Abstract
Ubiquitination regulates membrane events such as endocytosis, membrane trafficking and endoplasmic-reticulum-associated degradation (ERAD). Although the involvement of membrane-associated ubiquitin-conjugating enzymes and ligases in these processes is well documented, their regulation by ubiquitin deconjugases is less well understood. By screening a database of human deubiquitinating enzymes (DUBs), we have identified a putative transmembrane domain in ubiquitin-specific protease (USP)19. We show that USP19 is a tail-anchored ubiquitin-specific protease localized to the ER and is a target of the unfolded protein response. USP19 rescues the ERAD substrates cystic fibrosis transmembrane conductance regulator (CFTR)ΔF508 and T-cell receptor-α (TCRα) from proteasomal degradation. A catalytically inactive USP19 was still able to partly rescue TCRα but not CFTRΔF508, suggesting that USP19 might also exert a non-catalytic function on specific ERAD substrates. Thus, USP19 is the first example of a membrane-anchored DUB involved in the turnover of ERAD substrates.
Keywords: deubiquitinating enzyme, ubiquitin-specific protease 19, endoplasmic reticulum, ER-associated degradation, unfolded protein response
Introduction
Protein modifications by ubiquitin control protein stability and function, which are essential for cellular processes such as transcription, DNA repair, cell-cycle progression and apoptosis (Ciechanover, 2005). Ubiquitination is a reversible process performed by deubiquitinating enzymes (DUBs), of which only a minority has been functionally characterized (Nijman et al, 2005). Their roles are diverse, varying from maintaining the pool of free ubiquitin to regulatory activities on specific substrates or signalling pathways, making them potentially interesting as therapeutic targets (Nalepa et al, 2006; Daviet & Colland, 2008). Five sub-families of DUBs are known, including: ubiquitin-specific proteases, carboxy-terminal hydrolases, otubain proteases, the Machado–Joseph disease proteases and the metallo-proteases referred to as JAMM (JAB1/MPN/Mov34).
Ubiquitination of membrane proteins controls processes such as endocytosis, membrane trafficking (Hicke & Dunn, 2003), Golgi reassembly after mitosis and endoplasmic-reticulum-associated degradation (ERAD; Meusser et al, 2005). The regulatory function of DUBs in these processes is becoming increasingly clear; however, few DUBs are known to be membrane spanning. Only UBP16 was shown to be exclusively associated with membranes in yeast (Kinner & Kolling, 2003), and recently its human homologue, USP30, was shown to be important for maintaining proper mitochondrial morphology (Nakamura & Hirose, 2008).
Using a bioinformatics approach, we have identified a transmembrane (TM) domain in the ubiquitin-specific protease 19 (USP19). We show that USP19 is an ER-resident tail-anchored DUB that is upregulated during the unfolded protein response (UPR) and rescues the ERAD substrates green fluorescent protein (GFP)-cystic fibrosis transmembrane conductance regulator (CFTR)Δ508 and FLAG-T-cell receptor (TCR)α from proteasomal degradation. Thus, USP19 seems to be a new player in ERAD.
Results And Discussion
Prediction of human DUBs containing TM domains
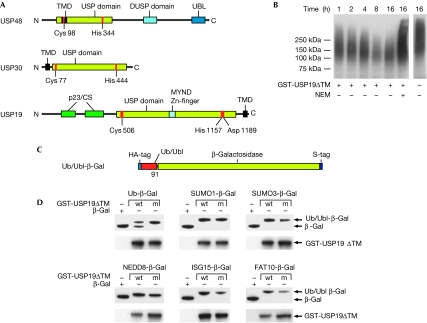
A search among human DUB sequences with the TM-HMM 2.0 algorithm (Krogh et al, 2001) identified three candidates with putative transmembrane domains: USP48 (also known as USP31), USP30 and USP19. USP48 is unlikely to be associated with the membrane as the putative transmembrane domain overlaps with the DUB active site and has a low probability score (Fig 1A; supplementary Fig 1 online). USP30 was recently identified as a mitochondria-associated protein (Nakamura & Hirose, 2008). USP19 is known to modulate cell proliferation through the regulation of Kip1 ubiquitination-promoting complex 1 (KPC1), a ubiquitin ligase for the cyclin-dependent kinase inhibitor p27(Kip1) (Lu et al, 2009) and for its upregulation during muscle atrophy (Combaret et al, 2005). USP19 also contains a myeloid translocation protein 8, Nervy and Deaf1 (MYND) domain that might mediate protein–protein interactions (Gross & McGinnis, 1996) and CHORD and SGT1 (CS/p23) domains, which suggest chaperone functions (Freeman et al, 1996; Garcia-Ranea et al, 2002). A bioinformatics search for USP19 orthologues showed that USP19 is conserved in higher eukaryotes, whereas no homologues could be identified in yeast (data not shown).
