Abstract
Background and Purpose
Emerging data suggest that neuroglobin (Ngb) may protect against hypoxic/ischemic neuronal insults. However, the underlying mechanisms in vivo and implications for long-term outcomes are still not well understood.
Methods
Using our newly created Ngb overexpressing transgenic (Ngb-Tg) mice, we measured brain infarction on day 1 and day 14 after transient focal cerebral ischemia and performed neurobehavioral assessments in sensorimotor deficits on days 1, 3, 7, and 14. To test the hypothesis that Ngb may play a role in reducing oxidative stress after stroke, intracellular malondialdehyde levels were measured and compared in Ngb-Tg and wild-type mice.
Results
Increased Ngb mRNA and protein levels were identified in Ngb-Tg brains. Malondialdehyde levels in ischemic hemispheres of Ngb-Tg were significantly reduced compared with wild-type controls at 8 hours and 22 hours after transient focal cerebral ischemia. Compared with wild-type controls, brain infarction volumes 1 day and 14 days after transient focal cerebral ischemia were significantly reduced in Ngb-Tg mice. However, there were no significant improvements in sensorimotor deficits for up to 14 days after stroke in Ngb-Tg mice compared with wild-type controls.
Conclusions
Ngb reduces tissue infarction and markers of oxidative stress after stroke. Tissue protection by overexpressing Ngb can be sustained for up to 2 weeks.
Keywords: neuroglobin, neuroprotection, oxidative stress, stroke
Neuroglobin (Ngb) is a recently discovered tissue globin with a high affinity for oxygen that is widely expressed in neurons of vertebrate central and peripheral nervous systems, retina, and endocrine tissues.1–5 It was recently shown that Ngb was elevated in both transcript and protein levels in cultured primary cortical neurons during the acute phases of hypoxia.6 An age-related decline in Ngb expression in rat brain was found and suggested that loss of this protein may have a role in increasing susceptibility to age-related neurological disorders.7
As a newly discovered member of the globin family, Ngb has been considered as the equivalent of brain or nerve tissue hemoglobin.8 The distribution is indicative of a function of Ngb in metabolically active, oxygen-consuming cell types. In general, tissue globins mediate multiple cellular and molecular responses to hypoxic/ischemic insults. For example, myoglobin in cardiomyocytes and oxidative skeletal myofibers helps facilitate oxygen transport, maintain nitric oxide homoeostasis, and scavenge reactive oxygen species.9–11 It is possible that Ngb has similar actions in brain. Greenberg and colleagues showed that overexpression of Ngb protects against hypoxic neuron injury in cell culture and reduces acute 1-day ischemic brain damage in vivo.6,12 However, the effects of Ngb overexpression on long-term neurological outcomes have not been validated and underlying neuroprotective mechanisms also remain unknown. In this study, we used our newly created Ngb-overexpressing transgenic mouse to examine neuroprotection of Ngb in both acute and prolonged times after focal cerebral ischemia and tested the hypothesis that Ngb promotes neuron survival in part by reducing oxidative stress.
Materials and Methods
All animal experiments were performed following protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Generation of Neuroglobin-Overexpressing Transgenic Mice
To test our hypotheses, we generated Ngb transgenic (Ngb-Tg) mice following standard methods. In brief, the full-length cDNA sequence of mouse Ngb was cloned following bioinformatic analysis and the rapid amplification of cDNA ends technique. The full-length of Ngb cDNA (GenBank Accession No NM 022414) was used to amplify the cDNA sequence. To create a fusion of Ngb gene and the N-terminal hemagglutinin epitope tag, the mouse Ngb gene was subcloned into the Sal I and Kpn I sites of the MCS of pCMV-myc (Clontech) plasmid in frame with the hemagglutinin coding sequence. The subcloned eukaryotic expression plasmid, pCMV-HA-mouse-Ngb, capable of encoding mouse Ngb tagged with hemagglutinin, respectively, was used to release the Ngb gene. The full length of the pCMV-Ngb transgene containing the Ngb gene and polyadenylation signals in the 3′ portion of the construct was released from the vector with Sph I and Mfe I. The purified plasmid was obtained by sucrose density gradient centrifugation and sequenced to determine the orientation of the subcloned Ngb product. The purified DNA fragment was used for generating Ngb-Tg mice in the Transgenic Core Facility at Massachusetts General Hospital. Pronuclear microinjection was performed by using standard techniques. Tg mice were initially created with B6C3F1 background and thereafter crossing with C57BL/6 mice. All animals used in this study were 6 to 9 generations; they were Ngb-Tg mice and wild-type (WT) littermates.
