Abstract
The stereoselective syntheses of the (+)-D and (−)-L enantiomers of racemic iso-methanocarbathymidine (iso-MCT) was achieved through two independent linear approaches that converged on two antipodal enantiomers, common to a key precursor utilized in the synthesis of racemic iso-MCT. In this study we identified (+)-3 [D-iso-(+)-MCT] as the active enantiomer that was exclusively recognized by the herpes 1 thymidine kinase (HSV1-tk) as was predicted by molecular modeling. For this purpose, a human osteosarcoma (HOS) cell line modified to contain, and express, HSV1-tk from herpes simplex virus (HSV1) was utilized to determine the cytotoxicity of the compounds via an assay that measures the level of ATP in the cells. The work demonstrates that changes in the substitution pattern of rigid bicyclo[3.1.0]hexane nucleosides, which relative to normal nucleosides appear unconventional, can lead to the spatial optimization of pharmacophores and a vastly improved substrate recognition.
Keywords: substituted bicyclo[3.1.0]hexanes, carbocyclic nucleosides, conformationally restricted nucleosides, asymmetric synthesis, antiviral agents, herpes virus thymidine kinase (HSV-tk), biological discrimination of enantiomers
Introduction
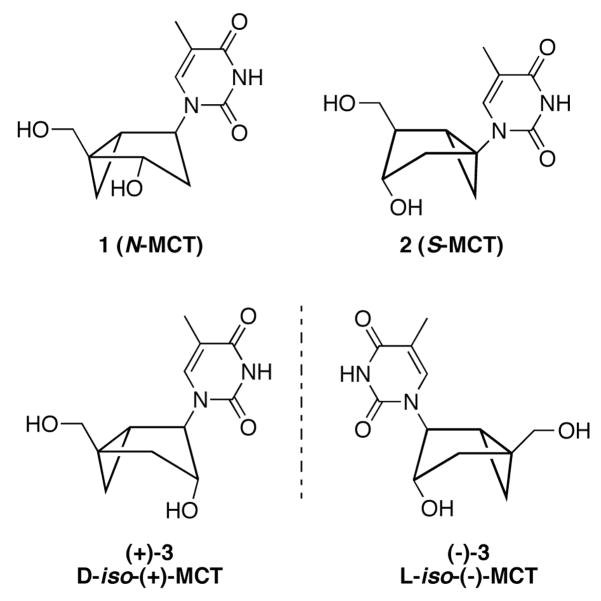
In two earlier reports we demonstrated that changes in the substitution pattern of a thymidine analogue built on a rigid bicyclo[3.1.0]hexane template resulted in greatly improved phosphorylation relative to the two canonical, locked North and South methanocarbathymidine analogues 1 (N-MCT) and 2 (S-MCT).[1,2] The superior phosphorylation of (±)-3 suggested that this new thymidine analogue had all the structural attributes necessary for optimal recognition by the viral kinase (Figure 1). However, because the original synthesis of (±)-3 produced a racemic compound,[2] and docking of the individual enantiomers at the active site of HSV1-tk indicated that only the D-enantiomer [(+)-3] would fit into the active site,[2] the synthesis and biological testing of each individual enantiomer was necessary to verify the identity of the ideal substrate. The present investigation establishes that the biologically active enantiomer, as predicted by computer docking, is indeed the D-enantiomer, (+)-3 [D-iso-(+)-MCT].
Figure 1.
Structures of 1 (N-MCT), 2 (S-MCT), and the D-[(+)-3] and L-[(−)-3] enantiomers of iso-MCT.
