Mitochondria are versatile organelles that provide most of the ATP required for cellular endoergonic processes, coordinate the biosynthesis of several metabolites, and shape and amplify Ca2+ signalling and apoptosis (Dimmer & Scorrano, 2006). These multifaceted tasks are mirrored by their complex morphology and ultrastructure (Frey & Mannella, 2000), which affect processes as diverse as apoptosis, Ca2+ signalling, formation of dendritic spines and chemotaxis. The tight regulation of mitochondrial morphology is of crucial importance for cellular homeostasis, as exemplified by the fact that mutations in the core mitochondria-shaping proteins are associated with neurodegenerative diseases (Dimmer & Scorrano, 2006).
Mitochondrial morphology is determined by a dynamic equilibrium between fission and fusion. In mammalian cells, fusion is controlled by the outer membrane proteins mitofusin 1 and mitofusin 2, and the inner membrane protein optic atrophy 1 (Dimmer & Scorrano, 2006). Dynamin-related protein 1 (DRP1) is an evolutionarily conserved large dynamin-like GTPase that participates in the multistep process of mitochondrial fission. DRP1 is recruited to the outer membrane and undergoes self-assembly into a ring-like oligomeric structure that wraps around the prospective fission site to fragment the organelle (van der Bliek, 2000). The precise mechanisms that regulate this process remain elusive. Cellular cues, including Ca2+ and cyclic AMP signals, can lead to a change in mitochondrial shape by inducing the post-translational modification of DRP1, thereby influencing its localization and activity (Jahani-Asl & Slack, 2007; Cereghetti et al, 2008). After translocation to mitochondria, the levels of DRP1 are controlled by the counterbalancing processes of ubiquitination and SUMOylation. In this issue of EMBO reports, Braschi and colleagues (2009) report the identification of the small ubiquitin-like modifier (SUMO) E3 ligase that mediates DRP1 SUMOylation and, therefore, regulates mitochondrial fission.
Ubiquitination is an important post-translational modification that regulates, among other things, protein degradation. It is catalysed in a three-step reaction that involves ubiquitin-activating (E1), ubiquitin-conjugating (E2) and ubiquitin-ligating (E3) enzymes. Polyubiquitinated proteins are usually degraded by the 26S proteasome complex, whereas other types of ubiquitination—such as mono-ubiquitination—can be involved in proteasomal-independent or lysosomal protein degradation (Hershko & Ciechanover, 1998). Notably, ubiquitination regulates the turnover not only of cytoplasmic, soluble proteins, but also of substrates that are integral components of the endoplasmic reticulum membrane (Werner et al, 1996). Furthermore, the stability of several mitochondrial proteins that are localized in different subcompartments of the organelle is influenced by proteasomal inhibition, suggesting a role for ubiquitination in their turnover (Neutzner et al, 2008). Indeed, earlier studies reported the existence of E1 and E2 enzymes within mitochondria (Magnani et al, 1991; Schwartz et al, 1992). In addition, a more recent and growing body of evidence shows that mitochondria also contain specific E3 ligases with a prominent role in the regulation of mitochondrial dynamics. In particular, Mitol/March5—a ubiquitin E3 ligase embedded in the outer membrane—participates in the ubiquitination of fission 1 (FIS1; Yonashiro et al, 2006), DRP1 and mitofusin 2 (Nakamura et al, 2006). Notably, mitochondrial DRP1 is stabilized when the catalytic domain of Mitol/March5 is mutated (Karbowski et al, 2007), highlighting the role of ubiquitination in the turnover of this protein and, therefore, in the regulation of mitochondrial morphology.
SUMO can also be conjugated to protein targets. Similar to ubiquitination, SUMOylation is performed by three enzymes—known as SUMO E1, SUMO E2 and SUMO E3, in analogy to ubiquitination—and it is readily reversed by specific proteases. Two classes of SUMO E3 ligases have recently been identified: the first has an essential RING-like domain with similarities to the RING finger of ubiquitin E3 ligases, whereas the second has no obvious similarity to other E3 ligases. The specificity of SUMO conjugation and proteolysis seems to be achieved by confining SUMO ligases and proteases to restricted subcellular compartments. SUMOylation regulates a wide range of cellular processes, from cell-cycle control to transcriptional regulation, apoptosis and signal transduction (Verger et al, 2003).
