Abstract
For much of the last century, the differentiated state that characterizes the many cell types of an adult organism was thought to be stable and abrogated only in rare instances by transdifferentiation, metaplasia or cancer. This stability was thought to reside in the autoregulatory molecular circuitry that exists between the cytoplasm and the nucleus, a status quo that could be disrupted during somatic cell nuclear transfer, to reprogramme cells to a pluripotent state. Pioneering work in the 1980s showed that transdifferentiation of cell lineages could be induced by the addition of transcription factors. However, these conversions were usually confined to cell types from the same germ layer, and proof of conversion was difficult to obtain. This deficiency has now been overturned by demonstrations that exogenously added transcription factors can convert differentiated cell types into embryonic-like induced pluripotent stem cells. Here, we highlight the recent progress, and the implications of this work for our understanding of the relationship between the pluripotent and more differentiated cell states.
Keywords: iPS, reprogramming, stem cell, differentiation, pluripotency
See Glossary for abbreviations used in this article.
Glossary
ALK5 activin receptor-like kinase 5
ASCl1 achaete-scute complex homologue 1
BAY BayK8644
BIO 6-bromoindirubin-3-oxime
BIX BIX-01294
BMP4 bone morphogenetic protein 4
C/EBP ccaat enhancer binding protein
CDX2 caudal-type homeobox 2
Epi-SC epiblast stem cell
FAB-SC fibroblast growth factor/activin/BIO-stem cell
FGF2 fibroblast growth factor 2
GSK3 glycogen synthase kinase 3
H3K histone 3 lysine
KLF4 kruppel-like factor 4
LIF leukaemia inhibitory factor
MEK mitogen-activated protein kinase kinase
MyoD myogenic differentiation 1
NGN3 neurogenin 3
OCT4 octamer 4, also known as POU class 5 homeobox 1
OSKM OCT4, SOX2, KLF4, c-Myc
OSNL OCT4, SOX2, Nanog, LIN28
PDX1 pancreatic and duodenal homeobox 1
PPARγ peroxisome proliferator-activated receptor-γ
PRDM16 PR domain-containing protein 16
SCNT somatic cell nuclear transfer
SOX2 SRY-box 2
SSEA4 stage-specific embryonic antigen 4
TGF transforming growth factor
XIST inactive X chromosome-specific transcript
Introduction: discovery of induced pluripotent stem cells
In 2006, Takahashi and Yamanaka reported the reprogramming of mouse fibroblasts to a pluripotent-like state by viral transfection of four transcription factors, OCT4, SOX2, KLF4 and c-Myc (OSKM; Takahashi & Yamanaka, 2006). These factors had been selected as being involved in maintaining pluripotency. Since this groundbreaking report, induced pluripotent stem cell (iPSC) lines have been generated from human (Takahashi et al, 2007; Yu et al, 2007), monkey (Liu et al, 2008) and rat (Li et al, 2009; Liao et al, 2009) somatic sources, as well as from multiple cell types, using various vehicles such as doxycycline-inducible viral vectors, adenoviral and plasmid vectors, and excisable genetic inserts (transposon-based and Cre-based), to deliver the exogenous genes (Hanna et al, 2008; Maherali & Hochedlinger, 2008; Soldner et al, 2009; Woltjen et al, 2009; Yusa et al, 2009). Furthermore, alternative transcription factor genes, recombinant transcription factor proteins (Zhou et al, 2009), and small chemicals that substitute for specific transcription factors and/or enhance the efficiency of the reprogramming process have been identified (Feng et al, 2009). Many of these developments have been summarized in excellent recent reviews (Hanna et al, 2008; Maherali & Hochedlinger, 2008; Feng et al, 2009) and are not commented on further here. Instead, we focus on the nature and outcome of this reprogramming process, and compare it with other methods that reprogramme the genome to a pluripotent-like or other state.
Comparison of iPSCs with embryonic stem cells
Embryonic stem cells (ESCs) are derived by the in vitro culture of blastomeres from the inner cell mass (ICM) of the mammalian embryo. The cells of the ICM are in a transient state in vivo, whereas ESCs can be perpetuated continuously if given an adequate in vitro-culture environment; both cell types are pluripotent. Single-cell transcriptional analysis of the mouse ICM and ESCs revealed similarities, but also showed that they do not express an identical set of genes (Kaji et al, 2007). In addition, other states of pluripotency clearly exist that vary in their transcriptional profile and developmental potential (Fig 1; Brons et al, 2007; Tesar et al, 2007; Chou et al, 2008). These qualifications need to be kept in mind in any interpretation of genome-based comparisons between ESCs and iPSCs, as any observed differences could be functionally inconsequential.
Figure 1.