Results and Discussion
Synthesis
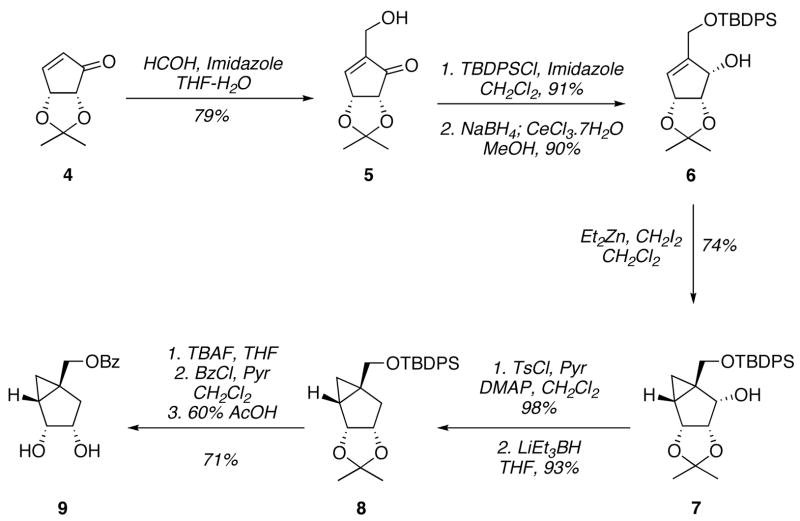
The synthetic strategy used for the preparation of (−)-3 was similar to the one already reported for the racemic counterpart, except that the radical deoxygenation step that led to the racemization was omitted and replaced by hydride reduction of the corresponding alkyl tosylate, which provided the desired deoxygenated compound as a single enantiomer. Thus, starting from the known chiral cyclopentanone 4,[3] the hydroxymethyl side chain was introduced by a Baylis-Hillman reaction in aqueous media generating 5 in high yield (Scheme 1).[2] The primary hydroxyl group was then protected as a silyl ether because the benzoate ester used in the racemic synthesis was not compatible with the reductive conditions envisioned for the deoxygenation.
Scheme 1.
Synthesis of the (−)-3-enantiomer pseudosugar precursor.
Reduction of the cyclopentenone ring under Luche conditions proceeded chemoselectively and stereoselectively to give quantitatively the allylic alcohol 6. The newly generated hydroxyl group was then used to direct the stereoselective cyclopropanation to the endo face of the molecule under modified Simmons-Smith conditions. This reaction afforded compound 7 in very good yield despite the anticipated high steric hindrance presented by the bicyclic substrate. Formation of the tosylate ester proceeded quantitatively under standard conditions and following treatment with ”Super Hydride”[4] the desired deoxygenated product 8 was obtained after column chromatography. Following the exchange of protecting groups, replacing the silyl ether for a benzoyl group, and cleavage of the acetal moiety provided diol 9, which is a common intermediate with the previous racemic synthesis.[2]
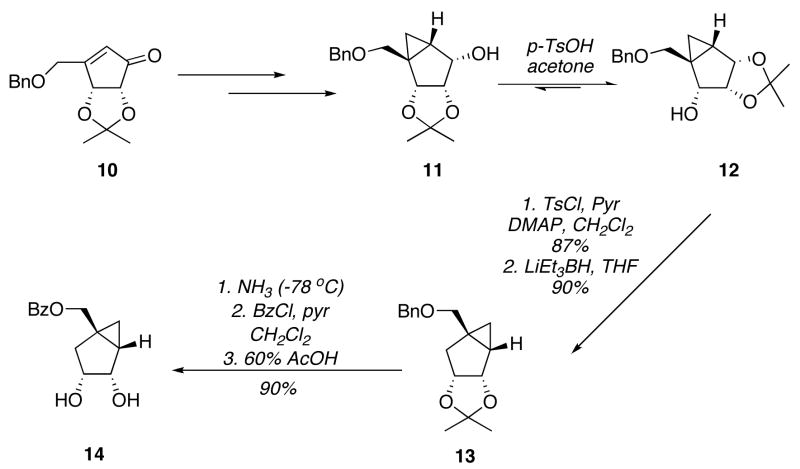
For the synthesis of (+)-3, the known chiral cyclopentenone 10[5] was employed as starting material (Scheme 2). Under similar conditions, as describe before,[6,7] hydride reduction of the carbonyl group and hydroxyl-directed cyclopropanation afforded the known intermediate 11,[6,7] which in the presence of catalytic amounts of p-toluenesulfonic acid in acetone afforded a chromatographically separable mixture of regioisomers 11 and 12 in a 2:1 ratio in favor of the desired isomer 12. After separation, a second treatment under acidic conditions allowed further transformation of 11 into 12. The deoxygenation step, which was carried out in the same manner as described above for the synthesis of 9, provided intermediate 13 in 86% yield after two steps. Similarly, after the exchange of the primary alcohol protecting group and cleavage of the isopropylidene acetal, compound 14 was obtained in 90% yield after three steps. This compound is also a common intermediate to the syntheses of (±)-3 and (−)-3.