The first hint that mitochondria were not strangers to SUMOylation was put forward five years ago by the McBride group. In a founding paper, they showed that the pro-fission protein DRP1 interacts with SUMO1—which is concentrated at sites of mitochondrial fission—and that high levels of SUMO1 stabilize DRP1 and promote mitochondrial fragmentation (Harder et al, 2004). This finding unveiled an additional and crucial layer of regulation of mitochondrial dynamics. However, a keystone was missing from this construction: the identification of the SUMO E3 ligase that SUMOylates DRP1 in mitochondria. The McBride group now reports the identification of this ligase, thereby closing the circle (Braschi et al, 2009). They had previously shown that mitochondria have a membrane-anchored protein ligase that contains a RING domain. Being true Canadians, they christened this protein MAPL, for mitochondrial-anchored protein ligase (Neuspiel et al, 2008)! However, as it has a RING domain, MAPL could be either a ubiquitin or a SUMO ligase. Now, by using synthetic peptides containing the SUMO1 consensus sequence as well as purified organelles, Braschi and colleagues show that the RING-finger domain of MAPL has SUMO E3 ligase activity, although, in the presence of high concentrations of ubiquitinating enzymes, MAPL can also be subject to auto-ubiquitination. Nonetheless, the downregulation of MAPL leads to a reduction of SUMO conjugates without significantly affecting the ubiquitination status of cellular proteins, indicating that SUMOylation is the predominant physiological activity of MAPL. The authors were able to show DRP1 SUMOylation in reconstituted in vitro assays, emphasizing the crucial involvement of this post-translational modification in the maintenance of mitochondrial morphology. This is in accordance with previous results indicating that the overexpression of MAPL enhances mitochondrial fragmentation in a RING-domain-dependent manner (Neuspiel et al, 2008). Furthermore, DRP1 has been shown to be de-SUMOylated by the protease SUMO1/sentrin-specific peptidase 5 (SENP5), resulting in decreased mitochondrial fission (Zunino et al, 2007).
MAPL silencing leads to reduced levels of DRP1 without significantly changing mitochondrial morphology, which suggests that although MAPL positively regulates mitochondrial fission, it might not be obligatory in this process. Alternatively, the effect of MAPL downregulation on fission could be counterbalanced by reduced mitochondrial fusion if MAPL also stabilizes other mitochondria-shaping proteins such as mitofusins. This is not just a remote possibility, as Braschi and colleagues found additional mitochondrial substrates of this SUMO E3 ligase and showed a specific reduction in the rate of mitochondrial fusion after MAPL knockdown. Furthermore, SUMOylation could be involved in the definition of interorganellar cross-talk, which, in the case of the juxtaposition between mitochondria and the endoplasmic reticulum, is crucially influenced by the levels of mitofusin 2 (de Brito & Scorrano, 2008). Notably, MAPL is selectively included in mitochondria-derived vesicles that bud from the organelle and are transported to peroxisomes. An open and unexplored issue is whether peroxisomes are the final destination of mitochondrial proteins that are SUMOylated by MAPL, which would be enclosed with their SUMO ligase in mitochondria-derived vesicles.
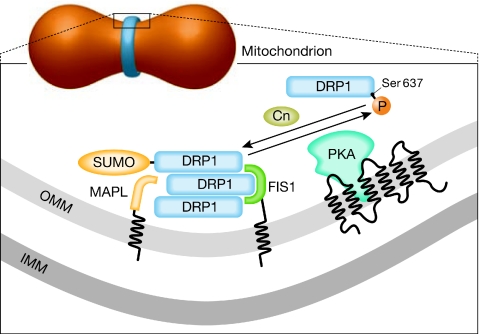
How does MAPL recognize DRP1? In principle, this could depend on proximity—that is, MAPL SUMOylates only the mitochondrial pool of DRP1. As the translocation of DRP1 is driven by the dephosphorylation of Ser 637 (Cereghetti et al, 2008), it is tempting to speculate that dephosphorylation and SUMOylation cooperate to determine the amount of active mitochondrial DRP1. However, SUMOylation usually requires a phosphorylated residue immediately downstream of the consensus sequence, rather than dephosphorylation. Interestingly, the Ser 637 site can be phosphorylated by protein kinase A (PKA), which is anchored to the mitochondrial outer membrane (Affaitati et al, 2003). A complex regulatory network could therefore be envisioned in which SUMOylation occurs to retain DRP1 on mitochondria only when it has been rephosphorylated by mitochondrial PKA. This scenario introduces the possibility of a versatile regulation of mitochondrial dynamics by multiple signalling events that emanate from the cytoplasm and converge on the outer membrane (Fig 1).
