Abstract
BACKGROUND
Fetal cells are shed from the regressing chorionic villi and it is possible to retrieve extravillous cytotrophoblast cells by transcervical sampling. The abundance of trophoblast cells in transcervical samples suggests that this non-invasive approach could distinguish between normal and abnormal pregnancies, such as an ectopic pregnancy (EP) and blighted ovum (BO). We aim to identify and quantify fetal trophoblast cells in the cervical canal during the first trimester to assess their usefulness to predict an abnormal pregnancy.
METHODS
Patients, age 18–45, presenting with a normal intrauterine pregnancy (IUP; n = 37), diagnosis of EP (n = 10) or BO (n = 5) were enrolled for collection of transcervical specimens using a cytobrush and fixative rinse. Non-pregnant, nulliparous women (n = 7) were included as negative controls. Cells were cleared of mucus by acidification, prepared on microscope slides and labeled with a monoclonal antibody recognizing the trophoblast marker, human leukocyte antigen (HLA)-G. HLA-G positive and negative cells were counted to calculate the ratio of trophoblast cells to total cervical cells.
RESULTS
Trophoblast cells were observed in 35/37 normal IUP, 6/10 EP and 4/5 BO specimens. The average frequency of HLA-G positive cells in the normal IUP cervical samples was ∼1 in 2000, which was 4-fold higher than samples from patients with EP or BO (P < 0.001). Receiver operating characteristic analysis showed that EP and BO pregnancies were distinguishable from normal pregnancies with 93% sensitivity, 95% specificity, 97% positive predictive value and 87% negative predictive value.
CONCLUSIONS
This pilot study presents evidence that trophoblast cells can be reliably obtained and identified among cervical cells in the first trimester by immunohistochemical staining for HLA-G, and suggests for the first time that abnormal pregnancies may be predictable based on the abundance of trophoblast cells in the cervical canal.
Keywords: trophoblast, cervix, first trimester, ectopic pregnancy, blighted ovum
Introduction
Fetal cells are shed from the regressing chorionic villi into the lower uterine pole and accumulate behind the cervical mucus at the level of the internal os (Bulmer et al., 1995). It is also possible to retrieve extravillous cytotrophoblast cells by transcervical sampling (Bulmer et al., 2003). Transcervical sampling and collection of the maternal mucus by aspiration, lavage or cytological brush has resulted in the identification of fetal cells with a success rate that varies from 40 to 90% (Adinolfi et al., 1995; Rodeck et al., 1995; Tutschek et al., 1995; Massari et al., 1996; Miller et al., 1999; Fejgin et al., 2001; Bussani et al., 2002; Cioni et al., 2003). This disparity in frequency could be due to the gestational age, method of collection, operator variability, detection sensitivity and status of pregnancy (normal versus abnormal). Historically, the identification of fetal cells has been accomplished using several approaches. The first method uses a combination of morphological identification and micromanipulation to isolate cell clumps that appear trophoblast-like (Tutschek et al., 1995; Sherlock et al., 1997; Bussani et al., 2002, 2007). The second and most recent method involves the use of monoclonal antibody G233 (anti-HLA-G) and laser microdissection to detect extravillous cytotrophoblast cellular elements (Bulmer et al., 2003). Other trophoblast markers have been used, including various cytokeratins and antigens expressed by villous and extravillous trophoblast cells (Bulmer et al., 1995; Miller et al., 1999; Katz-Jaffe et al., 2005). There is agreement that among cytokeratin subtypes, cytokeratin 7 is most useful as a marker for all human trophoblast phenotypes (Shiverick et al., 2001).
The isolation of fetal cells by transcervical sampling has been suggested as a non-invasive procedure for prenatal diagnosis of chromosomal abnormalities in fetuses and the standardization of this clinical tool is still under investigation. However, no attempt has been made to use trophoblast-enriched mucus in the endocervix during the first trimester for the diagnosis of obstetric abnormalities beyond genetic problems. The abundance of trophoblast cells in transcervical samples and their identification by immunohistochemical staining for HLA-G suggests that this non-invasive approach could distinguish between normal and abnormal pregnancies, such as an ectopic pregnancy (EP) or blighted ovum (BO).
