Abstract
BACKGROUND
Gonadotrophin releasing hormone (GnRH) analogues are widely used in IVF programmes as a method of suppressing the luteinizing hormone (LH) surge prior to ovarian stimulation, but their roles outside the pituitary remain relatively unknown. A 2002 Cochrane review (Al-Inany et al. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev 2006;3:CD001750) described lower pregnancy rates in women administered with GnRH antagonist, compared with those using an agonist, as part of an IVF programme, despite the fact that GnRH antagonist is a more effective repressor of LH. This study aimed to analyse the in-vitro effects of GnRH analogues on the decidualizing endometrium, blastocyst invasion and GnRH receptor expression in fertile women.
METHODS
We analysed the in-vitro decidualization capacity of endometrial stromal cells, derived from fertile women during the implantation window, in the presence of GnRH analogues. The influence of GnRH analogues on GnRH receptor expression and blastocyst invasion was assessed by in-vitro assays of biomedical marker secretion, immunoblots and blastocyst attachment to the stromal extracellular matrix.
RESULTS
We demonstrate that, at the concentrations and time periods used, GnRH analogues did not significantly influence the extent of decidualization of endometrial stromal cells. In addition, no adverse effect of GnRH analogues was seen on human blastocyst invasion.
CONCLUSIONS
We suggest that GnRH analogues affect neither the capacity of the endometrium to support invasion nor the invasive potential of the blastocyst in the early stages of implantation.
Keywords: GnRH analogues, blastocyst invasion, endometrium
Introduction
The use of a human gonadotrophin releasing hormone (GnRH) agonist to prevent the premature release of pituitary luteinizing hormone (LH) during controlled ovarian stimulation in in-vitro fertilization (IVF) is well established. More recently the use of a GnRH antagonist that induces a rapid decrease in LH and follicle stimulating hormone thus preventing and interrupting LH surges is advocated. The GnRH antagonist, unlike the agonist, does not require a desensitization period and thus can be used in the late follicular phase. This avoidance of a desensitization period and thus a much shorter IVF ‘cycle’ is of obvious patient advantage.
Early reports have indicated that implantation rates are lower in IVF cycles compared with natural cycles (Macklon and Fauser, 2000) and that ovarian stimulation therapy alters the endometrial receptivity (Bourgain and Devroey, 2003). Furthermore, a recent Cochrane review demonstrated significantly fewer clinical pregnancies in patients treated with a GnRH antagonist compared with patients treated with a GnRH agonist (Al-Inany et al., 2006).
Both GnRH and GnRH receptors (GnRH-R) have been shown to be present in preimplantation embryos and the fallopian tubes at both messenger ribonucleic acid (mRNA) and protein levels (Casan et al., 2000). Two forms of GnRH-R (GnRH-R I and GnRH-R II) appear to exist and have functional roles in multiple and diverse extra-pituitary mammalian tissues and cells (Cheng and Leung, 2005), including the endometrium. GnRH can modulate matrix metalloproteinases and their endogenous inhibitors, tissue-specific inhibitors, both of which play a key role in cyclic remodelling events in the human endometrium in preparation for implantation (Chou et al., 2003). GnRH agonists induce apoptosis in endometrial cells in vitro (Meresman et al., 2003), and GnRH-R have also been shown to be present in the endometrium with highest levels reported in the secretory phase (Shemesh, 2001) followed by a decline in GnRH-R numbers in the decidua (Takeuchi et al., 1998).
Thus GnRH analogues administered during IVF have the potential to act on the endometrium and on the embryo during the very early stages of implantation via GnRH-R. Here we have investigated the effect of GnRH analogues, buserelin and Cetrotide, on the capacity of endometrial stromal cells to undergo decidualization, and on trophoblast invasion of human embryos in an in-vitro model.