Various states of pluripotency. mESCs are derived from E3.5 mouse blastocysts in the presence of BMP + LIF. Epi-SCs are derived from E5.5–E5.75 post-implantation epiblasts in the presence of activin and bFGF. FAB-SCs can be derived from mouse blastocysts in the presence of bFGF, activin, BIO and an anti-LIF antibody. Cell-fate changes induced by cell-culture conditions are indicated by a red dotted arrow. hiPSCs derived in the presence of the four factors alone, and hESCs derived from human blastocysts, more closely resemble Epi-SCs (black dotted line). hiPSCs derived in the presence of hLIF and the signalling pathway inhibitors PD0325901, A-83-01 and CHIR99021, resemble mESCs (black dotted line). The developmental potential of the cell types is colour coded as follows: dark green depicts fully pluripotent cells, light green depicts cells that cannot form chimaeras and yellow depicts cells that are not pluripotent (based on embryoid body and teratoma-formation assays). The pluripotency of hESCs and hiPSCs can be judged only by embryoid body and teratoma-formation assays. The limited differentiation potential of FAB-SCs can be rescued by culturing them in the presence of LIF/BMP4 or by overexpression of E-cadherin. Epi-SCs can acquire an ESC fate by KLF4 overexpression in conjunction with mESC culture conditions. Notably, reprogramming to an earlier developmental state or transdifferentiation—that is, cell-fate changes between somatic lineages—seems to require ectopic gene expression, whereas forward conversion—that is, ESC to Epi-SC—needs only a change in culture conditions. Ab, antibody; bFGF, basic fibroblast growth factor; BIO, 6-bromoindirubin-3-oxime; BMP, bone morphogenetic protein; E, embryonic day; Epi-SC, epiblast stem cell; FAB-SC, fibroblast growth factor/activin/BIO-stem cell; hESC, human embryonic stem cell; hiPSC, human induced pluripotent stem cell; hLIF, human leukaemia inhibitory factor; KLF4, kruppel-like factor 4; LIF, leukaemia inhibitory factor; mESC, murine embryonic stem cell; OCT4, octamer 4.
Several functional tests have been used to assess the similarity of iPSCs to ESCs. These include in vitro tests assessing differentiation, cell fusion and X inactivation, and analysing the status of DNA methylation, and in vivo tests such as teratoma formation, chimaera formation, germ-line transmission and tetraploid aggregation. Generally, ESCs and iPSCs seem to have similar functional properties; however, the following findings are of note. ESCs and iPSCs can contribute to both chimaera formation and germ-line transmission, but there is currently no report of live births following the tetraploid aggregation of iPSCs—the latest developmental stage that has been achieved so far using this technique is 13.5–14.5 days post coitum (Woltjen et al, 2009; Wernig et al, 2007; Meissner et al, 2007). Both cell types give rise to teratomas containing cell types from all germ layers; although this is generally thought to be an indication of pluripotency, and is one of few such available assays for human iPSCs (hiPSCs); incompletely reprogrammed iPSCs also generate teratomas (Takahashi & Yamanaka, 2006).
In parallel with functional testing, a series of genome-based analytical comparisons has been made between ESCs and iPSCs. The 5′-promoter regions of many transcriptional units in the mammalian genome contain clusters of the dinucleotide CpG, which are methylated at transcriptionally silent genes and demethylated upon transcriptional activation. In differentiated cells, the Oct4, Nanog and Sox2 promoter regions are silent and methylated, whereas in murine ESCs (mESCs) and human ESCs (hESCs) these promoters are active and unmethylated. During reprogramming, almost complete demethylation of these promoters has been reported (Maherali et al, 2007; Mikkelsen et al, 2008; Okita et al, 2007; Wernig et al, 2007). This demethylation was specific, as allele-specific DNA methylation, which marks the presence of parentally imprinted genes and is present at all stages from the fertilized egg to the adult mammal, was unaffected by reprogramming (Wernig et al, 2007).
In mammals, female cells have two X chromosomes, whereas male cells have only one. The imbalance of X-linked genes between the two sexes is compensated for by the inactivation of one X chromosome. In the mouse, this process begins at the epiblast stage and female mESCs that are isolated from the ICM have two active X chromosomes. iPSCs derived from female fibroblasts also have two active X chromosomes, showing that reprogramming reactivates the inactive X chromosome (Maherali et al, 2007). During the in vitro differentiation of miPSCs, random inactivation of the X chromosome is observed. However, the situation for hESCs/hiPSCs might be different: a screen of 59 hESC lines (Adewumi et al, 2007) concluded that many of the female lines expressed significant levels of XIST RNA, which is indicative of an inactive X chromosome. This could signify that within some hESC lines, de novo X inactivation had begun to occur, as hESCs have several characteristics that resemble cells of the epiblast more closely than those of the ICM (see below).
H3K methylation is another epigenetic modification that influences gene expression and chromatin structure. Historically, the trimethylation of H3K4 and H3K27 has been associated with the promoter regions of active and inactive genes, respectively. The degree and distribution of H3K4 and H3K27 methylation across the genome provides a distinctive signature for different cell types. ESC and iPSC profiles in both human and murine cells are comparable, and differ from the original fibroblast profiles (Mikkelsen et al, 2008). Taken together, these results indicate that DNA and histone H3 methylation profiles are similar between iPSCs and ESCs, and very different from those of their somatic cell progenitors (Meissner et al, 2008).