EP complicates about 2% of all pregnancies (Ventura et al., 2008) and occurs when the developing blastocyst implants at a site other than the fundus of the uterine cavity, most commonly in the fallopian tube. The diagnosis of an EP is supported by clinical, laboratory and ultrasonographic findings. We hypothesized that the number of trophoblast cells in transcervical sampling from patients with an EP or BO, if present, should be significantly lower than those found in normal intrauterine pregnancies (IUPs), depending on the exact location of the EP or mass of the BO conceptus.
In this report, we demonstrate a non-invasive procedure involving the collection, identification and quantification of trophoblast cells from maternal cervical mucus in the first trimester to compare the number of trophoblast cells obtained from normal pregnancies with those obtained from women with a tubal EP or BO.
Materials and Methods
Patient selection and transcervical sampling
With approval from the Wayne State University Human Investigation Committee, written informed consent was obtained from the participating women. Patients, age 18–45, presenting at our obstetrics clinic for early prenatal care with a normal IUP (n = 37), or at the emergency room with a diagnosis of tubal EP (n = 10) or BO (n = 5), as well as non-pregnant, nulliparous women (n = 7) undergoing evaluation and treatment for primary infertility in our reproductive endocrinology and infertility clinic were enrolled for collection of transcervical specimens using a ThinPrep® kit (Hologic Inc., Marlborough, MA). Patients were placed in the lithotomy position and a speculum was introduced into the vagina to expose the cervix. Approximately 2 cm of a cytological brush was introduced into the endocervical canal and rotated 360° as it was withdrawn to collect the specimen. If cervical mucus was not obtained with the first attempt, the cytobrush was reintroduced to collect the dislodged mucus. Contact with the cervical wall was reduced to minimize maternal contamination and avoid bleeding and collection of excess epithelial cells.
For those women presenting for their first prenatal visit, gestational age was estimated, based on the first day of their last menstrual period, and they received a routine papanicolaou (PAP) test and a bedside ultrasound. Patients with EP were diagnosed according to clinical symptoms and ultrasonographic findings of no IUP with or without an adnexa mass. These were subsequently confirmed by laparoscopy in nine cases that were managed surgically; the 10th case was managed medically. BO was diagnosed according to clinical symptoms, ultrasonographic findings of an intrauterine gestational sac without fetal pole and beta human chorionic gonadotrophin levels of ≥5000 mIU/ml.
Specimen processing
Cells were rinsed from the cytological brush into 20 ml PreservCyt® (Hologic) fixative solution immediately after removal from the cervix. Specimens were transported to the laboratory for processing within 6 h of collection. Laboratory personnel were blinded to the diagnosis of each sample. The specimens were cleared of mucus by acidification with 600 µl of 3% acetic acid. After a 10 min incubation, the cells were washed three times by centrifugation at 190 g and resuspension in phosphate buffered saline (PBS). The sample was brought to 0.05% sodium azide in a volume of 10 ml and stored at 4°C.
Immunohistochemistry
A 1 ml aliquot of each processed specimen was prepared for microscopy using a Shandon Cytoslide® and Cytofunnel® to centrifuge the cells at 134 g for 10 min in a Shandon Cytospin® 3 centrifuge (Thermo Shandon Inc., Pittsburgh, PA). Approximately 50 000–200 000 cells were distributed over a 1 cm diameter area of the slides. Slides were labeled with 10 µg/ml of monoclonal antibody G233 (Exbio, Prague, Czech Republic) recognizing human HLA-G (Loke et al., 1997) using a DAKO (Carpinteria, CA) autostainer universal staining system. Anti-HLA-G binding was detected using an EnVision Systems Peroxidase kit from DAKO. A light hematoxylin stain was applied to counterstain the nuclei. Slides were viewed at 400× magnification using a Leica (Wetzlar, Germany) DM IRB inverted microscope and imaged with a SPOT Jr color digital camera (Diagnostic Research, Sterling Heights, MI).