Materials and Methods
Tissue collection and endometrial stromal cell culture
Endometrium was obtained at day 20–24 of the menstrual cycle from fertile women (n = 6) aged 32–44 years; the samples were obtained by pipelle biopsy from women undergoing diagnostic laparoscopy or sterilization or by endometrial curettage of the bisected uteri obtained at hysterectomy for benign indications. None of the women had received hormonal medication in the preceding 3 months. Endometrial stromal cells were isolated by collagenase digestion, purified through a percoll gradient and maintained in Dulbecco's modified essential medium (DMEM; Invitrogen, Paisley, UK) supplemented with 10% heat-inactivated fetal bovine serum (Autogen Bioclear Ltd, Calne, UK) and 50 IU/ml–50 µg/ml penicillin–streptomycin (Sigma, Poole, UK, DMEM complete) at 37°C in a humidified environment with 5% CO2 in air as described previously (Carver et al., 2003). Stromal cells were used between passages 2 and 6. They were 98% pure as assessed by Thy-1 and vimentin expression, with <2% contaminating epithelial and bone-marrow-derived cells, and they retained estrogen and progesterone receptors and CD10 expression in culture (Klemmt et al., 2006).
Treatments with GnRH analogues
In the experiments below, cells were treated with or without 2 µM cetrorelix acetate (GnRH antagonist, Cetrotide, Serono, Feltham, UK) or 0.1 µM buserelin acetate (GnRH agonist, Suprefact, Aventis Pharma, Frankfurt, Germany), generally for 2 or 3 days. The concentrations of the GnRH analogues were based on the physiological plasma serum levels after administration. The rational for the time periods was that decidualization initally peaks at day 3 and the embryo invasion assay could also be used at day 3.
In-vitro decidualization of endometrial stromal cells
Subconfluent endometrial stromal cells were seeded at 0.5 × 105 cells per well in duplicate into four-well plates in DMEM complete medium and cultured until confluent. Decidualization was induced by adding phenol red-free DMEM/F12 (Invitrogen, Paisley, UK) supplemented with 50 IU/ml–50 µg/ml penicillin–streptomycin, 10% FCS and 0.5 mM 8-Bromoadenosine 3′:5′-cyclic Monophosphate (8-Br-cAMP; Sigma), with or without 2 µM cetrorelix acetate or 0.1 µM buserelin acetate. The media were changed every 3 days. The culture supernatants were collected, centrifuged and stored at −20°C. The amount of total protein in the cell lysate was measured using the Coomassie Plus assay (Pierce Ltd, Cramlington, UK), and the amounts of prolactin (PRL) and insulin-like growth factor-binding protein-1 (IGFBP-1) in the supernatants were measured with the PRL Immunolite Kit (DPC Ltd, UK) and DuoSet ELISA Kit (R&D Systems, Abingdon, UK), respectively, according to the manufacturers' instructions. Levels of secreted PRL and IGFBP-1 were normalized to the amount of total protein present in each well and values were expressed as ng ± SEM/100 µg total protein. The detection levels of PRL and IGFBP-1 were 0.5 ng/ml and 60 ρg/ml, respectively.
Immunocytochemistry
Cultures of endometrial stromal cells seeded onto 13 mm glass coverslips (Chance Proper Ltd, Birmingham, UK) size 0 were decidualized for 3 days with or without the addition of either 2 µM cetrorelix acetate or 0.1 µM buserelin acetate as described above, and then fixed and stained by the use of immunofluorescent techniques as described previously (Chobotova et al., 2002). Specific antigens were detected by incubation with 10 µg/ml mouse anti-GnRH-R (clone GNRH03, LabVision, UK) followed by 15 µg/ml of donkey anti-mouse fluorescein isothiocyanate-conjugated IgG (Jackson ImmuoResearch Laboratories, Inc., West Grove, PA, USA) antibody. Mouse IgG was used in negative controls as appropriate. Coverslips were mounted in Vectashield medium with DAPI (Vector Laboratories, Peterborough, UK). The staining was observed using a Leitz DMRBE microscope (Leica Corp., Wetzlar, Germany) and Openlab imaging software (Improvision, Coventry, UK).