Genotyping of Neuroglobin Transgene by Polymerase Chain Reaction
To identify the Ngb transgene, mouse tail DNA was used for polymerase chain reaction (PCR) analysis with primers flanked by the cytomegalovirus (CMV) and Ngb sequence (5′ tccaagtctccaccccattgacg 3′ and 5′ ggctcatggcttgcaccacagct 3′). A 940-bp band of PCR production was the indicator for the presence of the mouse Ngb transgene.
Quantitative Real-Time Polymerase Chain Reaction Analysis
mRNA levels of Ngb were measured by real-time reverse transcription–PCR analysis following a standard method with minor modification. In brief, total brain tissue RNA was prepared for real-time PCR using the RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer's instructions. Reverse transcriptase was performed using Superscript II RNase H-Reverse Transcriptase (Invitrogen) to obtain cDNA. The primers for mouse Ngb were as follows: 5′-tacaatggccgccagttct-3′ and 5′-tggtcactgcagcatcaatca-3′. Real-time PCR was performed on an ABI prism 7000 sequence detection systems (Applied Biosystems). Data were analyzed according to the comparative threshold cycle method with glyceraldehydes-3-phosphate dehydrogenase expression for sample normalization. Melting curves for each PCR reaction were generated to ensure the purity of the amplification products.
Western Blot and Immunohistochemistry
To examine Ngb protein expression levels, customized polyclonal anti-Ngb antibody (GenScript Corporation) was produced by immunizing rabbits with 2 synthetic peptides (KLH coupled, FQYNGRQFSSPEDC and IRQSWRVVSRSPLEC) corresponding to mouse Ngb. The antibody was purified by protein A and peptide affinity chromatography. Western blot for Ngb was performed following the protocol as previously described.13 Relative Ngb protein expression levels were assessed by quantification of optical density of Ngb protein bands with National Institutes of Health Image software. For immunohistochemistry, mice were transcardially perfused with ice-cold phosphate-buffered saline (pH 7.4) and 4% paraformaldehyde. Brains were fixed in 4% paraformaldehyde solution and cytoprotected in 30% sucrose at 4°C overnight. We stained cryostat sections (10 μm) with antibodies against Ngb, neuronal nuclei (NeuN, 1:300; Chemicon), and glial fibrillary acidic protein (GFAP, 1:300; Chemicon) as we described previously.14 We used sections treated without primary antibodies as negative controls. Fluorescent images were observed and digitized under a fluorescent microscope (Model BX51; Olympus) with a digital image system.
Cerebral Blood Flow Measurements
Cerebral blood flow was assessed by hydrogen clearance for resting state values and laser Doppler flowmetry for perfusion levels during ischemia following standard techniques as previously described15,16 For hydrogen clearance, platinum wires were placed 2 mm deep into the cortex dorsal to the caudate putamen (1.25 mm lateral and 0.5 mm anterior to bregma). For laser Doppler flowmetry, probes were located at 3 mm lateral and 1 mm posterior to bregma.
Focal Cerebral Ischemic Models in Mice
The standard intraluminal middle cerebral artery occlusion method was used to make a transient focal cerebral ischemia as we previously described.13 All animals were assessed with laser Doppler flowmetry to confirm adequate induction of focal ischemia and successful reperfusion. Perfusion reductions during ischemia were assessed as a percentage of preischemia baselines. Physiological parameters, including blood pressure (mm Hg), heart rate (beat/min), arterial pH, pCO2, and pO2 (mm Hg), were monitored in randomly selected animals to ensure that parameters remain within normal limits. In this study, we used 2 types of transient focal cerebral ischemic models. First, in a 2-hour transient occlusion model, brain infarct was assessed at 24 hours after onset of ischemia. Second, in a 1-hour transient occlusion model, both histological and sensorimotor function measurements were assessed up to 14 days after ischemia in Ngb-Tg and WT mice. In all in vivo experiments, investigators were blinded to the experimental groups.
Brain Infarct Volumes
At 24 hours after a transient 2-hour focal cerebral ischemia, mice were euthanized; standard 2,3,5-triphenyltetrazolium hydrochloride staining was used, which reflects mitochondria damage was quantified as infarct areas. At 14 days after transient 1-hour focal cerebral ischemia, we used hematoxylin & eosin staining for 8 10-μm coronal sections (with a 1-mm interval). Infarct volumes were quantitated with a standard computer-assisted image analysis technique. To eliminate confounding effects of edema and swelling, the indirect method was used (contralateral volume minus uninfarcted ipsilateral volume). Overall swelling was also assessed by calculating the ipsilateral versus contralateral hemispheric ratios.