For immunofluorescence microscopy, cells were centrigued and resuspended in 0.15 M glycine (pH 7.0) for 10 min, re-centrifuged and washed three more times with PBS. They were then incubated in suspension overnight at 4° with the G233 antibody and 5 µg/ml of rabbit polyclonal antibody against human cytokeratin 7 (Abcam, Cambridge, MA) before incubation at room temperature for 1 h in a combination of Alexa Fluor® 594 goat anti-mouse immunoglobulin G (IgG) and Alexa Fluor® 488 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA), each at 4 µg/ml. The cells were counterstained with 1 µg/ml 4′,6-diamidino-2-phenylindole, HCl (DAPI), mounted on slides with coverslips and viewed by epifluorescence microscopy. Images were captured with an ORCA digital camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan) and processed using simple PIC (Hamamatsu) image analysis software to pseudocolor and merge images. Substitution of non-immune IgG for either of the primary antibodies produced no detectable labeling using fluorescent or peroxidase-conjugated secondary antibody (data not shown).
Data analysis and statistics
All HLA-G positive cells on each slide were identified by brown 3,3-diaminobenzidine staining and manually counted at 200× magnification. The total number of nucleated cells present on each slide was estimated by counting three microscopic fields at 400× magnification (average of 55 cells/field) and the ratio of HLA-G positive cells to total cells was calculated (trophoblast frequency). Three slides were prepared from each specimen and analyzed to determine the mean trophoblast frequency. Data were analyzed using the SPSS version 13.0 (SPSS, Chicago, IL) statistics program. Because of a non-uniform distribution, data were compared using a non-parametric analysis by the Kruskal Wallis test with the Mann–Whitney post-hoc test. The predictive accuracy of the data was assessed using a receiver operating characteristic (ROC) analysis. The correlation between trophoblast frequency and gestational age was determined using a linear regression analysis separately for normal IUP and abnormal pregnancies (EP and BO).
Results
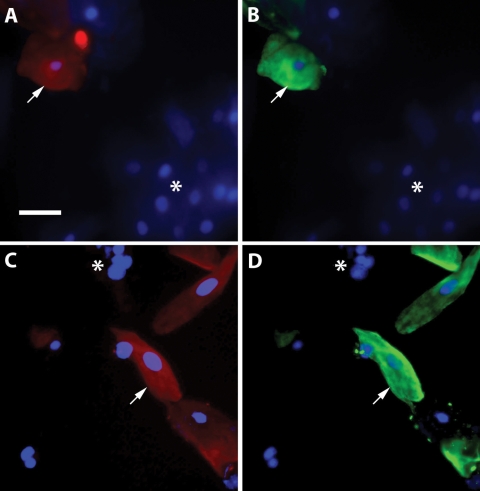
Cytotrophoblast cells were identified in cervical samples obtained from women during the first trimester of pregnancy using immunofluorescence microscopy with two antigenic markers, HLA-G and cytokeratin 7. Double labeled preparations revealed cytotrophoblast cells that were positively labeled by each antibody (Fig. 1, arrows) and groups of cervical cells that were non-reactive to both antibodies (Fig. 1, asterisks). Occasionally, cells expressing cytokeratin 7 were observed that were HLA-G negative (not shown), which may have represented syncytial fragments or a subpopulation of cervical cells. However, HLA-G positive cells were always also positive for cytokeratin 7. Therefore, HLA-G was used in subsequent analyses of cervical specimens to specifically identify cytotrophoblast cells.
Figure 1.
Identification of cytotrophoblast cells in cervical samples by immunofluorescence microscopy. Cervical cells were labeled with antibodies against HLA-G (A, C; red) and cytokeratin 7 (B, D; green), as well as with DAPI to stain nuclei (shown in all panels; blue). Cells were identified as cytotrophoblasts by their labeling for both HLA-G and cytokeratin (indicated with arrows). Resident cervical cells appear as DAPI stained cells that were not labeled by either antibody (indicated by asterisks). Bar, 50 µm.