The widespread epigenetic changes referred to above can be accentuated by the use of chemical epigenetic modifiers that improve reprogramming and sometimes substitute for transcription factors. The DNA methyltransferase inhibitor 5-aza-cytidine and the histone deacetylase inhibitor valproic acid enhance the efficiency of mouse fibroblast reprogramming (Huangfu et al, 2008a, b; Mikkelsen et al, 2008). In the presence of valproic acid, the transduction with OS was sufficient to generate hiPSCs (Huangfu et al, 2008b). Furthermore, chemical screens have identified three compounds, BIX, RG108 and BAY, which, in conjunction with OK, enhanced the reprogramming of neural progenitors (Shi et al, 2008a) and mouse embryonic fibroblasts (MEFs; Shi et al, 2008b). BIX inhibits the G9a histone methyltransferases, which are thought to silence OCT4 and REX1, whereas RG108 inhibits a DNA methyltransferase. BIX can replace OCT4 in the presence of KSM, although the efficiency of reprogramming is reduced. BAY is an L-channel calcium agonist (Shi et al, 2008a) with an interesting mode of action: it might exert its effect through upstream signalling pathways rather than direct epigenetic remodelling.
Comparative analyses of the ESC and iPSC transcriptomes have been completed for human and mouse lines. Mikkelsen and colleagues reported that gene-expression profiles of iPSCs and ESCs of the same species are no more different than those of individual ESC lines (Mikkelsen et al, 2008). Soldner and colleagues compared the transcriptional profiles of hiPSC lines, in which the exogenous viral sequences had been excised (factor-free hiPSCs), with those of hiPSCs before transgene excision (Soldner et al, 2009). The transcriptomes of factor-free hiPSCs more closely resemble those of hESCs than the parental hiPSCs. This convergence was attributed to several possible causes, including the loss of any downstream gene activation caused by the residual expression of the exogenous transcription factors and/or the loss of the epigenetic memory of the somatic state that survived the initial reprogramming event. However, it remains difficult to compare these differences, as most groups used hESCs that were genetically unrelated to the hiPSCs. A way of clarifying this issue would be to generate factor-containing and factor-free hiPSCs from the differentiated progeny of an hESC line.
A comparison of mESC and hESC—and, by inference, miPSC and hiPSC—transcriptomes shows many differences (Ginis et al, 2004), possibly because, from a functional point of view, hESCs more closely resemble murine Epi-SCs (Brons et al, 2007; Tesar et al, 2007). This issue might be resolved shortly, as fibroblasts have now been reprogrammed to iPSCs that show a stable mESC-like phenotype (Li et al, 2009) using OSNL in combination with human LIF, PD0325901 (which is an MEK inhibitor), CHIR99021 (a GSK3β inhibitor) and ALK5 (a type 1 TGF-β inhibitor). This development might presage the generation of new hESC lines that more closely resemble mESCs.
Efficiency and kinetics of iPSC reprogramming
Depending on cell type, vectors, reprogramming mix and infection protocols, reprogramming efficiencies range from 0.001 to 11% (Maherali & Hochedlinger, 2008). The use of animals made from iPSCs that were established by doxycycline-inducible vectors provides further insight, as the problem of variable transfection efficiency is bypassed and viral integration sites are normalized for all tissues. Mouse fibroblasts differentiated in vitro from such secondary iPSC lines showed that the maximum degree of reprogramming is approximately 2–4%, compared with approximately 0.05% in primary reprogramming (Wernig et al, 2007, 2008a). One group reported a reprogramming efficiency of more than 11% for mouse fibroblasts treated with valproic acid, which led these authors to conclude that most cells receiving the four factors were reprogrammed (Huangfu et al, 2008a). However, a different group observed reduced reprogramming when valproic acid was used in conjunction with a transposon-based polycistronic construct (Yusa et al, 2009). This discrepancy could be explained if, in the Huangfu study, valproic acid was particularly beneficial to those cells within a population that received an incomplete set of reprogramming factors, which would not occur with a polycistronic transcription unit (Yusa et al, 2009; Kaji et al, 2009). The difficulties in comparing reprogramming efficiencies obtained in different laboratories can be compounded further by the varying criteria used for scoring iPSC formation (Maherali & Hochedlinger, 2008). Nevertheless, some trends are apparent: keratinocytes, stomach cells and liver cells are more easily converted than fibroblasts (Aasen et al, 2008; Aoi et al, 2008). These superior efficiencies might reflect better delivery and/or integration of factors, or indicate that the transcriptional circuitry of certain cell types might be more amenable to change.