Figure 1.
USP19 is a ubiquitin-specific protease containing a predicted transmembrane domain. (A) Domain overview of the identified human deubiquitinating enzymes with potential transmembrane (TM) domains. (B) Ubiquitin (Ub) conjugates isolated from haemagglutinin (HA)-Ub-expressing cells were incubated with GST-USP19ΔTM. The reaction was carried out for the indicated time in the presence or absence of the cystein protease inhibitor N-ethylmaleimide (NEM). Western blots using an anti-HA antibody are shown. (C) Schematic representation of the Ub/Ubl (Ub-like) reporters. (D) Western blot of samples from bacteria co-expressing the reporter constructs and GST-USP19ΔTM (wt) or GST-USP19MUTΔTM (m). Antibodies were used against the S-tag, to detect the reporters, and against GST to verify USP19 expression. CS, CHORD and SGT1; DUSP, domain in USPs; GST, glutathione S-transferase; MYND, myeloid translocation protein 8, Nervy and Deaf1; p23, domain named after the protein p23; TMD, transmembrane domain; UBL, ubiquitin-like domain; USP, ubiquitin-specific protease.
USP19 is a ubiquitin-specific protease
To verify the predicted DUB activity, recombinant USP19 was expressed in fusion with glutathione S-transferase (GST) and purified from bacteria. Only the wild-type purified protein and not a mutant in which the predicted catalytic cysteine had been substituted cleaved the fluorogenic substrate ubiquitin-AMC (supplementary Fig 2 online). Furthermore, incubation of authentic ubiquitin conjugates enriched by immunoprecipitation from cells expressing HA-ubiquitin with USP19ΔTM resulted in a time-dependent decrease of polyubiquitin chain length (Fig 1B). To test the specificity of USP19, we generated a panel of reporters expressing ubiquitin, SUMO1, SUMO3, NEDD8, ISG15 or FAT10 fused to β-galactosidase (Fig 1C). Co-expression of GST-USP19ΔTM with the reporter plasmids in bacteria resulted in specific cleavage only of ubiquitin-β-gal, showing that USP19 is specific for ubiquitin (Fig 1D).
USP19 is anchored to the ER facing the cytoplasm
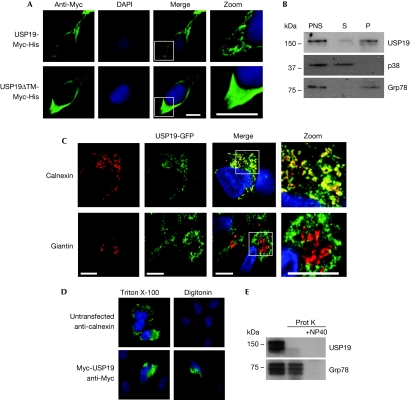
USP19 lacks a signal peptide and its predicted transmembrane domain is localized in the extreme C-terminus, suggesting that it is a member of the family of tail-anchored proteins that are post-translationally inserted into the outer mitochondrial or ER membrane (Borgese et al, 2007). Immunostainings of transfected human embryonic kidney (HEK)293 cells showed a reticular localization pattern of USP19-Myc, whereas the USP19ΔTM-Myc was evenly dispersed in the cytoplasm, suggesting that USP19 is indeed a membrane-anchored protein (Fig 2A). This was confirmed by the fractionation of cell lysates into cytosolic and microsomal fractions in which virtually all USP19 was recovered in the membrane fraction (Fig 2B). As the localization pattern of USP19 is reminiscent of a large cellular network, staining with two markers of the secretory pathway, calnexin for the ER and giantin for the cis- and mid-Golgi, was performed in cells expressing USP19-GFP. This revealed a clear co-localization with the ER but not with the Golgi (Fig 2C).