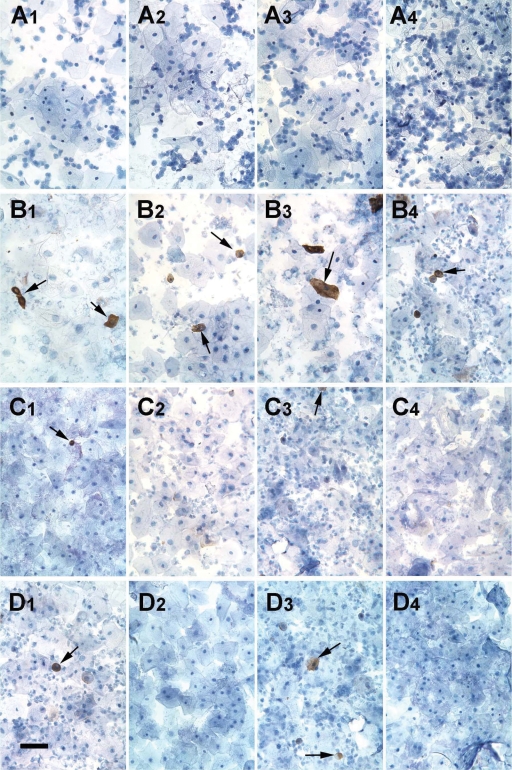
Immunohistochemical detection of HLA-G was performed on cervical cells from non-pregnant, nulliparous women or pregnant women during Weeks 6–14 of gestation. HLA-G positive cells were never observed in the specimens obtained from previously non-pregnant women (Fig. 2A), consistent with HLA-G being a specific marker for trophoblast cells. Cervical cells collected from women during early pregnancy contained large numbers of putative trophoblast cells that were HLA-G positive (Fig. 2B). However, HLA-G positive cells were relatively rare or absent in comparable samples from women with an EP (Fig. 2C) or a BO pregnancy (Fig. 2D). Trophoblast cells were observed in 35/37 (94.6%) of normal IUP, 6/10 (60%) of EP and 4/5 (80%) of BO specimens.
Figure 2.
Immunohistochemical detection of cytotrophoblast cells in cervical specimens labeled with anti-HLA-G. Slide preparations were made from cervical samples obtained from nulliparous women who were not pregnant (row A), as well as from women with normal intrauterine pregnancy (row B), tubal ectopic pregnancy (row C) or blighted ovum (row D). Each panel displays cells from a different patient. Antibody binding appears as a dark brown precipitate around HLA-G positive cells (indicated with arrows). Cells were counterstained with hematoxylin. Bar, 100 µm.
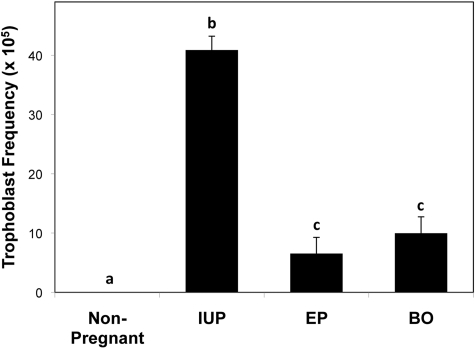
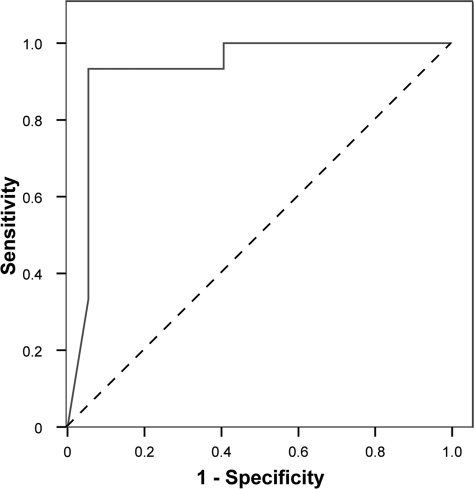
The frequency of HLA-G positive cells in the normal IUP cervical samples was ∼1 in 2000 cells, which was 4-fold higher (P < 0.001) than the average frequency in samples from patients with EP or BO (Fig. 3). The frequencies of HLA-G positive cells in the latter two groups were not significantly different. Specimens from non-pregnant women contained no HLA-G positive cells, which was significantly different from all other groups. Notably, even with the small number of subjects in this preliminary study, an ROC analysis revealed that normal IUP pregnancies were distinguishable from abnormal (EP and BO) pregnancies with 93% sensitivity and 95% specificity. The area under the ROC curve (Fig. 4) was 0.932 (P < 0.001). Using a cutoff value of 20.88 × 10−5, the positive predictive value for abnormal pregnancy was 97% and the negative predictive value was 87%.