Malondialdehyde Formation
Intracellular malondialdehyde (MDA) is a biomarker for lipid peroxidation. At 8 hours and 22 hours after a 2-hour period of transient focal cerebral ischemia, MDA levels in ischemic and nonischemic control hemispheres were measured by Bioxytech LPO-586 assay kit (oxisResearch) according to the manufacturer's introduction. Samples were measured on a spectrophotometer (Spectronic Unicam, Thermo) at 586 nM.
Sensorimotor Function Assessments
Assessments were made at 1, 3, 7, and 14 days after ischemic onset. All mice were initially trained for 2 days before the day of ischemia. Two hours before focal ischemic injury, mice were assessed to obtain preinjury baselines. Body weight was also measured on each test day. Mice were tested and scored for neurological deficits as previously described.17 The standard Rotorod test was performed with minor modification as we previously described.18 The foot fault test was performed following a standard protocol with minor modification.19 In brief, mice were put on a grid surface (30 cm long×35 cm wide×31 cm high); the grid openings were 2.5 cm2. During locomotion on the grid, the number of foot faults made by the ipsilateral and contralateral limbs was counted. Each test consisted of 3 trials lasting 1 minute each with an interval of 1 minute. Foot faults are expressed as the differences of number of errors made between ipsilateral and contralateral limbs. Mice were suspended by its forelimbs on a wire stretched between 2 posts 60 cm above a foam pillow. The time (in seconds) until the mouse fell was recorded, and the posture of the 4 limbs and tail and whether the mouse could walk were recorded as different scores (0 to 5). A score of zero was assigned if the mouse fell immediately and 60 seconds was the time-out period.20 Three trials were performed for each testing day.
Statistical Analysis
For parametric and continuous variable measurements, we used analysis of variance followed by Tukey-Kramer post hoc tests. For measurements taken over time, we used repeated-measures analysis of variance and Fisher partial least-square difference test. For nonparametric ordinal data (eg, functional outcomes), we used nonparametric Kruskal-Wallis followed by post hoc Mann-Whitney tests. Overall, P<0.05 was considered significant.
Results
Production and Characterization of Neuroglobin-Overexpressing Transgenic Mice
PCR analysis with primers flanking CMV and Ngb sequences detected a 940-bp band, thus identifying the presence of the mouse Ngb transgene in Ngb transgenic mouse tissue DNA (Figure 1A). Levels of Ngb mRNA were examined by quantitative real-time PCR. There was a 2.6-fold increase of Ngb mRNA level in Ngb-Tg mouse brains compared with WT brains (Figure 1B). Western blot detected the increase of Ngb protein in Ngb-Tg mouse brain compared with WT (Figure 1C). In the cerebral cortex, Ngb immunoreactivity was mainly colocalized with the neuron marker NeuN (Figure 1D, A–C) and not colocalized with the astrocyte marker GFAP (Figure 1D, D–F) in WT mice. By comparison, Ngb immunoreactivity was enhanced in neurons (Figure 1D, G–I) with some signals in astrocytes (Figure 1D, J–L) in Ngb-Tg mice. These elevations in Ngb protein in neurons and other cell types may be consistent with the nontissue-specific CMV promoter used in our Ngb-Tg mice.
Figure 1.