The doxycycline-inducible constructs facilitated studies to investigate the efficiency and kinetics of reprogramming in murine fibroblasts (Brambrink et al, 2008; Stadtfeld et al, 2008; Wernig et al, 2008a). By withdrawal of doxycycline after various periods, it was shown that the transgenes had to be expressed for a minimum of 10–12 days to initiate cellular reprogramming. Prolonged exposure to doxycyclin resulted in an increased number of reprogrammed cells (Brambrink et al, 2008; Stadtfeld et al, 2008). This system revealed the kinetics of marker appearance during reprogramming (Brambrink et al, 2008; Stadtfeld et al, 2008); alkaline phosphatase was found to be expressed early and OCT4 expression was found to occur late. The observation that individual mitotic daughter cells of a single transduced fibroblast activate endogenous OCT4-driven or Nanog-driven reporter genes at different times suggests that reprogramming involves stochastic events (Meissner et al, 2007).
An interesting recent experiment has shown that the integration of a single copy of each of the OSKM factors is sufficient to allow optimal reprogramming frequency and kinetics (Markoulaki et al, 2009). Furthermore, by using MEFs containing integrated doxycycline-inducible combinations of KM or OS, followed by infection with the complementary pair, it has been shown that iPSC colonies formed faster when MEFs were pretreated with K+M but not with O+S. This result indicates that KLF4 and c-Myc act earlier during reprogramming, conceivably by inducing epigenetic alterations that assist the binding of OCT4 and SOX2 to their target genes (Markoulaki et al, 2009). These conclusions were confirmed and extended by a study that specifically addressed the temporal role of the four factors during reprogramming (Sridharan et al, 2009), which showed that the ectopic expression of c-Myc is necessary only during the initial stages of reprogramming and can be substituted by valproic acid. c-Myc or valproic acid might facilitate the interaction of OSK with their target genes to repress somatic gene expression and initiate the gene-expression programme that marks the pluripotent state. Conversely, in the absence of c-Myc—or valproic acid—these hurdles are overcome only stochastically, thereby diminishing the efficiency of reprogramming (Sridharan et al, 2009; Nakagawa et al, 2008; Wernig et al, 2008b).
Mechanisms of reprogramming to the pluripotent state
Jaenisch and Young describe four strategies for reprogramming differentiated cells to an embryonic state: SCNT, cell fusion, culture-induced reprogramming and iPSC generation (Jaenisch & Young, 2008). Culture-induced reprogramming of embryonic germ cells and spermatogonial stem cells, although important, does not constitute an example of radical reprogramming. Cell fusion has not yet resulted in the production of pluripotent diploid cells, although it might do so in the future (Cowan et al, 2005). By contrast, both SCNT and iPSC formation have succeeded in this task. SCNT, cell fusion and iPSC formation can all be interrogated to address the kinetics and mechanisms of the observed reprogramming. SCNT and iPSC generation—the latter in the absence of chemical supplements—are both inefficient procedures. The best recorded efficiencies using mouse fibroblasts are similar: 3.4% for SCNT (Wakayama et al, 2001) and 1–3% for iPSC generation (Hockemeyer et al, 2008; Maherali et al, 2008; Wernig et al, 2008a). We have commented above on the stochastic nature of iPSC reprogramming that could, in the absence of further modification, set an upper limit on the maximum efficiency. iPSC lines can arise over a protracted timeline and, therefore, inappropriate changes might be reversed over time. In other ways, SCNT, cell fusion and iPSC reprogramming are clearly different processes. In SCNT, the time required for reprogramming before initiation of development is short. OCT4 was detectable in mouse SCNT blastocysts within 12–24 h although the reactivation of many embryonic genes was erratic and variable between embryos (Boiani et al, 2002). Rapid OCT4 and SSEA4 expression is also seen in the somatic nucleus of 13–16% of mouse ESC-somatic cell heterokaryons (Pereira et al, 2008). In both processes, significant and rapid nuclear swelling, which is thought to indicate chromatin decondensation, precedes reprogramming. By contrast, during iPSC formation, OCT4 becomes detectable only after roughly 2 weeks (Brambrink et al, 2008; Stadtfeld et al, 2008) and only in a small proportion of treated cells. These data indicate that the processes share a stochastic nature but that, in SCNT and cell fusion, reprogramming occurs much faster.