Figure 3.
Frequency of trophoblast cells in cervical samples. Cervical specimens collected from non-pregnant women (n = 7) or pregnant women with a normal intrauterine pregnancy (IUP; n = 37), ectopic pregnancy (EP; n = 10) or blighted ovum (BO; n = 5) were labeled with antibody to HLA-G and counterstained with hematoxylin. The number of trophoblast cells per total cervical cells (trophoblast frequency) was determined and the means with SE (error bars) are shown for each group. Bars labeled with different letters are significantly different (P < 0.05).
Figure 4.
Receiver operating characteristic curve for the predictive value of endocervical trophoblast cell frequency. The ability of trophoblast cell frequency to predict a normal intrauterine pregnancy was tested against abnormal pregnancies (ectopic pregnancy or blighted ovum).
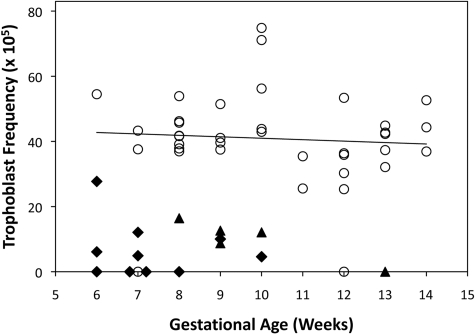
Among the pregnant women sampled in this study, the mean (range) gestational ages at the time of collection for normal IUP, EP and BO were 9 (6–14), 8 (6–10) and 10 (8–13) weeks, respectively. The trophoblast frequencies were unaffected by the gestational age in normal IUP between Weeks 6 and 14 (Fig. 5). The regression analysis of trophoblast frequency versus gestational age revealed non-significant Pearson correlation coefficients of −0.072 for normal IUP and −0.22 for combined EP and BO. With few exceptions, the range and distribution of values for normal IUP were well separated from abnormal (EP and BO) pregnancies (Fig. 5). Two normal IUP samples that contained no trophoblast cells were obtained at 7 and 12 weeks of gestation and both specimens contained lower numbers of total cells than all other normal IUP samples. There was also a single outlier among the EP samples with a trophoblast frequency of 27.7 × 10−5, which was just within the normal range. This was the only tubal EP positioned as cornual, according to the post-operative report. Of the specimens that contained trophoblast cells, the triplicate slide readings produced a greater coefficient of variation for EP and BO specimens (0.36) than for the normal IUP (0.14). The total number of cervical cells on the slides did not differ (P = 0.4) between the normal IUP group (70 350 ± 20 100) and the combined EP and BO samples (67 000 ± 21 300). Of the 37 normal IUP, 35 delivered successfully and one had an elective termination three weeks after her specimen was collected. Patients did not report vaginal bleeding in excess of that expected for a routine PAP test in the first trimester. However, one patient who had been seen three times at the emergency room for first trimester bleeding prior to testing experienced bleeding 4 days after the procedure and underwent a spontaneous miscarriage 3 days later.
Figure 5.
Influence of gestational age on the endocervical trophoblast cell frequency. Trophoblast frequencies for individual women with normal intrauterine pregnancy (open circles), ectopic pregnancy (closed diamonds) or blighted ovum (closed triangles) are shown with respect to the gestational age at the time of collection. A linear regression line drawn through the normal intrauterine pregnancy values is shown and had an R2 value of 0.005.