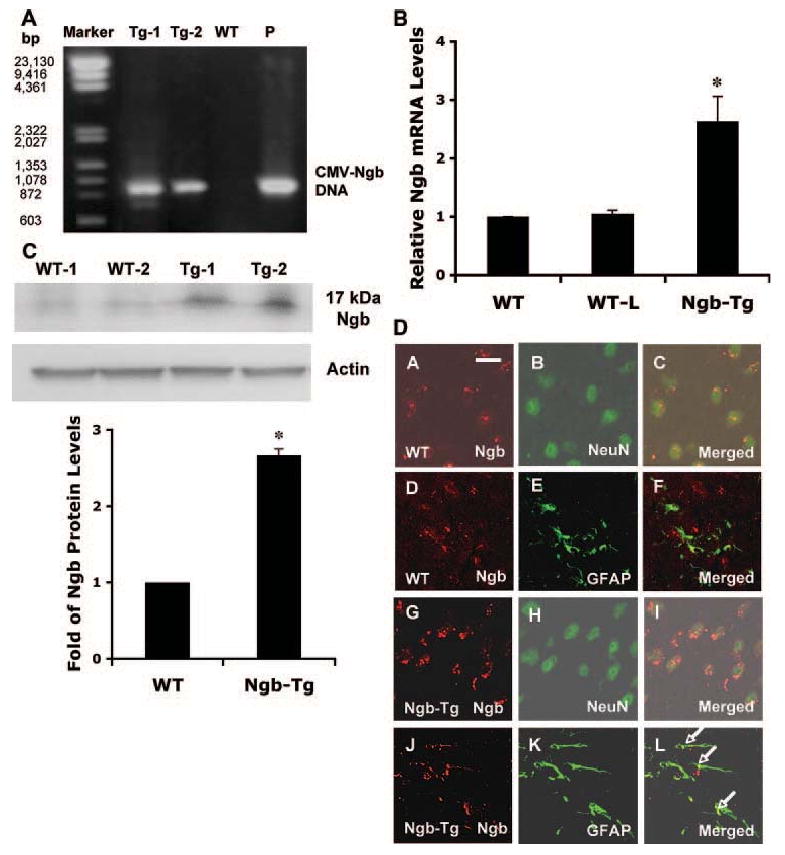
Production and characterization of Ngb-overexpressing transgenic mice. A, Tail DNA was used for PCR analysis with primers flanked CMV and Ngb sequence. The detected 940-bp band of PCR production indicates the presence of mouse Ngb transgene. DNA markers (Marker) appeared on the first lane; pCMV-Ngb plasmid served as a positive control (P). B, Brain RNA was extracted for measuring Ngb mRNA levels by quantitative real-time PCR. There was an approximately 2.6-fold increases of Ngb mRNA levels in Ngb-Tg mouse brains compared with WT and WT littermates (WT-L); similar Ngb mRNA levels were detected in WT (C57BL/6) and WT-L mouse brains. All animals used were 22 to 26 males between 10 and 12 weeks of age. Mean±SEM, n=6 per group, *P<0.05. C, Top panel is a representative Western blot showing the increase of Ngb protein in Ngb-Tg mouse brains (Tg) compared with WT controls. Actin served as equal loading controls. Lower panel shows relative Ngb protein expression levels quantified by optical density of Ngb protein bands. Mean±SEM, n=4 per group, *P<0.05. D, In cerebral cortex, immunohistochemistry showed Ngb immunoreactivity was mainly colocalized with neuron marker neuronal nuclei (D, A–C), not colocalized with astrocyte marker glial fibrillary acidic protein (D, D–F) in WT mice. By comparison, Ngb immunoreactivity was enhanced in neurons (D, G–I), some colocalized with astrocyte marker glial fibrillary acidic protein as pointed out by arrows (D, J–L) in Ngb-Tg mice. Bar: 50 μmol/L.
Measurements of Cerebral Blood Flow
Hydrogen clearance was used to compare resting cerebral blood flow, and laser Doppler flowmetry was used to measure reduction of perfusion levels during ischemia and reperfusion. In both measurements, there were no statistically significant differences between WT and Ngb-Tg mice (Table 1).
Table 1. Cerebral Blood Flow Measurements.
| Measurements | WT | Ngb-Tg | ||
|---|---|---|---|---|
| Hydrogen clearance | 83.3±3.2 | 88.5±8.9 | ||
| Resting cerebral blood flow, mL/100 g/min | ||||
| Laser Doppler flowmetry | Ischemia | Reperfusion | Ischemia | Reperfusion |
| Cerebral blood flow, % of preischemia baseline | 17.5±1.5 | 96.5±3.9 | 14.8±2.3 | 96.2±4.7 |
Hydrogen clearance was used to measure resting cerebral blood flow; platinum wire was positioned under cortex caudate putamen nuclei. Laser Doppler flowmetry was used to measure cerebral blood flow during ischemia and reperfusion in Ngb-Tg and WT mice. Results are expressed as mean±SEM. N=5 per group for hydrogen clearance and n=10 per group for laser Doppler flowmetry measurement, respectively. All animals used were 23- to 27-g male mice. In both measurements, there were no statistically significant differences between WT and Ngb-Tg mice.
Reduction of Acute Ischemic Infarction in Neuroglobin Transgenic Mouse Brains
To test the effect of Ngb overexpression in acute ischemic damage, brain infarction was examined at 24 hours after a transient 2-hour focal cerebral ischemia by standard 2,3,5-triphenyltetrazolium hydrochloride staining. Total infarct volumes were significantly smaller in Ngb-Tg mice compared with WT controls with the main reductions occurring mostly in cortical regions (Figure 2A). As expected, there was also some hemispheric swelling in ischemic brain, but there was no detectable difference between WT versus Ngb-Tg brains: 9.54±2.35% for WT and 7.44±2.31% (mean±SEM, n=8) for Ngb-Tg mice, respectively. Physiological parameters, including blood pressure (mm Hg), arterial pH, pCO2, and pO2 (mm Hg), were monitored in randomly selected animals; there was no significant differences between the 2 animal groups (Table 2).