Scheme 2.
Synthesis of the (+)-3-enantiomer pseudosugar precursor.
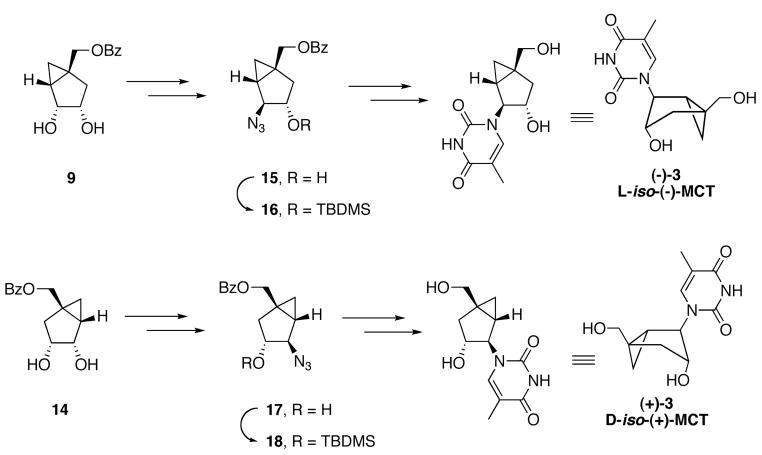
With both enantiopure alcohols 9 and 14 at hand, the two target thymidine analogues (−)-3 [L-iso-(−)-MCT] and (+)-3 [D-iso-(+)-MCT] were synthesized following the same procedure described previously for the synthesis of the racemate (Scheme 3).[2]
Scheme 3.
Synthesis of (−)-3 and (+)-3.
Biological results and molecular modeling
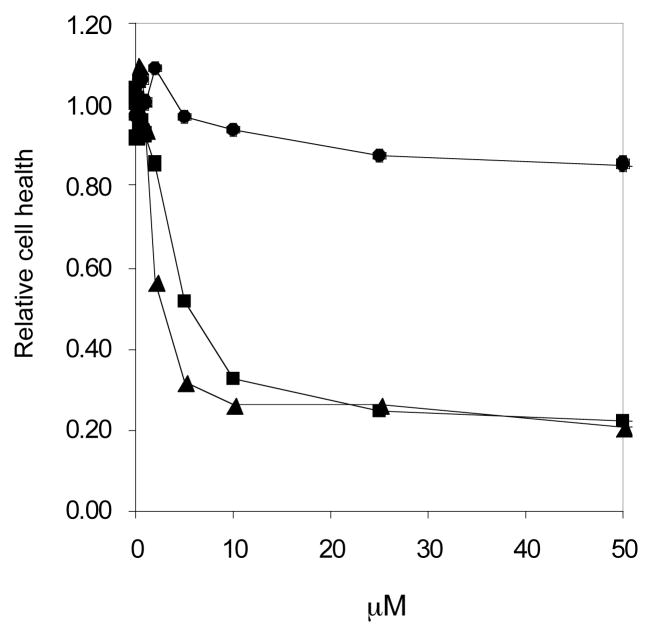
To determine the activation of these compounds by HSV1-tk, we utilized conventional human osteosarcoma (HOS) cells and HOS cells modified to contain, and express, HSV1-tk from herpes simplex virus (HSV1).[8] The cell line expressing the HSV1-tk has been designated HOS-313. Either HOS or HOS-313 cells were plated in 96 well luminescence cell culture plates and treated with each compound at different concentrations. After 48 h, cytotoxicity was determined by measuring the amount of ATP in the cells using a luciferase-based assay.[9] Light is produced by the reaction of ATP with added luciferase and D-luciferin, the amount of light is proportional to ATP concentration. Because ATP concentration declines rapidly when cells undergo necrosis or apoptosis monitoring ATP is a good indicator of cytotoxicity. The results were normalized to a value of 1.0 representing the luciferase signal in the absence of compound. No cytotoxicity was detected in the parent HOS cell line-lacking HSV1-tk for any of the compounds tested (data not shown). However, in the HOS-313 cells, which express HSV1-tk, (+)-3 [D-iso-(+)-MCT] was cytotoxic with a CC50 of 2.11 μM, while (−)-3 [L-iso-(−)-MCT] was not cytotoxic (Figure 2). It is interesting to note that at higher concentrations the cytotoxicity of (±)-3 matched that of the active enantiomer, (+)-3 [D-iso-(+)-MCT].