Immunoblotting
Confluent stromal cell cultures in six well plates were decidualized as described above with or without the addition of either 2 µM cetrorelix acetate or 0.1 µM buserelin acetate for 3 days. The plates were placed on ice and washed twice with ice-cold PBS. Cells were lysed for 30 min in 1× Triton lysis buffer (10 mM Tris–HCL pH 8.0, 2.5 mM MgCl2, 5 mM EGTA pH 8.0, 0.5% Triton X-100) supplemented with phosphatase inhibitors and protease complete before scraping. The cell lysates were clarified by centrifugation. The total concentration of protein in each lysate was measured using a BioRad DC Protein Assay Kit (BioRad, UK) according to the manufacturer's instructions. Proteins (10 µg/sample) were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane (BioRad, Hemel Hempstead, UK). GnRH-R was detected by incubation in 2 µg/ml antibody clone GNRH03 (LabVision, UK) and β-actin, for a loading control, with 80 ng/ml antibody clone mAbcam 8226 (Abcam, Cambridge, UK). Bound antibodies were detected by incubation with a 1 in 2500 dilution of sheep anti-mouse IgG (Sigma, Poole, UK) conjugated with HRP, with the use of ECL (Amersham Biosciences, UK) according to the manufacturers' instructions. The densities of the GnRH-R bands were normalized to the densities of the loading control β-actin bands and expressed as arbitrary units GnRH-R.
Embryo collection and culture
The experiments were performed with ethical approval of the Oxfordshire Research Ethics Committee and the Human Fertilization and Embryology Authority. Embryos were donated for research with informed consent from patients attending the Oxford Fertility Unit, John Radcliffe Hospital. The embryos were generated following a long protocol, controlled, ovarian-stimulated IVF cycle. Down-regulation was achieved using Synarel® (Nafarelin Acetate, Pfizer, UK) with controlled ovarian stimulation using Gonal-f® (follitropin alfa, Serono, Feltham, UK) injection. Embryos were cultured in groups with 15 µl BlastAssist (MediCult, Surrey, UK), overlaid with 2.5 ml light paraffin oil (MediCult, Surrey, UK) and maintained in culture. Assisted hatching of embryos that reached the blastocyst stage (day 6 post-insemination) was performed if required by incubation in acidified Tyrodes solution (Sigma, Poole, UK) to remove the zona pellucida. Expanded, hatched blastocysts were transferred onto endometrial stromal cell cultures.
Embryo co-culture assays
Human blastocysts were transferred onto pre-decidualized endometrial stromal cells from fertile women (coverslips) and cultured in 500 µl of DMEM supplemented with 10% FCS and either 2 µM cetrorelix acetate or 0.1 µM buserelin acetate for 48 h. The blastocysts attached to the stromal cell layer were fixed and the degree of trophoblast invasion and spreading into the endometrial stromal cell monolayer was assessed. Secretion of hCG by blastocysts into the culture medium was determined using the hCG Immunolite Kit (DPC Ltd, UK) according to the manufacturer's instructions. The hCG production by each embryo was expressed in mIU/ml.
Statistical analyses
The data were assessed by one-way analysis of variance (ANOVA) followed by either Tukey's Multiple Comparison post test or Dunnett's Multiple post test where applicable. Differences with an α level of <0.05 were considered significant.
Results
The GnRH analogues, cetrorelix acetate and buserilin, have no adverse effect on in-vitro decidualization
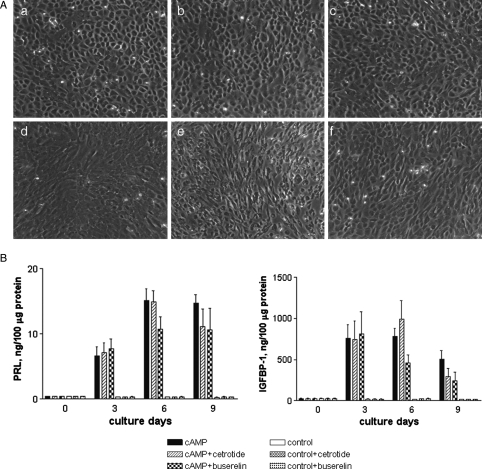
We investigated the potential influence of GnRH analogues on endometrial stromal cell decidualization capacity prior to embryo implantation. Phase-contrast microscopy (Fig. 1A) confirmed that 8 Br-cAMP induced the characteristic change in morphology from non-differentiated bipolar fibroblasts to polygonal pre-decidual endometrial stromal cells in cultures in the presence or absence of either analogue. The presence of GnRH analogues thus did not appear to affect the decidual reaction, nor did either compound induce decidualization in the absence of cAMP.