Figure 2.
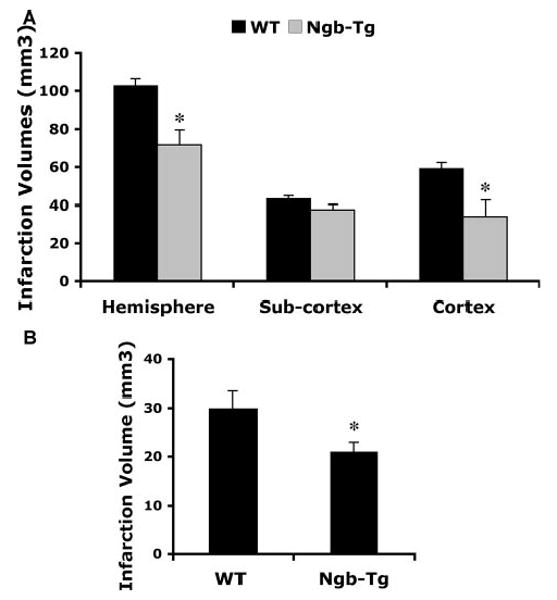
A, Reduction of acute ischemic infarction in Ngb transgenic mice after focal cerebral ischemia. After 2 hours transient focal cerebral ischemia followed by 22 hours reperfusion, 2,3,5-triphenyltetrazolium hydrochloride infarction volume in the whole hemisphere and cortical regions was significantly reduced in Ngb-Tg mice compared with matching WT littermates. Mean±SEM, n=8 per group, *P<0.05. B, Reduction of late ischemic infarction in Ngb-Tg mouse brains. At 14 days after transient 1-hour focal cerebral ischemia, brain infarction was measured by hematoxylin & eosin staining and image analysis. The infarction was only located in subcortical regions, and the volume was significantly reduced in Ngb-Tg mice compared with WT mice. Mean±SEM, n=6 for Tg and 7 for WT per group, *P<0.05. All mice were 22 to 27 g males between 8 and 9 weeks old.
Table 2. Physiological Parameter Measurements.
| Parameters | pO2 | pCO2 | pH | Blood Pressure |
|---|---|---|---|---|
| WT | ||||
| Preischemia | 126.2±9.7 | 31.3±1.4 | 7.38±0.05 | 88.2±4.4 |
| Postischemia | 95.4±10.5 | 46.9±1.7 | 7.34±0.08 | 90.2±5.1 |
| Ngb-Tg | ||||
| Preischemia | 123.1±9.2 | 30.5±1.5 | 7.37±0.06 | 89.7±4.5 |
| Postischemia | 98.3±8.9 | 47.3±2.4 | 7.35±0.07 | 86.8±5.7 |
Physiological parameters, including arterial pO2 (mm Hg), pCO2, pH, and blood pressure (mm Hg), were monitored before focal cerebral ischemia (Preischemia) and 1 hour after ischemia (Postischemia) in randomly selected WT and Ngb-Tg mice. Data are mean±SD, n=4 per group.
Reduction of Late Ischemic Infarction in Neuroglobin Transgenic Mouse Brains
Because ischemic lesion evolution may continue beyond 24 hours after stroke, hematoxylin & eosin staining analysis was used to measure brain infarction at 14 days after a 1-hour period of transient focal cerebral ischemia. Infarction volumes in Ngb-Tg mice remained significantly smaller compared with WT controls even at this delayed time poststroke (Figure 2B).
Reduction of Malondialdehyde Production in Ngb-Tg Mouse Brains After Stroke
MDA levels were measured as a surrogate biomarker of reactive oxygen species generation after stroke. In nonischemic control brains, MDA levels were not significantly different between Tg and WT mice indicating similar baseline conditions. However, MDA production was significantly reduced in Tg mice compared with WT controls at both early 8-hour and late 22-hour time points after transient focal cerebral ischemia (Figure 3).
Figure 3.