Figure 2.
Cytotoxicity of (+)-3 [D-iso-(+)-MCT] (▲); (−)-3 [L-iso-(−)-MCT] ( ) and racemic iso-(±)-MCT (■) in HOS-313 cells. Luciferase activity in the absence of compounds was considered to be 1.
) and racemic iso-(±)-MCT (■) in HOS-313 cells. Luciferase activity in the absence of compounds was considered to be 1.
Because N-MCT (1) showed potent anti-HIV activity in HOS-313 cells in assays using an HIV-1 viral vector, a similar assay was done to test the effects of (+)-3 [D-iso-(+)-MCT] and (−)-3 [L-iso-(−)-MCT] on viral replication.[10] The vector expresses the luciferase gene from the nef reading frame, and luciferase activity is used as a measure of the ability of the virus to infect the cells. Although (+)-3 [D-iso-(+)-MCT] effectively blocked HIV-1 replication in this assay, the IC50 was 2.15 μM (data not shown), which is very similar to the CC50 concentration of 2.11 μM in the HOS-313 cell line. The observation that the concentration of the compound that is required to reduce HIV infectivity by 50% (IC50) and the concentration that kills approximately half of the cells (CC50) are quite similar suggests that it is likely that reduction in HIV infectivity seen in cell-based assay is the result of the effect of the compound on the cells rather than a specific effect of the compound on HIV replication. Although it is possible that there is a specific effect on HIV replication that is masked by the toxicity of the compound, the toxicity makes the compound unsuitable for drug development.
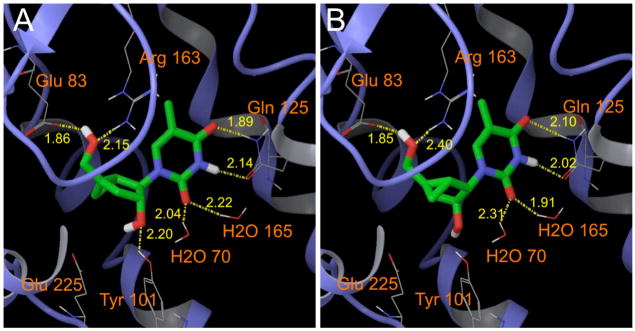
The above experiments confirmed that the active enantiomer of iso-MCT was, as predicted by the modeling, the D-(+)-enantiomer.[2] Figure 3 displays the docked poses for both enantiomers and suggest that the loss of a key hydrogen bond to Tyr101 in (−)-3 [L-iso-(−)-MCT] might be the critical difference between these two enantiomers. Therefore, (+)-3 [D-iso-(+)-MCT] appears to be an ideal substrate for HSV1-tk and thus an important tool for future biological studies.
Figure 3.
Docking of (+)-3 [D-iso-(+)-MCT] (A) and (−)-3 [L-iso-(−)-MCT] (B) at the active site of HSV-tk. The crystal structure of S-MCT (2) in complex with HSV-tk was used for the construction of the active site.[2]
Experimental Section
Cytotoxicity assay
The human osteosarcoma cell line HOS was obtained from Dr. Richard Schwartz (Michigan State University; Lansing, MI). The HOS-313 cell line was derived from HOS cells by infecting HOS cells with the previously described MLV vector KD-1.[7] In order to ensure selection of the HSV1-tk containing cells, the vector contains a gene that encodes for G418 (geneticin) resistance. Individual G418-resistant colonies were isolated, expanded, and tested for ganciclovir sensitivity; the HOS-313 cells are, as expected, ganciclovir sensitive. HOS cells were maintained in Dulbecco’s modified Eagle’s medium (Life Technologies) supplemented with 5% (v/v) fetal bovine serum, 5% newborn calf serum, and penicillin (50 units/mL) plus streptomycin (50 mg/ml) (Quality Biological). The HOS-derived cell 313 was maintained in media containing G418 at a concentration of 500 μg/mL. HOS or HOS-313 cells were plated in 96 well luminescence cell culture plates at a density of 4000 cells in 100 mL per well the day prior to treatment with the test compounds. Cytotoxicity was determined by measuring the effect of the compounds on the ATP level in the cells using the ATPlite assay (PerkinEler Waltham, MA). ATP levels were determined by treating batches of cells with different concentrations of each compound for 48 h. Luminescence was measured using a microplate reader normalized to HOS or HOS-313 data in the absence of compounds.[9] NFit (University of Texas, Galveston, Texas) was used to perform regression analysis on the data. CC50 values were determined from the fit model.