Figure 2.
USP19 is anchored to the endoplasmic reticulum facing the cytoplasm. (A) Human embryonic kidney (HEK)293 cells expressing USP19-Myc-His or USP19ΔTM-Myc-His, stained with a Myc antibody. White squares indicate the zoomed areas. The nucleus was visualized by DAPI. Scale bars, 20 μm. (B) Lysates of HEK293T cells were fractionated into a soluble cytosolic fraction (S) and a microsomal fraction (P). The mitogen-activated protein kinase p38 and the endoplasmic reticulum chaperone Grp78/Bip were used as markers for soluble and membrane proteins, respectively. Equal amounts of proteins were loaded in each lane. (C) HEK293 cells stably expressing USP19-GFP (green) stained against calnexin (red, upper panel) or giantin (red, lower panel). Scale bars, 20 μm. (D) Untransfected HeLa cells stained with a calnexin antibody (upper panel) and Myc-USP19-expressing cells stained anti-Myc (lower panel). The cells were treated with 0.1% Triton X-100 to permeabilize all membranes or 0.15% Digitonin to selectively permeabilize only the outer membranes. (E) Western blot of microsomal fractions from HEK293T cells treated with 0.4 μg/ml proteinase K±1% NP40. BiP, immunoglobulin heavy chain-binding protein; GFP, green fluorescent protein; Grp78, 78 kDa glucose-regulated protein precursor; PNS, post-nuclear supernatant; TM, transmembrane; USP, ubiquitin-specific protease.
To determine the membrane orientation of USP19, cells were transfected with Myc-USP19 and fixed in formaldehyde and then treated with 0.1% Triton X-100 for 10 min, for permeabilization of both the outer and inner cellular membranes, or 0.15% Digitonin for 1 min, for selective permeabilization of the plasma membrane. The efficiency of the procedure was confirmed by the selective staining of the ER-resident protein calnexin in cells treated with Triton X-100. The amino-terminally tagged Myc-USP19 was readily stained under both conditions, indicating that all the functional domains are facing the cytoplasm (Fig 2D). Consistent with this, efficient proteinase K digestion of USP19 did not require permeabilization of microsomes with NP40, confirming that the protein faces the cytosol (Fig 2E).
USP19 expression is induced by ER stress
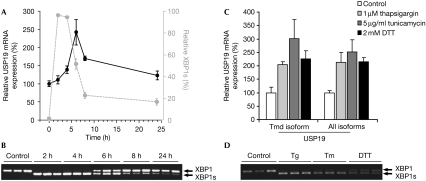
A significant proportion of newly synthesized ER proteins fail to acquire a native conformation and are subject to ERAD, which involves retrotranslocation, ubiquitination and proteasomal degradation of the aberrant protein. Excessive production of misfolded proteins causes ER stress. Adaptation to this stress requires activation of the UPR, which entails inhibition of translation and upregulation of ER chaperones and ERAD components (Schroder & Kaufman, 2005). Because of the ER localization of USP19, we hypothesized that it might be part of the UPR and therefore regulated in response to ER stress. To test this possibility, USP19 mRNA was quantified by using a real-time PCR in HEK293 cells treated with the ER stress agent tunicamycin (inhibitor of N-linked glycosylation; Fig 3A). The induction of spliced X-box binding protein 1 (XBP1s) was tested in parallel to confirm the efficiency of the treatment (Fig 3B). USP19 mRNA peaked approximately 2 h after XBP1s, which is in line with XBP1s being an immediate-early response to ER stress, preceding the general transcriptional response. Similar results were obtained with the other ER stress agents, thapsigargin (Ca2+ ATPase inhibitor) and dithiothreitol (DTT) reducing agent (Fig 3C,D). These findings are in agreement with the upregulation of USP19 during muscle atrophy that bears many similarities to ER stress (Hunter et al, 2001). The Ensembl database predicts seven USP19 transcripts, of which two contain the transmembrane domain (http://www.ensembl.org/index.html). PCR analysis using primer sets to distinguish between these revealed no difference, showing that only USP19 containing the transmembrane domain is upregulated after ER stress (Fig 3C).