Figure 1.
(A) Morphology of in-vitro decidualized endometrial stromal cells from fertile women treated with (a–c) or without (d–f) 0.5 mM of 8 Br-cAMP for 3 days in the presence of either cetrorelix acetate (b, e) or buserelin acetate (c, f).
Endometrial stromal cells underwent morphological changes from bipolar fibroblasts into polygonal decidual cells in the presence of 8 Br-cAMP while untreated cells retained a fibroblast-like, spindle shape appearance irrespective of the presence of GnRH analogues. (B) Time-dependent secretion of PRL and IGFBP-1 in endometrial stromal cells from fertile women (n = 3) in response to 0.5 mM of 8-Br-cAMP for 9 days with or without GnRH analogues. The PRL and IGFBP-1 secretion by stromal cells into supernatants was measured every 3 days and normalized to total protein contents. The presence of GnRH analogues neither hindered the decidual reaction nor did it induce decidualization in the absence of 8 Br-cAMP. Magnification ×20.
The secretion of the biochemical markers PRL and IGFBP-1 (as quantitative indicators of decidualization) into supernatants of 8 Br-cAMP treated cells compared with cells in the absence of 8 Br-cAMP is higher from day 3 onwards (at day 6: 15.2 ± 4.9 and 782.9 ± 179.5 ng/100 µg protein, respectively; Fig. 1B). The secretion of PRL and IGFBP-1 was similar in the presence of the GnRH analogues compared with 8 Br-cAMP alone. The GnRH analogues in the absence of 8 Br-cAMP did not induce differentiation. From these results, we conclude that GnRH analogues (antagonist or agonist) had no significant adverse, or otherwise, effect on decidualization of endometrial stromal cells.
GnRH analogues do not influence levels of GnRH-R on stromal cells
Since levels of endometrial GnRH-R have been reported to change during the menstrual cycle and in decidual tissue (Takeuchi et al., 1998; Shemesh, 2001), we investigated the levels of GnRH-R in non-decidualized and decidualized endometrial stromal cells. Both undifferentiated and decidualized endometrial stromal cells from fertile women expressed GnRH-R (Fig. 2A). Addition of either of the GnRH analogues did not appear to alter GnRH-R expression as detected by immunocytochemistry.
Figure 2.
(A) Endometrial stromal cells from fertile women (n = 3) were grown on glass coverslips to confluence followed by incubation with (a–c) or without (d–f) 8 Br-cAMP for 3 days in the presence of either cetrorelix acetate (b, e) or buserelin acetate (c, f) and stained for the GnRH receptor using immunofluorescence.
GnRH-R was expressed in the cytoplasm and cell membrane in both 8 Br-cAMP treated and untreated cells irrespective of the presence of GnRH analogues. (B) Immunoblots (10 µg cell lysate per lane) of endometrial stromal cells (lane 1–6) were probed with 2 µg/ml GnRH-R (clone GNRH03). Cell extracts from the T47D cell line was used as a positive control. (C) The densities of the bands corresponding to GnRH-R (n = 3) were normalized to those of the β-actin loading control bands and expressed as arbitrary units GnRH-R. Magnification ×20.
Immunoblotting was performed to quantify the levels of GnRH-R in decidualized endometrial stromal cells: a representative Western blot is shown in Fig. 2B and the histogram combining data for all three cell lines is shown in Fig. 2C. The cells contained approximately 2-fold higher levels of GnRH-R in the presence of 8 Br-cAMP compared with non-decidualized cells (Fig. 2C). The addition of either GnRH analogue did not alter the expression level of GnRH-R.