In all situations, it remains unclear whether the reactivation of the embryonic genome seen during reprogramming requires DNA replication and cell division. In the frog, the activation of embryonic or specific pluripotency-associated genes does not require the host cell or donor nucleus to progress through the cell cycle or undergo DNA replication (De Robertis & Gurdon, 1977; Byrne et al, 2003). Similarly, the expression of various pluripotency-associated genes can be detected in the somatic components of heterokaryons before nuclear fusion (Pereira et al, 2008). By contrast, iPSCs have always been made from proliferative somatic cell populations, and whether DNA replication and/or cell division are essential for reprogramming remains untested. In conclusion, cell fusion and SCNT initiate a faster activation of pluripotency-associated genes in the absence of cell division. This could be a consequence of the oocyte or ESC cytoplasm and/or nucleoplasm providing a more complete set of factors that facilitate more rapid reprogramming than a defined group of transcription factors, or of inherent differences in the reprogramming mechanism. So far, the design of iPSC-reprogramming experiments has made it impossible to exclude the necessity of DNA replication and cell division. Gurdon & Melton (2008) remind us of the extensive SCNT literature indicating that a radical resetting of the differentiated state is less efficient as cells become more differentiated. Owing to methodological differences, it is difficult to determine from published results whether such a discernible difference in reprogramming efficiencies applies to iPSCs that are made from a diverse range of cell types. Recent data comparing factor-mediated secondary reprogramming of embryonic and adult mouse fibroblasts indicate a 2.5-fold to fourfold advantage in favour of the MEFs (Markoulaki et al, 2009).
A final point of comparison concerns the fate of epigenetic memory that is possessed by the donor cell nuclei. We should distinguish two forms of such memory: one owing to parental imprinting of specific genes according to their gametic origin—that is, sperm or egg—and one owing to the developmental imprinting that leads to random X-inactivation and the patterns of gene expression that uniquely identify each cell type. In SCNT, the parental imprinting present in the somatic donor nucleus can survive the technique, at least within the fetus (Inoue et al, 2002), whereas the re-expression of embryonic genes is relatively chaotic (Boiani et al, 2002; Bortvin et al, 2003). The parental imprint also survives iPSC formation (Wernig et al, 2007). SCNT has highlighted an interestingdifference between somatic cell and ESC nuclear donors: the memory of the specific inactive X in the somatic donor is retained during trophectoderm formation, irrespective of the gametic origin of that X chromosome (Eggan et al, 2000). It will be interesting to see whether this ‘mark' is retained if iPSC donors are used. Finally, it remains unclear whether any epigenetic memory of the original differentiated state remains after iPSC formation. By using endodermal and neuroectodermal nuclear donors, Ng & Gurdon (2005) showed that epigenetic memory can be retained during SCNT and might lead to abnormalities after the procedure.
The stability of the differentiated state
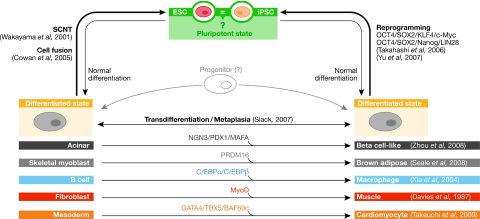
Historically, stability was regarded as one of the enduring characteristics of the differentiated state. It was believed that the molecular circuitry established between the cytoplasm and nucleus maintained cell identity and resisted change. Exceptions such as the regeneration of a newt lens from the dorsal pigmented epithelial cells of the iris were rare (Reyer, 1954). SCNT and cell fusion were thought to work precisely because they broke the tight relationship between the nucleus and the cytoplasm. Therefore, the production of iPSCs from somatic cells has captured the imagination of scientists everywhere. What does this reprogramming tour de force tell us about the nature of the pluripotent state and its relationship to all other differentiated states? In iPSC formation, OCT4 and SOX2 seem to be essential, although SOX2 is dispensable when neural stem cells are reprogrammed (Kim et al, 2008). An exogenous provision of Nanog is dispensable as the other factors activate the Nanog gene. These three factors are intricately linked to the maintenance of the pluripotent state. Chromatin immunoprecipitation followed by DNA microarray analysis in hESCs (Boyer et al, 2005) showed that 353 genes were jointly occupied by OCT4, SOX2 and Nanog. Active genes included transcription factors, signal transduction components and chromatin-modifying enzymes; inactive genes included a large set of transcription factors associated with specific lineages. Notably, OCT4, SOX2 and Nanog all occupied each other's promoters, and OCT4 and Nanog have been shown to interact physically (Wang et al, 2006). These three factors are believed to act in an autoregulatory loop to maintain their own expression, while repressing genes that are pertinent to the establishment of other cell states. There are many examples in which the overexpression of transcription factors modulates cell fate both in vitro and in vivo; however, the observed transitions occur mainly between embryologically related lineages, perhaps reflecting shared and similar epigenetic states. In the classical work of Davis and colleagues (1987), fibroblasts were turned into myoblast-like cells by exogenous MyoD expression (Fig 2). Overexpression of PRDM16, which interacts with PPARγ—a master regulator of adipogenesis—in myoblasts, facilitates their differentiation into brown fat cells (Seale et al, 2008). The expression of ASCL1 in proliferating adult hippocampal stem/progenitor cells induced differentiation towards the oligodendrocyte lineage in vivo (Jessberger et al, 2008), whereas the overexpression of C/EBPα and C/EBPβ converted differentiated B cells into macrophages (Xie et al, 2004). Finally, Zhou et al (2008) converted murine pancreatic acinar cells into β-like cells in situ by viral transduction with three transcription factor genes (PDX1, MAFA and NGN3). We speculate that the stability of the newly acquired state is mediated by the activation of factors, which, in turn, stabilize their own levels of expression. In combination, these factors initiate the lineage-specific gene programme, and regulate each other and their own expression. Such autoregulatory loops could represent a widespread method of ‘fixing' and stabilizing differentiated states. One might envisage all separate cellular states as metastable and interconvertible (Fig 2), but is there a hierarchy in the ease with which conversion to some states can be effected? The observation that cell fusion between ES-like cells and somatic cells leads to heterokaryons that manifest a pluripotent phenotype has been interpreted as indicating that the pluripotent state trumps more differentiated ones—it is a default state. However, in all such examples, the culture media were chosen to favour the pluripotent cell type. The in vitro-culture medium or in vivo niche is fundamental to cell homeostasis. Perturbations of the external environment can trigger cell-fate changes. Difficulties in simulating these in vivo conditions might explain why many differentiated cell types fare badly in vitro. ESC are an in vitro creation whose properties and maintenance are dependent on the specialized culture conditions that are used to prepare and maintain them (Fig 1). Mouse cells can be propagated in media containing LIF and BMP4. By contrast, hESCs cannot be maintained in these media and require activin A, FGF2 and other additions (Yu & Thomson, 2008). Similar growth conditions have been shown to work with Epi-SCs, and mESCs can be induced to transition into Epi-SCs by a simple change in medium (Fig 1; Guo et al, 2009). This conversion cannot be reversed by a simple medium change, although the forced expression of exogenous KLF4 and mESC media can effect the reversion (Guo et al, 2009). Cell-culture changes can have more enigmatic results: when mouse blastocysts are plated on mouse fibroblast feeders in the presence of FGF2, activin, BIO (which is a GSK3 kinase inhibitor) and an anti-LIF antibody, the resultant FAB-SC lines are unable to generate embryoid bodies or teratomas under any circumstances. However, on exposure of FAB-SCs to BMP4 and LIF media, or forced expression of E-cadherin, these cells assume properties that are similar to those of mESCs (Fig 1; Chou et al, 2008).
Figure 2.
Interconvertible cellular states. The reprogramming of differentiated cells towards pluripotency by SCNT, cell fusion and reprogramming with the combination of either OSKM or OSNL. Transdifferentiation between somatic cell fates is seen in pathological situations (metaplasia) and can be experimentally induced by overexpression of factors such as MyoD, PRDM16, PDX1, MAFA, NGN3, C/EBPα and C/EBPβ. A progenitor intermediate might be involved in these transitions. BAF60c, cardiac specific subunit of BAF chromatin-remodelling complexes; C/EBP, ccaat enhancer binding protein; ESC, embryonic stem cell; GATA4, GATA-binding protein 4; iPSC, induced pluripotent stem cell; KLF4, kruppel-like factor 4; MyoD, myogenic differentiation 1; NGN3, neurogenin 3; OCT4, octamer 4; OSKM, OCT4, SOX2, KLF4, c-Myc; OSNL, OCT4, SOX2, Nanog, LIN28; PDX1, pancreatic and duodenal homeobox 1; PRDM16, PR domain-containing protein 16; SCNT, somatic cell nuclear transfer; SOX2, SRY-box 2; TBX5, T-box 5.
A major factor contributing to the success of iPSC formation was the use of ES culture media. hiPSCs and hESCs differ significantly in their media growth requirements from miPSCs and mESCs, and are reported to be more similar in this aspect to mEpi-SCs. Recently, hiPSCs have been generated using miPSC/mESC media after supplementation with ESC signalling pathway inhibitors (Li et al, 2009). We speculate that any judicious choice of defined transcription factor, in combination with the appropriate chromatin remodelling, could ensure the conversion and maintenance of any cell type, given that the in vitro culture conditions or the in vivo milieu are permissive for their establishment.
An intriguing question is whether, if a transition between different cell types can be effected by the overexpression of only a few transcription factors, do such events occur in vivo and lead to pathological consequences. Metaplasias occur as a result of tissue damage, chronic trauma, abnormal hormonal stimulation or infection, and predispose to specific cancers (Eberhard & Tosh, 2008; Slack, 2007). Some metaplasias can be experimentally induced by the manipulation of certain factors: PDX1, TGF-α and interferon-γ induce acinar-to-ductal cell metaplasia; CDX2 leads to Barrett metaplasia; and p63 results in vaginal adenosis. However, as many of these transitions occur between developmentally related lineages, it is impossible to exclude the involvement of a common progenitor without well-designed lineage-tracing experiments.