Figure 3.
USP19 messenger RNA expression is induced by endoplasmic reticulum stress. (A) USP19 mRNA levels were analysed by real-time (RT)–PCR in human embryonic kidney (HEK)293 cells treated with tunicamycin (tm; 5 μg/ml) for the indicated time (black line). Values are expressed as USP19 expression relative to the control GAPDH±s.e.m. Values in grey illustrate the spliced X-box binding protein 1 (XBP1s) relative to total XBP1 as quantified by densitometry of the semi-quantitative RT–PCR shown in (B). Mean±s.d. of experiments in triplicate. (B) Agarose gel of RT–PCR showing the level of XBP1s in the samples shown in (A). (C) Real-time PCR of USP19 mRNA expression in HEK293 cells treated with thapsigargin (tg; 1 μM), tunicamycin (5 μg/ml) or dithiothreitol (DTT; 2 mM) for 6 h. Expression levels relative to GAPDH±s.e.m. of three experiments. Primer sets specific for USP19 containing the transmembrane domain (Tmd isoform) and all predicted USP19 transcripts (all isoforms) were used. (D) Agarose gel of RT–PCR showing XBP1 and XBP1s of samples used in (C). USP, ubiquitin-specific protease.
Membrane-associated USP19 rescues ERAD substrates
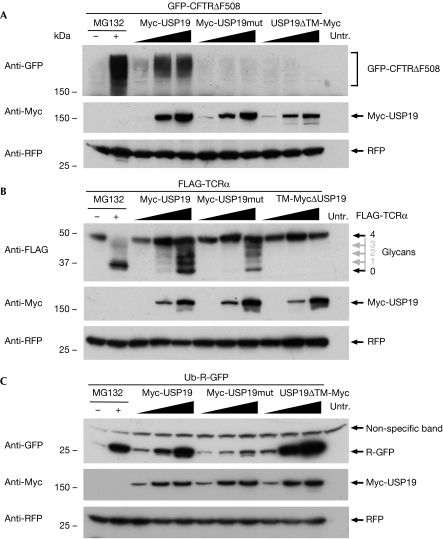
The ER localization and upregulation after ER stress suggest that USP19 might function in ERAD. This was investigated by testing the effect of USP19 on the degradation of various ERAD substrates. CFTR has several transmembrane and cytosolic domains and undergoes slow co-translational folding and quality control at the ER (Kleizen et al, 2005), whereas the CFTRΔF508 is degraded by ERAD. GFP-CFTRΔF508 accumulated in response to treatment with the proteasome inhibitor MG132 (10 μM), confirming that it is a proteasomal substrate (Fig 4A). The effect of USP19 on the turnover of GFP-CFTRΔF508 was investigated by co-transfection with increasing amounts of Myc-USP19, Myc-USP19mut or USP19ΔTM-Myc. The accumulation of GFP-CFTRΔF508 was observed only in cells expressing the wild-type USP19, suggesting that both DUB activity and ER localization are crucially required to rescue this ERAD substrate (Fig 4A). A careful analysis using GFP-wtCFTR as a reference suggests that the core-glycosylated immature form (B-form) of CFTR is preferentially accumulated in response to USP19 expression (supplementary Fig 3 online).
Figure 4.