Discussion
The observed 95% trophoblast detection rate (35/37 in normal IUP) from transcervical collections obtained using the cytological brush suggests that this non-invasive technique is highly reliable for prenatal testing. The detection rates reported by other authors using similar methods are lower and inconsistent (Fejgin et al., 2001; Cioni et al., 2003, 2005). The variability among studies could be related to the methods used for cell collection, specimen processing or trophoblast identification. The present study employed a standardized kit for cervical cell procurement, a processing protocol that largely eliminated interfering mucus and a highly reliable and robust antibody marker for human trophoblast cells. Although HLA-G is expressed principally by the extravillous subpopulation of trophoblast cells (Chumbley et al., 1994; Loke et al., 1997), it is a highly selective marker that was verified by co-labeling with anti-cytokeratin 7 and not present in any cell populations of specimens obtained from non-pregnant, nulliparous women. The limitation of not detecting syncytial fragments using this antibody was offset by the absence of false identification of resident cervical cells. Indeed, the G233 antibody was reported to react with no human tissues other than extravillous trophoblast (Loke et al., 1997). Provision of molecular proof that the HLA-G positive cells are of fetal origin would bolster confidence in the reliability of this marker, but was outside the scope of this pilot study. However, Bulmer et al. (2003) used the same antibody to identify trophoblastic elements in transcervical samples and showed by laser capture microdissection and quantitative PCR that the isolated cells indeed contained fetal DNA markers.
We have quantified trophoblast cells in cervical samples collected from normal ongoing pregnancies not intended for termination. The ideal method for obtaining transcervical samples from pregnant women should have no negative impact on the ongoing pregnancy and be free from infectious or traumatic complications. It should also be simple to perform and cost effective, with minimal inter-observer variability. A number of techniques have been devised to retrieve transcervical samples from the endocervical canal and the endometrial cavity; these include smears obtained with cotton swabs or a cytological brush, aspiration of cervical mucus with a catheter, endometrial biopsy with a Pipelle and lavage of the endocervical canal or uterine cavity (Adinolfi et al., 1995; Rodeck et al., 1995; Tutschek et al., 1995; Massari et al., 1996; Miller et al., 1999; Fejgin et al., 2001; Bussani et al., 2002; Cioni et al., 2003). Although techniques like endocervical and intrauterine lavage have been reported to yield high numbers of trophoblast cells (ErgIn et al., 2001; Bussani et al., 2002; Bulmer et al., 2003; Cioni et al., 2005; Fang et al., 2005), these methods are more invasive and difficult to perform, with the possibility of harming the conceptus. Intrauterine lavage may preferentially retrieve villous trophoblast cells and syncytial fragments shed into the lower uterine pole, while trophoblast cells populating the cervix could be predominantly motile extravillous trophoblast cells. This may explain the consistent performance of the cytobrush in our study compared with intrauterine lavage (Bulmer et al., 2003) for obtaining HLA-G positive cytotrophoblast cells.
Examination of 37 women with normal IUP established that ∼1 in 2000 cells obtained from the endocervical canal expressed HLA-G. Variations in the size of HLA-G labeled cells were noted. Clumps of trophoblast cells have been reported previously in cervical specimens (Sherlock et al., 1997; Miller et al., 1999; Bussani et al., 2002), although the dispersion of mucus by acidification appears to have eliminated much of this problem. More commonly, labeled cells were found that appeared smaller than would be expected of trophoblast cells. These may represent fragmented trophoblast cells generated during slide preparation. No positively stained cells were found among seven non-pregnant nulliparous women, consistent with the assumption that HLA-G expressing cells are of fetal origin. However, two of the normal IUP specimens yielded no trophoblast cells. These samples were at the lower extreme (36 000–37 000) of the range in total cell number per slide, suggesting a possible error in the performance of the transcervical sampling rather than the absence of cervical trophoblast cells. Repeated analyses of these specimens with positive controls confirmed the negative results, refuting any failure in the immunohistochemical procedure. A low total cell count may, therefore, be indicative of suboptimal sample collection and is recommended as an exclusion criterion in the analysis of transcervical specimens for trophoblast frequency.