GnRH analogues have no detrimental effect on trophoblast invasion
Implantation studies in rodent models have indicated that treatment with a GnRH antagonist reduces embryo development and the number of implantation sites, in contrast to GnRH agonist treatment which promotes embryo development and implantation rates (Kawamura et al., 2005; Torres Mde et al., 2005). We analysed the effects of GnRH analogue treatment on early human embryo implantation events using an in-vitro human implantation model (Carver et al., 2003). Embryos cultured in the presence of buserelin (n = 9), or Cetrolix acetate (n = 9) or those receiving no treatment (n = 10) all attached to the cell layer, and showed a similar degree of invasion and trophoblast spreading (Table I). The morphology of the invaded and spread blastocysts in the presence of GnRH analogues compared with that of untreated blastocysts is shown in Fig. 3A. The range and mean values of the levels of hCG, a marker of trophoblast development, secreted by the implanting blastocysts were similar for the three experimental groups (Fig. 3B).
Table I.
Effect of GnRH analogues on implantation after 48 h
| Treatment | Attached | Spread (+) | Spread (++) | Detached |
|---|---|---|---|---|
| Buserelin, 0.1 µM | 8/9 | 2/9 | 6/9 | 1/9 |
| Cetrorelix acetate, 2 µM | 6/9 | 2/9 | 4/9 | 3/9 |
| No treatment | 7/10 | 1/10 | 6/10 | 3/10 |
Figure 3.
(A) Blastocysts were transferred onto pre-decidualized endometrial stromal cells derived from fertile women in the presence of either 0.1 µM buserelin (a) or 2 µM cetrorelix acetate (b) for 48 h.
The presence of GnRH analogues had no detrimental effect on blastocyst attachment and spreading when compared with those in the absence of GnRH analogues (c). The dotted lines indicate the area of trophoblast spreading. (B) Levels of hCG in supernatants from control, buserilin or cetrotide co-cultures. Magnification ×20.
Discussion
The use of a GnRH antagonist for controlled ovarian stimulation in IVF is desirable, however, the small but significant decrease in the clinical pregnancy rate in these patients suggests that this treatment may have adverse effects on embryo implantation. In this study, we have investigated the effects of a GnRH agonist and a GnRH antagonist on endometrial decidualization and invasion of the endometrial stroma by the post-implantation early embryo.
Results from rodent models indicate that GnRH and its analogues play an important role in the development of peri-implantation embryos and influence implantation rates (Kawamura et al., 2005; Torres Mde et al., 2005). It was reported that GnRH agonists were potent promoters of embryo development and implantation while GnRH antagonists had detrimental effects on both. Here we have shown that the addition of physiologically relevant serum concentrations of GnRH analogues at the time of implantation in-vitro does not affect the extent of trophoblast invasion into stromal cells in day 6–9 embryos. Our experiments, however, do not exclude the possibility that detrimental effects of these treatments might occur at earlier or later stages of embryo development and the implantation process. Clinical studies suggest that the use of GnRH analogues in IVF cycles cause luteal phase defects (Bourgain and Devroey, 2003).
We demonstrated that, at the concentrations used, GnRH analogues do not compromise the degree of endometrial stromal cell differentiation in our system. Previously it has been shown that GnRH-R levels are higher in endometrial epithelium compared with stromal compartments at the time of implantation (Shemesh, 2001). However, our data show clearly that GnRH-R is localized to the cell membrane in endometrial stromal cells, and furthermore that levels of GnRH-R increase with decidualization.
Three possible explanations for the decrease in clinical pregnancy following the use of a GnRH antagonist are that there is (i) an adverse effect on the autocrine and paracrine regulation of folliculogenesis (Choi et al., 2006; Ferrari et al., 2006); (ii) a profound suppression of endogenous LH production (Kolibianakis et al., 2006); or (iii) a direct effect on the endometrium or on the embryo (Zikopoulos et al., 2004; Papanikolaou et al., 2006). This lead us to investigate this third possibility, that a GnRH analogue has an effect on the endometrium or the embryo or both, during early implantation events.