Membrane-associated USP19 rescues endoplasmic reticulum associated degradation substrates from degradation. (A) Western blot of human embryonic kidney (HEK)293T cells transfected with a constant amount of GFP-CFTRΔF508 and titrated amounts of the various USP19 constructs as indicated. A plasmid expressing the red fluorescent protein (RFP) was co-transfected to control for equal transfection efficiency. (B) Western blot of HEK293T cells transfected with FLAG-TCRα and the various USP19 constructs as indicated. The glycosylation of FLAG-TCRα is indicated and the numbers of glycans marked in grey are predicted on the basis of the earlier literature (Hirsch et al, 2003). RFP was used as a transfection control. (C) Western blot of HEK293T cells transfected with the soluble cytosolic proteasome substrate ubiquitin (Ub)-R-GFP and the various USP19 constructs as indicated. RFP was used as a transfection control. CFTR, cystic fibrosis transmembrane conductance regulator; GFP, green fluorescent protein; TCRα, T-cell receptor α; TM, transmembrane; Untr., untransfected; USP, ubiquitin-specific protease.
We next tested the effect of USP19 on the degradation of the TCRα that, unlike CFTRΔF508, is a single transmembrane domain glycoprotein facing the ER lumen and therefore is likely to show various requirements for retrotranslocation and degradation. Co-expression of FLAG-TCRα with the various USP19 variants resulted in the accumulation of deglycosylated FLAG-TCRα and slower migrating bands representing partly deglycosylated forms (Fig 4B). Partial stabilization of FLAG-TCRα was also achieved with the mutant USP19 suggesting that non-DUB-related functions might contribute to the rescue of this ERAD substrate. Attempts to study the role of endogenous USP19 in ERAD were hampered by the severity of the knockdown phenotype in which reduced cell growth and increased cell death were observed (data not shown), which is in line with the recent report of USP19 being a cell-cycle regulator (Lu et al, 2009). The localization-dependent rescue of ERAD substrates raised the question whether USP19 might also affect soluble cytosolic substrates. Supporting the importance of localization for USP19 function, co-expression of the soluble N-end rule substrate ubiquitin-R-GFP (Dantuma et al, 2000) with the USP19 constructs resulted in efficient stabilization of the reporter in cells expressing soluble USP19ΔTM-Myc (Fig 4C). In line with the observation that several ERAD components are also acting promiscuously on cytosolic substrates (Ravid et al, 2006; Yeung et al, 2008), a rescue was achieved with the ER-anchored Myc-USP19, whereas the catalytic mutant had no effect.
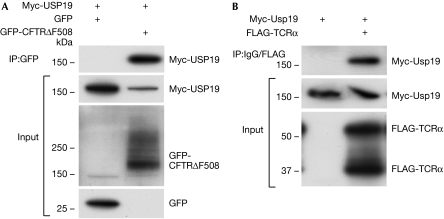
Co-immunoprecipitation experiments revealed that USP19 interacts with both GFP-CFTRΔF508 and FLAG-TCRα (Fig 5), thus suggesting that a close association of USP19 with these substrates is required for their rescue.
Figure 5.
Myc-USP19 associates with endoplasmic reticulum-associated degradation substrates. (A) Western blot of human embryonic kidney 293T cells where Myc-USP19 was co-transfected with GFP-CFTRΔF508 or GFP as control. Immunoprecipitations from whole-cell lysates were performed using a GFP antibody. The input is shown in the lower panels. (B) Western blot of co-immunoprecipitation experiments where Myc-USP19 was co-transfected with FLAG-TCRα. Immunoprecipitations were performed using a FLAG antibody or an isotype-matched antibody as control. The input is shown in the lower panels. CFTR, cystic fibrosis transmembrane conductance regulator; GFP, green fluorescent protein; TCRα, T-cell receptor α; USP, ubiquitin-specific protease.