The abundance of trophoblast cells in the transcervical canal correlated to the pathological status of pregnancy. Specimens collected from patients with tubal EP or BO contained significantly fewer fetal cells. Abnormal embryonic implantation and development leading to an EP or BO reduced the trophoblast frequency by ∼4-fold. In a substantial number of abnormal pregnancies (20–40%), no trophoblast cells were found. The reduced amount of fetal tissue present in EP and BO and the location of the conceptus away from the uterine cavity in tubal EP likely contributed to the sharp reduction in the presence of endocervical trophoblast cells. Indeed, the one EP in a cornual location was distinguishable by an elevated trophoblast frequency compared with the other EP cervical specimens. An ROC analysis indicated that abnormal pregnancies could be distinguished from normal IUP with 93% sensitivity and 95% specificity. This pilot study included only a limited number of EP and BO specimens, constraining our ability to draw firm conclusions. Refinements in sample collection procedures and identification of inadequate specimens (e.g. based on total cell number) could further improve the predictive value of the method. While these specimens were obtained after diagnosis of the pathology by standard practices, it is possible that useful prognostic information could be obtained by determining the abundance of trophoblast cells in transcervical samples earlier in pregnancy. Prospective studies are required to confirm this view and develop strategies to address heterogeneity between patients and optimization of specimen procurement.
Based on this pilot study of 59 women, it appears that trophoblast cells can be reliably identified among cervical cells in the first trimester by immunohistochemical staining for HLA-G. This non-invasive procedure could provide a useful platform for prenatal diagnosis of pregnancy outcomes that could eventually be adapted for genetic analysis after further investigation to establish its efficacy. As a screen to distinguish between normal and abnormal pregnancies, direct comparison with current modalities, such as ultrasound and serum beta-human chorionic gonadotrophin concentration, are needed in a large clinical trial before the efficacy of this novel approach can be established.
Funding
The study was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health DHHS.
Acknowledgements
We are grateful to Mr Michael Kruger for help with statistical analysis and Drs Marcos Cordoba and Holly Yettaw for assistance with transcervical sample collection.
References
- Adinolfi M, Sherlock J, Tutschek B, Halder A, Delhanty J, Rodeck C. Detection of fetal cells in transcervical samples and prenatal diagnosis of chromosomal abnormalities. Prenat Diagn. 1995;15:943–949. doi: 10.1002/pd.1970151009. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Rodeck C, Adinolfi M. Immunohistochemical characterization of cells retrieved by transcervical sampling in early pregnancy. Prenat Diagn. 1995;15:1143–1153. doi: 10.1002/pd.1970151211. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Cioni R, Bussani C, Cirigliano V, Sole F, Costa C, Garcia P, Adinolfi M. HLA-G positive trophoblastic cells in transcervical samples and their isolation and analysis by laser microdissection and QF-PCR. Prenat Diagn. 2003;23:34–39. doi: 10.1002/pd.511. [DOI] [PubMed] [Google Scholar]
- Bussani C, Cioni R, Scarselli B, Barciulli F, Bucciantini S, Simi P, Fogli A, Scarselli G. Strategies for the isolation and detection of fetal cells in transcervical samples. Prenat Diagn. 2002;22:1098–1101. doi: 10.1002/pd.469. [DOI] [PubMed] [Google Scholar]
- Bussani C, Cioni R, Mattei A, Fambrini M, Marchionni M, Scarselli G. Prenatal diagnosis of common aneuploidies in transcervical samples using quantitative fluorescent-PCR analysis. Mol Diagn Ther. 2007;11:117–121. doi: 10.1007/BF03256231. [DOI] [PubMed] [Google Scholar]