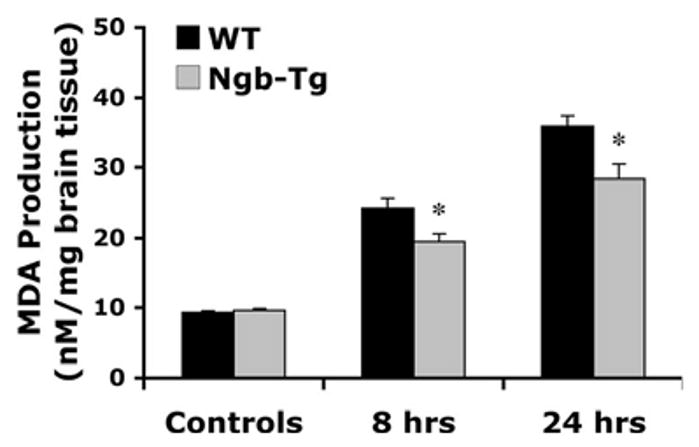
Reduction of MDA production in Ngb-Tg mouse brains after acute focal stroke. In nonischemic control hemispheres (controls), MDA levels were not significantly different between WT and Tg mice, whereas MDA production was significantly decreased in Ngb-Tg mice compared with WT controls at 8 hours (n=10 per group) and 22 hours (n=4 per group) after 2-hour transient focal cerebral ischemia. Mean±SEM, *P<0.05.
Sensorimotor Function Assessments
Four assessments, including neurological score, Rotorod test, hanging wire, and foot fault test, were performed at 1, 3, 7, and 14 days after ischemic onset. Two hours before focal ischemic injury, mice were assessed to obtain preinjury baselines. Body weight loss was also measured on each test day. Significant deficits were observed in all tests from day 1 to day 7 after ischemia; the deficits reached maximal levels at day 1 for Rotorod and hanging wire tests, day 1 to day 3 for neurological score, day 7 for the foot fault test, and day 3 to day 7 for body weight losses. All scores recovered close to preinjury baselines by day 14 after onset of ischemia. No statistically significant differences were detectable between WT and Ngb-Tg mice in all assessments during the 2-week stroke recovery period (Table 3).
Table 3. Sensorimotor Function Assessments.
| Tests | Group | Day 0 | Day 1 | Day 3 | Day 7 | Day 14 |
|---|---|---|---|---|---|---|
| Neurological score median (25th to 75th percentiles) | WT | 0 | 1 (0.25–1) | 1 (1–2) | 1 (1–2) | 1 (0.25–1) |
| Ngb-Tg | 0 | 1 (1–2) | 2 (1.5–2) | 1 (0–1) | 1 (0–1) | |
| Rotorod test, % of day 0 | WT | 100 | 70.5±10.8 | 78.5±10.6 | 90.5±9.7 | 104.3±3.3 |
| Ngb-Tg | 100 | 68.7±12.8 | 78.1±10.9 | 84.5±17.4 | 110.7±2.5 | |
| Hanging wire median (25th to 75th percentiles) | WT | 5 (4.75–5) | 2.5 (1–4) | 3.5 (2–4.25) | 4 (4–5) | 4.5 (4–5) |
| Ngb-Tg | 5 (4.5–5) | 3 (1.5–3) | 4 (2.5–4) | 4 (3.5–4) | 4 (4–5) | |
| Foot fault test median (25th to 75th percentiles) | WT | 0 (0–1) | 3 (0–5.75) | 4 (0–5.25) | 5 (4–5) | 2 (1–2) |
| Ngb-Tg | 1 (0–1.5) | 2 (1–5) | 4 (2–6) | 4 (3.5–4.5) | 1 (1–1.5) | |
| Body weight, % of day 0 | WT | 100 | 91.7±0.57 | 82.1±1.21 | 81.8±3.04 | 91.3±1.54 |
| Ngb-Tg | 100 | 91.5±0.73 | 80.1±1.58 | 81.2±3.45 | 91.9±1.55 |
Sensorimotor deficits and body weight loss were assessed before (day 0) and at days 1, 3, 7, and 14 after transient (1-hour) focal cerebral ischemia. Results are expressed as median values and 25th to 75th percentiles for assessments of neurological score, hanging wire, and foot fault tests; and mean±SEM for assessments of Rotorod test and body weight loss. N=6 for Ngb-Tg and 7 for WT per group; 23- to 27-g male mice. In all assessments, there were no statistical significant differences between WT and Ngb-Tg groups.