DUBs might regulate ERAD in several ways. First, a DUB might counteract ERAD by enabling stabilization and further trafficking of proteins into the secretory pathway, as exemplified by USP4, which promotes increased cell-surface expression of the G-protein-coupled adenosin 2A receptor (Milojevic et al, 2006). A DUB can also regulate ERAD by controlling retrotranslocation as in the case of the DUB ataxin 3 that interacts with the p97–derlin 1–VIMP complex (Zhong & Pittman, 2006). A DUB might also act close to the proteasome by regulating the rate of unfolding and degradation of the substrate. Finally, DUBs might rescue luminal proteins that possibly need to survive retrotranslocation to exert a function outside the ER. There are few examples of retrotranslocation without degradation, including some hormone- and G-coupled receptors that need to leave the ER to reach the nucleus (Liao & Carpenter, 2007). The role of ubiquitination in this process is, however, unexplored.
In conclusion, our data identify USP19 as the first example of a transmembrane DUB having a function in ERAD. The requirement for membrane anchoring, together with the preferential rescue of the core-glycosylated immature form of GFP-CFTRΔF508 and deglycosylated forms of TCRα, suggests that USP19 could participate in a late step of the protein quality-control machinery by rescuing ERAD substrates that have been retrotranslocated to the cytosol. The presence of two CS/p23 domains might shed some light on USP19 function and the surprising effect of USP19mut on TCRα degradation. The CS/p23 domain is named after the protein p23, which is a small co-chaperone of heat-shock protein (HSP)90 that can bind to partly folded proteins, maintaining folding competence and preventing aggregation in vitro (Freeman et al, 1996). Through the CS/p23 domains, USP19 might promote folding by interacting with HSP90 or the domains might confer the capacity to USP19 to act as an independent chaperone. These activities of USP19 might be important for the proper folding of ER-resident proteins and for the rescue of proteins that might need to survive retrotranslocation.
Methods
Database searches. Protein sequences of human DUBs were extracted from the UniProt/Swiss-Prot database version 51.2 and the TM-HMM 2.0 algorithm (Krogh et al, 2001) was used to identify candidates with putative transmembrane domains. Domain mapping was performed using the CDART (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml), SMART (http://smart.embl-heidelberg.de/) and Pfam (http://www.sanger.ac.uk/Software/Pfam/) algorithms and databases.
Plasmids. USP19 was PCR amplified from cDNA (Kazusa DNA Research Institute) and cloned in the pcDNA4/Myc-His-B vector (Invitrogen, Carlsbad, CA, USA). The active site mutant USP19C506S was generated using the Quickchange mutagenesis kit (Stratagene; www.stratagene.com). GST- and GFP-tagged USP19 was cloned in the pGEX-5X-1 vector (Amersham) and pEGFP-N2 vector (Clontech, Mountain View, CA, USA), respectively. For generation of the ubiquitin or ubiquitin-like (Ubl)–β-galactosidase (β-gal) fusion proteins, the lacZ gene was PCR amplified from pYC6-LacZ (Invitrogen, V8255-20) and cloned into pACYCDuet1; Novagen, Darmstadt, Germany, 71147-3). Ubl cDNAs were then cloned in frame with the β-gal reporter generating the pACYC-Ub/Nedd8/SUMO1/SUMO3/ISG15/FAT10–LacZ constructs. The primers used for cloning are listed in supplementary Table 1 online.
Membrane fractionation and proteinase K digestion. Lysates from HEK293T cells were prepared using 50 strokes with a dounce homogenizer in a buffer containing 50 mM Tris pH 7.4, 250 mM sucrose, 25 mM KCl, 0.5 mM MgCl2 and protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Cell debris and nuclei were removed by 10 min centrifugation at 1,500 g, +4°C, generating the post-nuclear supernatant from which microsomes were enriched by centrifugation at 100,000 g for 1 h, +4°C. The microsome fraction was treated with 0.4 μg/ml proteinase K for 20 min on ice ±1% NP40 (Igepal, Sigma, St Louis, MO, USA).