Here, we paint a picture of the organism as comprising a myriad of potentially labile differentiated states, each secured internally by autoregulatory transcriptional circuits and protected externally by its specialized niche. When, in the nineteenth century, Claude Bernard invoked homeostasis of the internal environment within the body as being a requisite for an independent existence, he was referring to parameters such as the temperature and the composition of bodily fluids. It is ironic to think that, at the level of integration of the activities of all tissues and organs towards healthy bodily function, he was correct; however, when one looks within those tissues and organs at the activities of individual cells or groups of cells, a picture emerges of local, controlled microenvironments that differ from one another in ways that are essential to cell function and, we argue, to the maintenance of cell identity.
Conclusions
The molecular mechanisms that allow terminally differentiated cells to regain pluripotency remain a mystery (see Sidebar A). We do not currently know whether the reprogramming route retraces the developmental pathway. If pluripotency is indeed a ‘default' state, then many avenues might lead there. Similarly, in the case of transdifferentiation, does the process pass through some common progenitor-like state or is it more direct? Certainly, the efficiency and speed of the acinar-to-β-cell transdifferentiation (Zhou et al, 2008) would seem to rule out a pluripotent intermediate. Irrespective of which mechanisms apply, further detailed investigation of these processes should provide an insight into how cellular identity is established and maintained, and pave the road for the generation of more efficient reprogramming and transdifferentiation protocols. It might even provide new strategies for regenerative medicine. Whatever might transpire, the aura of the ‘differentiated' state as inviolable has vanished.
Sidebar A | In need of answers.
What is the molecular mechanism of reprogramming and what role does each factor have?
How similar are embryonic stem cells and induced pluripotent stem cells? Do they have equal differentiation capabilities?
Can reprogramming be achieved solely with chemical compounds?
Is epigenetic memory completely erased during reprogramming?
Could chromatin modifiers such as valproic acid enhance transdifferentiation?
Does cell division enhance the ability of a cell to change fate?
Note added in proof. During the laying out of this article,Takeuchi & Bruneau (2009) showed that the ectopic expression of the cardiac transcription factors Gata4 and Tbx5, in addition to the cardiac-specific subunit of BAF chromatin-remodelling complexes, Baf60c (also know as Smarcd3), facilitates the differentiation of mouse mesoderm into beating cardiomyocytes, thereby providing yet another example of the interconvertibility of cellular states.

Alan Colman (left) & Oliver Dreesen
Acknowledgments
The authors thank R. Pedersen for useful advice.
References
- Aasen T et al. (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26: 1276–1284 [DOI] [PubMed] [Google Scholar]
- Adewumi O et al. (2007) Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol 25: 803–816 [DOI] [PubMed] [Google Scholar]
- Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321: 699–702 [DOI] [PubMed] [Google Scholar]
- Boiani M, Eckardt S, Scholer HR, McLaughlin KJ (2002) Oct4 distribution and level in mouse clones: consequences for pluripotency. Genes Dev 16: 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A, Eggan K, Skaletsky H, Akutsu H, Berry DL, Yanagimachi R, Page DC, Jaenisch R (2003) Incomplete reactivation of Oct4-related genes in mouse embryos cloned from somatic nuclei. Development 130: 1673–1680 [DOI] [PubMed] [Google Scholar]
- Boyer LA et al. (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122: 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H, Jaenisch R (2008) Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brons IG et al. (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448: 191–195 [DOI] [PubMed] [Google Scholar]
- Byrne JA, Simonsson S, Western PS, Gurdon JB (2003) Nuclei of adult mammalian somatic cells are directly reprogrammed to oct-4 stem cell gene expression by amphibian oocytes. Curr Biol 13: 1206–1213 [DOI] [PubMed] [Google Scholar]
- Chou YF, Chen HH, Eijpe M, Yabuuchi A, Chenoweth JG, Tesar P, Lu J, McKay RDG, Geijsen N (2008) The growth factor environment defines distinct pluripotent ground states in novel blastocyst-derived stem cells. Cell 135: 449–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CA, Atienza J, Melton DA, Eggan K (2005) Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309: 1369–1373 [DOI] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB (1987) Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51: 987–1000 [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Gurdon JB (1977) Gene activation in somatic nuclei after injection into amphibian oocytes. Proc Natl Acad Sci USA 74: 2470–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard D, Tosh D (2008) Transdifferentiation and metaplasia as a paradigm for understanding development and disease. Cell Mol Life Sci 65: 33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Hochedlinger K, Rideout W 3rd, Yanagimachi R, Jaenisch R (2000) X-Chromosome inactivation in cloned mouse embryos. Science 290: 1578–1581 [DOI] [PubMed] [Google Scholar]
- Feng B, Ng JH, Heng JC, Ng HH (2009) Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell 4: 301–312 [DOI] [PubMed] [Google Scholar]
- Ginis I et al. (2004) Differences between human and mouse embryonic stem cells. Dev Biol 269: 360–380 [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Evres I, Mansfield W, Smith A (2009) Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 136: 1063–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Melton DA (2008) Nuclear reprogramming in cells. Science 322: 1811–1815 [DOI] [PubMed] [Google Scholar]
- Hanna J, Carey BW, Jaenisch R (2008) Reprogramming of somatic cell identity. Cold Spring Harb Symp Quant Biol 73: 147–155 [DOI] [PubMed]
- Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R (2008) A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell 3: 346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA (2008a) Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol 26: 795–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA (2008b) Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol 26: 1269–1275 [DOI] [PubMed] [Google Scholar]
- Inoue K, Kohda T, Lee J, Ogonuki N, Mochida K, Noguchi Y, Tanemura K, Kaneko-Ishino T, Ishino F, Ogura A (2002) Faithful expression of imprinted genes in cloned mice. Science 295: 297. [DOI] [PubMed] [Google Scholar]
- Jaenisch R, Young R (2008) Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 132: 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S, Toni N, Clemenson GD, Ray J, Gage FH (2008) Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci 11: 888–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Nichols J, Hendrich B (2007) Mbd3, a component of the NuRD co-repressor complex, is required for development of pluripotent cells. Development 134: 1123–1132 [DOI] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K (2009) Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 9: 771–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB et al. (2008) Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 454: 646–650 [DOI] [PubMed] [Google Scholar]
- Li W, Wei W, Zhu S, Zhu J, Shi Y, Lin T, Hao E, Hayek A, Deng H, Ding S (2009) Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4: 16–19 [DOI] [PubMed] [Google Scholar]
- Liao J et al. (2009) Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell 4: 11–15 [DOI] [PubMed] [Google Scholar]
- Liu H et al. (2008) Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 3: 587–590 [DOI] [PubMed] [Google Scholar]
- Maherali N, Hochedlinger K (2008) Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell 3: 595–605 [DOI] [PubMed] [Google Scholar]
- Maherali N et al. (2007) Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 1: 55–70 [DOI] [PubMed] [Google Scholar]
- Maherali N, Ahfeldt T, Rigamonti A, Utikal J, Cowan C, Hochedlinger K (2008) A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell 3: 340–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markoulaki S et al. (2009) Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nat Biotechnol 27: 169–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A et al. (2008) Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature 454: 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner A, Wernig M, Jaenisch R (2007) Direct reprogramming of genetically unmodified fibroblasts into pluripotent stem cells. Nat Biotechnol 25: 1177–1181 [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P, Bernstein BE, Jaenisch R, Lander ES, Meissner A (2008) Dissecting direct reprogramming through integrative genomic analysis. Nature 454: 794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M et al. (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26: 101–106 [DOI] [PubMed] [Google Scholar]
- Ng RK, Gurdon JB (2005) Epigenetic memory of active gene transcription is inherited through somatic cell nuclear transfer. Proc Natl Acad Sci USA 102: 1957–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448: 313–317 [DOI] [PubMed] [Google Scholar]
- Pereira CF, Terranova R, Ryan NK, Santos J, Morris KJ, Cui W, Merkenschlager M, Fisher AG (2008) Heterokaryon-based reprogramming of human B lymphocytes for pluripotency requires Oct4 but not Sox2. PLoS Genet 4: e1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyer RW (1954) Regeneration of the lens in the amphibian eye. Q Rev Biol 29: 1–46 [DOI] [PubMed] [Google Scholar]
- Seale P et al. (2008) PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Scholer HR, Ding S (2008a) Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3: 568–574 [DOI] [PubMed] [Google Scholar]
- Shi Y, Do JT, Desponts C, Hahm HS, Scholer HR, Ding S (2008b) A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell 2: 525–528 [DOI] [PubMed] [Google Scholar]
- Slack JM (2007) Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol 8: 369–378 [DOI] [PubMed] [Google Scholar]
- Soldner F et al. (2009) Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136: 964–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan R et al. (2009) Role of the murine reprogramming factors in the induction of pluripotency. Cell 136: 364–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Maherali N, Breault DT, Hochedlinger K (2008) Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2: 230–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG (2009) Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459: 708–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448: 196–199 [DOI] [PubMed] [Google Scholar]
- Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P (2001) Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science 292: 740–743 [DOI] [PubMed] [Google Scholar]
- Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW, Orkin SH (2006) A protein interaction network for pluripotency of embryonic stem cells. Nature 444: 364–368 [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448: 318–324 [DOI] [PubMed] [Google Scholar]
- Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R (2008a) A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol 26: 916–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Cassady JP, Jaenisch R (2008b) c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Nat Biotechnol 2: 10–12 [DOI] [PubMed] [Google Scholar]
- Woltjen K et al. (2009) piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458: 766–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Ye M, Feng R, Graf T (2004) Stepwise reprogramming of B cells into macrophages. Cell 117: 663–676 [DOI] [PubMed] [Google Scholar]
- Yu J, Thomson JA (2008) Pluripotent stem cell lines. Genes Dev 22: 1987–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Yusa K, Rad R, Takeda J, Bradley A (2009) Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods 6: 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H et al. (2009) Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4: 381–384 [DOI] [PMC free article] [PubMed]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA (2008) In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455: 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]