A reduction of clinical pregnancies at high doses of a GnRH antagonist has been reported in an early study of the effect of increasing doses of the antagonist. However, this reduction was not observed in the subsequent frozen embryo replacement cycle for embryos generated with the use of a high dose GnRH analogue (Kol et al., 1999). This is consistent with the fact that when the GnRH analogue is absent, there is no adverse effect on the endometrium in unstimulated, frozen cycles. However, if there was an adverse effect on the embryo (generated in a GnRH analogue-stimulated cycle), it would have been likely to persist in the unstimulated, frozen cycle. Furthermore, there is evidence to suggest that the clinical pregnancy rate is lower, and first trimester pregnancy loss is greater, following cycles using high doses of GnRH agonist in the GnRH antagonist ovarian stimulation protocol (Greisinger et al., 2006).
Early implantation is a complex process involving many cellular events and signalling cascades, and disruption of any one of these events could result in inhibition of the implantation process. We conclude from the data we report here, that at the concentrations and time-periods used, GnRH analogues, agonist or antagonist, have no detrimental effect on the specific events of trophoblast invasion and stromal cell decidualization in vitro. In addition, levels of GnRH-R are elevated in decidualized stromal cells suggesting a function for the GnRH–GnRH-R pathway in these early implantation events.
Funding
This research was funded by The Medical Research Council, the Wellcome Trust and Serono.
Acknowledgements
We are extremely grateful to the patients, Karen Turner and the staff of the Oxford Infertility Clinic, John Radcliffe Hospital, for their valuable contribution to this work.
References
- Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;3:CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- Bourgain C, Devroey P. The endometrium in stimulated cycles for IVF. Hum Reprod Update. 2003;9:515–522. doi: 10.1093/humupd/dmg045. [DOI] [PubMed] [Google Scholar]
- Carver J, Martin K, Spyropoulou I, Barlow D, Sargent I, Mardon H. An in-vitro model for stromal invasion during implantation of the human blastocyst. Hum Reprod. 2003;18:283–290. doi: 10.1093/humrep/deg072. [DOI] [PubMed] [Google Scholar]
- Casan EM, Raga F, Bonilla-Musoles F, Polan ML. Human oviductal gonadotropin-releasing hormone: possible implications in fertilization, early embryonic development, and implantation. J Clin Endocrinol Metab. 2000;85:1377–1381. doi: 10.1210/jcem.85.4.6503. [DOI] [PubMed] [Google Scholar]
- Cheng CK, Leung PC. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr Rev. 2005;26:283–306. doi: 10.1210/er.2003-0039. [DOI] [PubMed] [Google Scholar]
- Chobotova K, Muchmore ME, Carver J, Yoo HJ, Manek S, Gullick WJ, Barlow DH, Mardon HJ. The mitogenic potential of heparin-binding epidermal growth factor in the human endometrium is mediated by the epidermal growth factor receptor and is modulated by tumor necrosis factor-alpha. J Clin Endocrinol Metab. 2002;87:5769–5777. doi: 10.1210/jc.2002-020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Ku SY, Jee BC, Suh CS, Choi YM, Kim JG, Moon SY, Kim SH. Comparison of follicular fluid IGF-I, IGF-II, IGFBP-3, IGFBP-4 and PAPP-A concentrations and their ratios between GnRH agonist and GnRH antagonist protocols for controlled ovarian stimulation in IVF-embryo transfer patients. Hum Reprod. 2006;21:2015–2021. doi: 10.1093/humrep/del091. [DOI] [PubMed] [Google Scholar]