Antibodies and immunostaining. Antibodies against β-actin (AC-15), Myc (9E10), GST (B-14) and GFP (SC-8334) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA), FLAG (M2; Sigma), GFP (Ab290; Abcam, Cambridge, MA, USA), S-tag (71549-3; Novagen), Grp78/Bip and p38 (both from Cell Signaling, Beverly, MA, USA), USP19/ZMYND9 (Bethyl Laboratories, Montgomery, TX, USA), haemagglutinin (HA) (12CA5; Roche) and red fluorescent protein (from Jacques Neefjes, The Netherlands Cancer Institute) were used in western blots together with the appropriate horse radish peroxidase (HRP)-conjugated secondary antibody (Zymed, San Francisco, CA, USA). For immunofluorescence, HEK293 cells stably expressing tagged USP19 were grown on poly-L lysine (Sigma)-coated coverslips. The cells were fixed in 4% formaldehyde and stained with calnexin (AF18; Abcam) or giantin (PRB-114C; Biosite) antibodies diluted in 50 mM Tris, pH 7.5, 0.9% NaCl, 0.1% gelatin and 0.5% Triton X-100. The result was analysed using a Zeiss Axiovert confocal microscope.
DUB assays. Deconjugase activity was investigated in bacterial co-transformation assays as described earlier (Sompallae et al, 2008). Ubiquitin conjugates were enriched by immunoprecipitation from COS7 cells expressing HA-Ub. The immunoprecipitates were incubated with 0.2 μg GST-USP19ΔTM in a buffer containing 50 mM Tris pH 7.5, 150 mM NaCl, 2 mM EDTA pH 8.0, 2 mM DTT at 37°C for the indicated time.
Real-time PCR. Total RNA was isolated according to the RNeasy plus protocol (Qiagen, Valencia, CA, USA). cDNA was transcribed using QuantiTect™ SYBR®-green mastermix (Qiagen) and real-time PCR was carried out with the appropriate primer pairs (supplementary Table 2 online) in an ABI-Prism 7000 instrument (Applied Biosystems; www.appliedbiosystems.com). Real-time analysis of SYBR green chemistry values was performed as described earlier (Edstrom & Ulfhake, 2005). GAPDH and β-actin were used as internal controls to confirm RNA integrity.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figures and Tables
Acknowledgments
We thank I. Braakman and B. Kleizen (Utrecht University, The Netherlands), E. Wiertz (Utrecht University Medical Center, The Netherlands), J. Neefjes (The Netherlands Cancer Institute) Z.-Q. Pan (Mount Sinai Medical Center, New York, USA), F. Melchior (Göttingen University, Germany), B. Hassel (University of Maryland, Marlene and Stewart Greenbaum Cancer Center, USA) and M. Groettrup (University of Constance, Germany) for the kind gifts of antibodies and plasmids. This study was supported by research grants awarded by the Swedish Cancer Society, the Swedish Medical Research Council and Karolinska Institutet (to K.L.), the Swedish Brain Foundation (to M.A.) and by the European Community Network of Excellence RUBICON Project no LSHC-CT-2005-018683 (to M.G.M.).
Footnotes
The authors declare that they have no conflict of interest.
