wound care, hand and personal hygiene, and the importance of regular monitoring and control of diabetes, a common underlying condition with VRSA infection. Despite these efforts, questions remain unanswered, including why 7 of the 9 US VRSA cases occurred in Michigan. Before targeted prevention strategies can be developed, more research is needed to improve understanding of the microbiologic, clinical, and epidemiologic risk factors for VRSA.
Acknowledgments
We thank D.M. Sievert, J.T. Rudrick, J.B. Patel, L.C. McDonald, M.J. Wilkins, and J.B. Hageman (1) for their availability to discuss our investigations and advise us and for their continued work in organism identification and characterization. We also thank Katherine Allen-Bridson, James Michael Collins, Georgeann (Gigi) Kelm, Martin Levesque, and Rosemarie Maron for assistance with contact investigations; and Karen Flowers Anderson, Nancye C. Clark, Gregory E. Fosheim, David Lonsway, and Wenming Zhu for assistance with laboratory tests.
Biography
Dr Finks is a CDC Epidemic Intelligence Service officer assigned to the Michigan Department of Community Health. Her primary research interest is the epidemiology of antimicrobial drug–resistant infections.
Footnotes
Suggested citation for this article: Finks J, Wells E, Dyke TL, Husain N, Plizga L, Heddurshetti R, et al. Vancomycin-resistant Staphylococcus aureus, Michigan, USA, 2007. Emerg Infect Dis [serial on the Internet]. 2009 Jun [date cited]. Available from http://www.cdc.gov/EID/content/15/6/943.htm
References
- 1.Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JB. Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis. 2008;46:668–74. 10.1086/527392 [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically; approved standard M7-A7. 7th ed. Wayne (PA): The Institute; 2006. [Google Scholar]
- 3.Bannerman TL, Peacock SJ. Staphylococcus, Micrococcus, and other catalase-positive cocci that grow aerobically. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of clinical microbiology. 9th ed. Washington: ASM Press; 2007;1:390–411. [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. Wayne (PA): The Institute; 2008.
- 5.Vannuffel P, Gigi J, Ezzedine H, Vandercam B, Delmee M, Wauters G, et al. Specific detection of methicillin-resistant Staphylococcus species by multiplex PCR. J Clin Microbiol. 1995;33:2864–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–20. 10.1128/JCM.41.11.5113-5120.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu W, Clark NC, McDougal LK, Hageman J, McDonald LC, Patel JB. Vancomycin-resistant Staphylococcus aureus isolates associated with Inc18-like vanA plasmids in Michigan. Antimicrob Agents Chemother. 2008;52:452–7. 10.1128/AAC.00908-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hageman JC, Patel JB, Carey RC, Tenover FC, McDonald LC. Investigation and control of vancomycin-intermediate and -resistant Staphylococcus aureus; a guide for health departments and infection control personnel. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2006. [cited 2008 Sep 16]. Available from http://www.cdc.gov/ncidod/dhqp/pdf/ar/visa_vrsa_guide.pdf
- 9.Noble WC, Virani Z, Cree RG. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992;72:195–8. 10.1111/j.1574-6968.1992.tb05089.x [DOI] [PubMed] [Google Scholar]
- 10.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, Flannagan SE, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–71. 10.1126/science.1090956 [DOI] [PubMed] [Google Scholar]
- 11.Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2007. [cited 2008 Sep 16]. Available from http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Isolation2007.pdf