Discussion
Rapidly emerging data suggest that Ngb may function as an important neuroprotective molecule.5,12,21,22 However, the mechanisms underlying its actions remain to be fully elucidated. In the present study, we created a novel strain of Ngb-overexpressing transgenic mice and used this model system to show that Ngb is neuroprotective against cerebral ischemia in vivo. Acute focal infarction at 24 hours after transient 2-hour focal cerebral ischemia was significantly reduced approximately 30%, consistent with a most recent report from the Greenberg group.12 Importantly, our present study documented that tissue neuroprotection in the Ngb-Tg brains was sustained for up to 2 weeks. In terms of mechanisms, the reduction of MDA production in Ngb-Tg mouse brains suggests that Ngb promotes neuron survival in part by reducing oxidative stress after focal cerebral ischemia.
The functional role of Ngb in normal brain is not well understood.4,5,21–26 It has been suggested that Ngb may function as oxygen sensors associated with heme proteins and may respond to hypoxia by altering the rate of formation and release of reactive oxygen species that activate transcription factors.24,27,28 Furthermore, Ngb may function as a scavenger of nitric oxide or a regulator of reactive oxygen species.5,24,29 By altering these free radicals, Ngb may protect against oxidative stress in stroke. The present study demonstrated Ngb's neuroprotection against ischemia might occur through decreasing oxidative stress. In addition to its use to dissect pathophysiology, our newly generated Ngb-Tg mouse may also provide a useful tool to elucidate the functional roles and mechanisms of Ngb under physiological conditions.
There are a few caveats in this study. First, our Ngb-Tg mouse is not tissue- or cell-specific. DNA fragments containing mammalian cell promoter CMV and Ngb full-length cDNA were extracted for generating Ngb transgenic mice. Further studies using conditional or neuron-specific Ngb-Tg mice may be required. A second related issue is that as a constitutive mutant, adaptive compensations may have occurred. Although we saw no differences in resting (hydrogen clearance) or ischemic (laser Doppler flowmetry) cerebral blood flow, other spatially resolved techniques such as autoradiography or anatomic mapping of capillary density may be required to fully address these blood flow questions. A third issue in the present study is that we failed to detect significant differences in sensorimotor function recovery in our Ngb-Tg mice. In part this is because 60 minutes middle cerebral artery occlusion might not be severe enough to cause long-term sensorimotor deficits because these smaller lesions are mainly localized in subcortex areas. Alternatively, we may have simply lacked power and larger numbers of test animals would have helped. Further investigation is warranted to carefully define the role of Ngb in both long-term sensorimotor and cognitive deficits after stroke. The final caveat is related to mechanisms. We acknowledge that it will likely be impossible to unequivocally separate reactive oxygen species effects versus mitochondrial effects of Ngb. There are multiple and probably inextricable feedback loops between preservation of mitochondrial energetics versus direct radical scavenging.30–33 Both of these actions will yield the same result, ie, protection against ischemic insults. Deceased MDA production at 10 hours and 24 hours after focal ischemia in Ngb-Tg mice suggests there might be an antioxidative role for Ngb in vivo. However, for in vivo stroke models, it is difficult to unequivocally prove causality because reduced tissue damage may secondarily contribute to decreased oxidative stress and/or mitochondrial dysfunction.
In conclusion, this study documented the reduction of brain infarction in the Ngb-Tg brains was sustained for up to 2 weeks. This unique brain globin protects against ischemic injury by ameliorating oxidative stress. Further studies to test both sensorimotor and cognitive deficits are warranted in terms of fully evaluating the effect of Ngb in stroke long-term recovery.
Acknowledgments
We thank Dr David Greenberg for very helpful discussion and Drs Shuzhen Guo, Sun-Ryung Lee, and Changhong Xing for their excellent technical assistance.
Sources of Funding: This work was supported in part by National Institutes of Health grants R01-NS049476 (to X.W.), R01-NS37074, R01-NS48422, and P50-NS10828 (to E.H.L.), and a Scientist Development Grant, 0435087N, from the American Heart Association (to X.W.).
Footnotes
Disclosures: None.