- Chou CS, Tai CJ, MacCalman CD, Leung PC. Dose-dependent effects of gonadotropin releasing hormone on matrix metalloproteinase (MMP)-2, and MMP-9 and tissue specific inhibitor of metalloproteinases-1 messenger ribonucleic acid levels in human decidual Stromal cells in vitro. J Clin Endocrinol Metab. 2003;88:680–688. doi: 10.1210/jc.2002-021277. [DOI] [PubMed] [Google Scholar]
- Ferrari B, Pezzuto A, Barusi L, Coppola F. Follicular fluid vascular endothelial growth factor concentrations are increased during GnRH antagonist/FSH ovarian stimulation cycles. Eur J Obstet Gynecol Reprod Biol. 2006;124:70–76. doi: 10.1016/j.ejogrb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Greisinger G, Diedrich K, Devroey P, Kolibinianakis EM. GnRH agonist for triggering final oocyte maturation in the GnRH antagonist ovarian hyperstimulation protocol: a systematic review and meta- analysis. Hum Reprod Update. 2006;12:159–168. doi: 10.1093/humupd/dmi045. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Fukuda J, Kumagai J, Shimizu Y, Kodama H, Nakamura A, Tanaka T. Gonadotropin-releasing hormone I analog acts as an antiapoptotic factor in mouse blastocysts. Endocrinology. 2005;146:4105–4116. doi: 10.1210/en.2004-1646. [DOI] [PubMed] [Google Scholar]
- Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril. 2006;85:564–572. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol S, Lightman A, Hillensjo T, Devroey P, Fauser B, Tarlatzis B, Mannaerts B, Itskovitz-Eldor J. High doses of gonadotrophin-releasing hormone antagonist in in-vitro fertilization cycles do not adversely affect the outcome of subsequent freeze-thaw cycles. Hum Reprod. 1999;14:2242–2244. doi: 10.1093/humrep/14.9.2242. [DOI] [PubMed] [Google Scholar]
- Kolibianakis EM, Collins J, Tarlatzis B, Papanikolaou E, Devroey P. Are endogenous LH levels during ovarian stimulation for IVF using GnRH analogues associated with the probability of ongoing pregnancy? A systematic review. Hum Reprod Update. 2006;12:3–12. doi: 10.1093/humupd/dmi030. [DOI] [PubMed] [Google Scholar]
- Macklon NS, Fauser BC. Impact of ovarian hyperstimulation on the luteal phase. J Reprod Fertil Suppl. 2000;55:101–108. [PubMed] [Google Scholar]
- Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Baranao RI. Effect of GnRH analogues on apoptosis and release of interleukin-1beta and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Hum Reprod. 2003;18:1767–1771. doi: 10.1093/humrep/deg356. [DOI] [PubMed] [Google Scholar]
- Papanikolaou EG, Camus M, Fatemi HM, Tournaye H, Verheyen G, Van Steirteghem A, Devroey P. Early pregnancy loss is significantly higher after day 3 single embryo transfer than after day 5 single blastocyst transfer in GnRH antagonist stimulated IVF cycles. Reprod Biomed Online. 2006;12:60–65. doi: 10.1016/s1472-6483(10)60981-9. [DOI] [PubMed] [Google Scholar]
- Shemesh M. Actions of gonadotrophins on the uterus. Reproduction. 2001;121:835–842. doi: 10.1530/rep.0.1210835. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Futamura N, Minoura H, Toyoda N. Possible direct effect of gonadotropin releasing hormone on human endometrium and decidua. Life Sci. 1998;62:1187–1194. doi: 10.1016/s0024-3205(98)00045-9. [DOI] [PubMed] [Google Scholar]
- Torres Mde M, Donadio N, Donadio NF, Brandao AC, Heck B. Comparison of embryo implantation in Wistar rats that underwent ovarian stimulation using exogenous gonadotropins associated with cetrorelix acetate or leuprolide acetate. Fertil Steril. 2005;84:1235–1240. doi: 10.1016/j.fertnstert.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Zikopoulos K, Kolibianakis EM, Camus M, Tournaye H, Van den Abbeel E, Joris H, Van Steirteghem A, Devroey P. Duration of gonadotropin-releasing hormone antagonist administration does not affect the outcome of subsequent frozen-thawed cycles. Fertil Steril. 2004;81:473–475. doi: 10.1016/j.fertnstert.2003.08.009. [DOI] [PubMed] [Google Scholar]