Figure 1.
The dynamic trafficking of DRP1 at sites of mitochondrial fission. The mitochondrial fission site is enlarged in the boxed area. A putative network regulating DRP1 accumulation and assembly is shown, in which DRP1 translocation is controlled by calcineurin-mediated dephosphorylation of Ser 637. Mitochondrial PKA then rephosphorylates the same site, pushing DRP1 away from the organelle. However, MAPL-mediated SUMOylation stabilizes DRP1 on mitochondria and could prevent its re-translocation to the cytoplasm. Cn, calcineurin; DRP1, dynamin-related protein 1; FIS1, fission 1; IMM, inner mitochondrial membrane; MAPL, mitochondrial-anchored protein ligase; OMM, outer mitochondrial membrane; PKA, protein kinase A; SUMO, small ubiquitin-like modifier.
In addition, McBride and colleagues have identified several other mitochondrial substrates of MAPL-induced SUMOylation, which opens the exciting possibility of a role for SUMOylation in the regulation of mitochondrial protein turnover and/or function, whether by stabilization or by the coordination of trafficking to this organelle. In the coming years, we will undoubtedly learn whether MAPL is the only SUMO E3 ligase present in mitochondria, and how SUMOylation controls the physiology and pathology of this crucial organelle.

References
- Affaitati A, Cardone L, de Cristofaro T, Carlucci A, Ginsberg MD, Varrone S, Gottesman ME, Avvedimento EV, Feliciello A (2003) Essential role of A-kinase anchor protein 121 for cAMP signaling to mitochondria. J Biol Chem 278: 4286–4294 [DOI] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM (2009) MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep 10: 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghetti GM, Stangherlin A, de Brito OM, Chang CR, Blackstone C, Bernardi P, Scorrano L (2008) Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA 105: 15803–15808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610 [DOI] [PubMed] [Google Scholar]
- Dimmer KS, Scorrano L (2006) (De)constructing mitochondria: what for? Physiology 21: 233–241 [DOI] [PubMed] [Google Scholar]
- Frey TG, Mannella CA (2000) The internal structure of mitochondria. Trends Biochem Sci 25: 319–324 [DOI] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H (2004) Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol 14: 340–345 [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67: 425–479 [DOI] [PubMed] [Google Scholar]
- Jahani-Asl A, Slack RS (2007) The phosphorylation state of Drp1 determines cell fate. EMBO Rep 8: 912–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Neutzner A, Youle RJ (2007) The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J Cell Biol 178: 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani M, Serafini G, Antonelli A, Malatesta M, Gazzanelli G (1991) Evidence for a particulate location of ubiquitin conjugates and ubiquitin-conjugating enzymes in rabbit brain. J Biol Chem 266: 21018–21024 [PubMed] [Google Scholar]
- Nakamura N, Kimura Y, Tokuda M, Honda S, Hirose S (2006) MARCH-V is a novel mitofusin 2- and Drp1-binding protein able to change mitochondrial morphology. EMBO Rep 7: 1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuspiel M, Schauss AC, Braschi E, Zunino R, Rippstein P, Rachubinski RA, Andrade-Navarro MA, McBride HM (2008) Cargo-selected transport from the mitochondria to peroxisomes is mediated by vesicular carriers. Curr Biol 18: 102–108 [DOI] [PubMed] [Google Scholar]
- Neutzner A, Benard G, Youle RJ, Karbowski M (2008) Role of the ubiquitin conjugation system in the maintenance of mitochondrial homeostasis. Ann NY Acad Sci 1147: 242–253 [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Trausch JS, Ciechanover A, Slot JW, Geuze H (1992) Immunoelectron microscopic localization of the ubiquitin-activating enzyme E1 in HepG2 cells. Proc Natl Acad Sci USA 89: 5542–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM (2000) A mitochondrial division apparatus takes shape. J Cell Biol 151: F1–F4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger A, Perdomo J, Crossley M (2003) Modification with SUMO. A role in transcriptional regulation. EMBO Rep 4: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ED, Brodsky JL, McCracken AA (1996) Proteasome-dependent endoplasmic reticulum-associated protein degradation: an unconventional route to a familiar fate. Proc Natl Acad Sci USA 93: 13797–13801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonashiro R et al. (2006) A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J 25: 3618–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM (2007) The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci 120: 1178–1188 [DOI] [PubMed] [Google Scholar]