References
- Borgese N, Brambillasca S, Colombo S (2007) How tails guide tail-anchored proteins to their destinations. Curr Opin Cell Biol 19: 368–375 [DOI] [PubMed] [Google Scholar]
- Ciechanover A (2005) Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ 12: 1178–1190 [DOI] [PubMed] [Google Scholar]
- Combaret L, Adegoke OA, Bedard N, Baracos V, Attaix D, Wing SS (2005) USP19 is a ubiquitin-specific protease regulated in rat skeletal muscle during catabolic states. Am J Physiol Endocrinol Metab 288: E693–E700 [DOI] [PubMed] [Google Scholar]
- Dantuma NP, Lindsten K, Glas R, Jellne M, Masucci MG (2000) Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nat Biotechnol 18: 538–543 [DOI] [PubMed] [Google Scholar]
- Daviet L, Colland F (2008) Targeting ubiquitin specific proteases for drug discovery. Biochimie 90: 270–283 [DOI] [PubMed] [Google Scholar]
- Edstrom E, Ulfhake B (2005) Sarcopenia is not due to lack of regenerative drive in senescent skeletal muscle. Aging Cell 4: 65–77 [DOI] [PubMed] [Google Scholar]
- Freeman BC, Toft DO, Morimoto RI (1996) Molecular chaperone machines: chaperone activities of the cyclophilin Cyp-40 and the steroid aporeceptor-associated protein p23. Science 274: 1718–1720 [DOI] [PubMed] [Google Scholar]
- Garcia-Ranea JA, Mirey G, Camonis J, Valencia A (2002) p23 and HSP20/alpha-crystallin proteins define a conserved sequence domain present in other eukaryotic protein families. FEBS Lett 529: 162–167 [DOI] [PubMed] [Google Scholar]
- Gross CT, McGinnis W (1996) DEAF-1, a novel protein that binds an essential region in a deformed response element. EMBO J 15: 1961–1970 [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Dunn R (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19: 141–172 [DOI] [PubMed] [Google Scholar]
- Hirsch C, Blom D, Ploegh HL (2003) A role for N-glycanase in the cytosolic turnover of glycoproteins. EMBO J 22: 1036–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RB, Mitchell-Felton H, Essig DA, Kandarian SC (2001) Expression of endoplasmic reticulum stress proteins during skeletal muscle disuse atrophy. Am J Physiol Cell Physiol 281: C1285–C1290 [DOI] [PubMed] [Google Scholar]
- Kinner A, Kolling R (2003) The yeast deubiquitinating enzyme Ubp16 is anchored to the outer mitochondrial membrane. FEBS Lett 549: 135–140 [DOI] [PubMed] [Google Scholar]
- Kleizen B, van Vlijmen T, de Jonge HR, Braakman I (2005) Folding of CFTR is predominantly cotranslational. Mol Cell 20: 277–287 [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305: 567–580 [DOI] [PubMed] [Google Scholar]
- Liao HJ, Carpenter G (2007) Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 18: 1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Adegoke OA, Nepveu A, Nakayama KI, Bedard N, Cheng D, Peng J, Wing SS (2009) USP19 deubiquitinating enzyme supports cell proliferation by stabilizing KPC1, a ubiquitin ligase for p27Kip1. Mol Cell Biol 29: 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T (2005) ERAD: the long road to destruction. Nat Cell Biol 7: 766–772 [DOI] [PubMed] [Google Scholar]
- Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth M, Nanoff C (2006) The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol 69: 1083–1094 [DOI] [PubMed] [Google Scholar]
- Nakamura N, Hirose S (2008) Regulation of mitochondrial morphology by USP30, a deubiquitinating enzyme present in the mitochondrial outer membrane. Mol Biol Cell 19: 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalepa G, Rolfe M, Harper JW (2006) Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov 5: 596–613 [DOI] [PubMed] [Google Scholar]
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R (2005) A genomic and functional inventory of deubiquitinating enzymes. Cell 123: 773–786 [DOI] [PubMed] [Google Scholar]
- Ravid T, Kreft SG, Hochstrasser M (2006) Membrane and soluble substrates of the Doa10 ubiquitin ligase are degraded by distinct pathways. EMBO J 25: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Sompallae R, Gastaldello S, Hildebrand S, Zinin N, Hassink G, Lindsten K, Haas J, Persson B, Masucci MG (2008) Epstein–Barr virus encodes three bona fide ubiquitin-specific proteases. J Virol 82: 10477–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung HO, Kloppsteck P, Niwa H, Isaacson RL, Matthews S, Zhang X, Freemont PS (2008) Insights into adaptor binding to the AAA protein p97. Biochem Soc Trans 36: 62–67 [DOI] [PubMed] [Google Scholar]
- Zhong X, Pittman RN (2006) Ataxin-3 binds VCP/p97 and regulates retrotranslocation of ERAD substrates. Hum Mol Genet 15: 2409–2420 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures and Tables