- Chumbley G, King A, Gardner L, Howlett S, Holmes N, Loke YW. Generation of an antibody to HLA-G in transgenic mice and demonstration of the tissue reactivity of this antibody. J Reprod Immunol. 1994;27:173–186. doi: 10.1016/0165-0378(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Cioni R, Bussani C, Scarselli B, Bucciantini S, Barciulli F, Scarselli G. Fetal cells in cervical mucus in the first trimester of pregnancy. Prenat Diagn. 2003;23:168–171. doi: 10.1002/pd.562. [DOI] [PubMed] [Google Scholar]
- Cioni R, Bussani C, Scarselli B, Bucciantini S, Marchionni M, Scarselli G. Comparison of two techniques for transcervical cell sampling performed in the same study population. Prenat Diagn. 2005;25:198–202. doi: 10.1002/pd.1104. [DOI] [PubMed] [Google Scholar]
- ErgIn T, Baltaci V, Zeyneloglu HB, Duran EH, Ergenel IM, Batioglu S. Non-invasive early prenatal diagnosis using fluorescent in situ hybridization on transcervical cells: comparison of two different methods for retrieval. Eur J Obstet Gynecol Reprod Biol. 2001;95:37–41. doi: 10.1016/s0301-2115(00)00357-2. [DOI] [PubMed] [Google Scholar]
- Fang CN, Kan YY, Hsiao CC. Detection of fetal cells from transcervical mucus plug before first-trimester termination of pregnancy by cytokeratin-7 immunohistochemistry. J Obstet Gynaecol Res. 2005;31:500–507. doi: 10.1111/j.1447-0756.2005.00329.x. [DOI] [PubMed] [Google Scholar]
- Fejgin MD, Diukman R, Cotton Y, Weinstein G, Amiel A. Fetal cells in the uterine cervix: a source for early non-invasive prenatal diagnosis. Prenat Diagn. 2001;21:619–621. doi: 10.1002/pd.117. [DOI] [PubMed] [Google Scholar]
- Katz-Jaffe MG, Mantzaris D, Cram DS. DNA identification of fetal cells isolated from cervical mucus: potential for early non-invasive prenatal diagnosis. BJOG. 2005;112:595–600. doi: 10.1111/j.1471-0528.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- Loke YW, King A, Burrows T, Gardner L, Bowen M, Hiby S, Howlett S, Holmes N, Jacobs D. Evaluation of trophoblast HLA-G antigen with a specific monoclonal antibody. Tissue Antigens. 1997;50:135–146. doi: 10.1111/j.1399-0039.1997.tb02852.x. [DOI] [PubMed] [Google Scholar]
- Massari A, Novelli G, Colosimo A, Sangiuolo F, Palka G, Calabrese G, Camurri L, Ghirardini G, Milani G, Giorlandino C, et al. Non-invasive early prenatal molecular diagnosis using retrieved transcervical trophoblast cells. Hum Genet. 1996;97:150–155. doi: 10.1007/BF02265257. [DOI] [PubMed] [Google Scholar]
- Miller D, Briggs J, Rahman MS, Griffith-Jones M, Rane V, Everett M, Lilford RJ, Bulmer JN. Transcervical recovery of fetal cells from the lower uterine pole: reliability of recovery and histological/immunocytochemical analysis of recovered cell populations. Hum Reprod. 1999;14:521–531. doi: 10.1093/humrep/14.2.521. [DOI] [PubMed] [Google Scholar]
- Rodeck C, Tutschek B, Sherlock J, Kingdom J. Methods for the transcervical collection of fetal cells during the first trimester of pregnancy. Prenat Diagn. 1995;15:933–942. doi: 10.1002/pd.1970151008. [DOI] [PubMed] [Google Scholar]
- Sherlock J, Halder A, Tutschek B, Delhanty J, Rodeck C, Adinolfi M. Prenatal detection of fetal aneuploidies using transcervical cell samples. J Med Genet. 1997;34:302–305. doi: 10.1136/jmg.34.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiverick KT, King A, Frank H, Whitley GS, Cartwright JE, Schneider H. Cell culture models of human trophoblast II: trophoblast cell lines—a workshop report. Placenta. 2001;22:S104–S106. doi: 10.1053/plac.2001.0647. [DOI] [PubMed] [Google Scholar]
- Tutschek B, Sherlock J, Halder A, Delhanty J, Rodeck C, Adinolfi M. Isolation of fetal cells from transcervical samples by micromanipulation: molecular confirmation of their fetal origin and diagnosis of fetal aneuploidy. Prenat Diagn. 1995;15:951–960. doi: 10.1002/pd.1970151010. [DOI] [PubMed] [Google Scholar]
- Ventura SJ, Abma JC, Mosher WD, Henshaw SK. Estimated pregnancy rates by outcome for the United States, 1990–2004. Natl Vital Stat Rep. 2008;56:1–2528. [PubMed] [Google Scholar]