References
- 1.Awenius C, Hankeln T, Burmester T. Neuroglobins from the zebrafish Danio rerio and the pufferfish Tetraodon nigroviridis. Biochem Biophys Res Commun. 2001;287:418–421. doi: 10.1006/bbrc.2001.5614. [DOI] [PubMed] [Google Scholar]
- 2.Zhang CG, Li L, Deng MY, Xie F, Wang CL, Zhou WQ, Wang HY, He FC. Coding region cDNA sequence cloning of rat neuroglobin gene, its polymorphism feature and tissue expression profile analysis [in Chinese] Yi Chuan Xue Bao. 2001;28:997–1001. [PubMed] [Google Scholar]
- 3.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 4.Burmester T, Hankeln T. Neuroglobin: a respiratory protein of the nervous system. News Physiol Sci. 2004;19:110–113. doi: 10.1152/nips.01513.2003. [DOI] [PubMed] [Google Scholar]
- 5.Garry DJ, Mammen PP. Neuroprotection and the role of neuroglobin. Lancet. 2003;362:342–343. doi: 10.1016/S0140-6736(03)14055-X. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci U S A. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Y, Jin K, Mao XO, Xie L, Peel A, Childs JT, Logvinova A, Wang X, Greenberg DA. Effect of aging on neuroglobin expression in rodent brain. Neurobiol Aging. 2005;26:275–278. doi: 10.1016/j.neurobiolaging.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Mammen PP, Shelton JM, Goetsch SC, Williams SC, Richardson JA, Garry MG, Garry DJ. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J Histochem Cytochem. 2002;50:1591–1598. doi: 10.1177/002215540205001203. [DOI] [PubMed] [Google Scholar]
- 9.Garry DJ, Kanatous SB, Mammen PP. Emerging roles for myoglobin in the heart. Trends Cardiovasc Med. 2003;13:111–116. doi: 10.1016/s1050-1738(02)00256-6. [DOI] [PubMed] [Google Scholar]
- 10.Marcinek DJ, Ciesielski WA, Conley KE, Schenkman KA. Oxygen regulation and limitation to cellular respiration in mouse skeletal muscle in vivo. Am J Physiol Heart Circ Physiol. 2003;285:H1900–H1908. doi: 10.1152/ajpheart.00192.2003. [DOI] [PubMed] [Google Scholar]
- 11.Vasil'ieva VO, Korobov VM, Velykyik Role of myoglobin in supplying oxygen to tissues [in Ukranian] Ukr Biokhim Zh. 1996;68:45–55. [PubMed] [Google Scholar]
- 12.Khan AA, Wang Y, Sun Y, Mao XO, Xie L, Miles E, Graboski J, Chen S, Ellerby LM, Jin K, Greenberg DA. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci U S A. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 16.Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- 17.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with bb-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Jung J, Asahi M, Chwang W, Russo L, Moskowitz MA, Dixon CE, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on morphological and motor outcomes after traumatic brain injury. J Neurosci. 2000;20:7037–7042. doi: 10.1523/JNEUROSCI.20-18-07037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- 20.Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- 21.Wakasugi K, Kitatsuji C, Morishima I. Possible neuroprotective mechanism of human neuroglobin. Ann N Y Acad Sci. 2005;1053:220–230. doi: 10.1196/annals.1344.020. [DOI] [PubMed] [Google Scholar]
- 22.Herold S, Fago A. Reactions of peroxynitrite with globin proteins and their possible physiological role. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:124–129. doi: 10.1016/j.cbpb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Fago A, Mathews AJ, Dewilde S, Moens L, Brittain T. The reactions of neuroglobin with CO: evidence for two forms of the ferrous protein. J Inorg Biochem. 2006;100:1339–1343. doi: 10.1016/j.jinorgbio.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Brunori M, Giuffre A, Nienhaus K, Nienhaus GU, Scandurra FM, Vallone B. Neuroglobin, nitric oxide, and oxygen: functional pathways and conformational changes. Proc Natl Acad Sci U S A. 2005;102:8483–8488. doi: 10.1073/pnas.0408766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uno T, Ryu D, Tsutsumi H, Tomisugi Y, Ishikawa Y, Wilkinson AJ, Sato H, Hayashi T. Residues in the distal heme pocket of neuroglobin. Implications for the multiple ligand binding steps. J Biol Chem. 2004;279:5886–5893. doi: 10.1074/jbc.M311748200. [DOI] [PubMed] [Google Scholar]
- 26.Fago A, Hundahl C, Malte H, Weber RE. Functional properties of neuroglobin and cytoglobin. Insights into the ancestral physiological roles of globins. IUBMB Life. 2004;56:689–696. doi: 10.1080/15216540500037299. [DOI] [PubMed] [Google Scholar]
- 27.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76:839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 28.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 29.Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(iii) and Fe(ii)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- 30.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 31.Saito A, Maier CM, Narasimhan P, Nishi T, Song YS, Yu F, Liu J, Lee YS, Nito C, Kamada H, Dodd RL, Hsieh LB, Hassid B, Kim EE, Gonzalez M, Chan PH. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- 32.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Pinzon MA, Dave KR, Raval AP. Role of reactive oxygen species and protein kinase C in ischemic tolerance in the brain. Antioxid Redox Signal. 2005;7:1150–1157. doi: 10.1089/ars.2005.7.1150. [DOI] [PubMed] [Google Scholar]