Infectivity assay
HIV-1 viral vectors were prepared as previously reported[10] and diluted 1 to 3 in media for infectivity assays. Infectivity assays were carried out by treating batches of cells with each compound at different concentrations, incubating for 3 h, then infecting cultures with the vector. The vector expresses luciferase. After 48 h infectivity was determined using a luciferase reporter assay. Luciferase activity was measured using the PerkinElmer steadylyte plus assay; luminescence was measured using a microplate reader. Luminescence data was normalized to data obtained by infecting HOS cells in the absence of compounds. NFit (University of Texas, Galveston, Texas) was used to perform regression analysis on the data. EC50 values were determined from the fit model.
Molecular Modeling
Grid files representing the shape and properties of the receptor have been described.[2] The three dimensional structures of (+)-3 [D-iso-(+)-MCT] and (−)-3 [L-iso-(−)-MCT] were constructed and optimized as reported and the compounds were subjected to flexible docking using the precomputed grid files.[2] The distance between the hydrogen atom of the OH in Tyr101 and the oxygen atom of the secondary OH of (−)-3 [L-iso-(−)-MCT] is 3.38 Å, more than the ideal distance of 2.5 Å to form a hydrogen bond.
General Synthetic methods
All chemical reagents were commercially available. Melting points were taken on a Fisher-Jones apparatus and are uncorrected. Combi-flash column chromatography was performed on silica gel 60 (230–240 mesh) employing a Teledyne Isco instrument. Analytical TLC was performed on Analtech Uniplates silica gel GF. Unless otherwise indicated, NMR spectra were determined in CDCl3 (99.8%) with residual CHCl3 as the reference peak (7.26 and 77.0 ppm) and were recorded on a Varian 400 MHz spectrophotometer. The coupling constants are reported in Hertz, and the peak shifts are reported in the delta (ppm) scale; abbreviations s (singlet), d (doublet), dd (doublet-of-doublets), ddd (doublet-of-doublet-of-doublets), t (triplet), q (quartet) and m (multiplet). Infrared spectroscopy data was obtained neat with a Jasco. FT-IR/615 spectrometer. Specific optical rotations were measured in a Perkin-Elmer model 241 polarimeter. Positive-ion fast-bombardment mass spectra (FABMS) were obtained on a VG 7070E mass spectrometer at an accelerating voltage of 6 kV and a resolution of 2000. Glycerol was used as the sample matrix, and ionization was effected by a beam of xenon atoms. Elemental analyses were performed by Atlantic Microlab. Inc., Norcross, GA.
(4S,3aS,6aR)-5-[(2,2-dimethyl-1,1-diphenyl-1-silapropoxy)methyl]-2,2-dimethyl-4,3a,6a-trihydrocyclopenta[1,2-d]1,3-dioxolan-4-ol (6)
To a stirred solution of 5[2] (2.10 g, 11.38 mmol) in anhydrous CH2Cl2 (100 mL) under argon at 0 °C, imidazole (1.72 g, 25.04 mmol) and TBDPSCl (3.6 mL, 13.66 mmol) were successively added. The reaction mixture was stirred for 1 h at the indicated temperature when brine (50 mL) was added and the aqueous phase was extracted with CH2Cl2 (3 × 30 mL). The combined organic phases were washed with brine (30 mL), dried (MgSO4) and concentrated under reduced pressure. The residue was purified by combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (19:1) as eluant to afford 4.39 g (91% yield) of the silyl ether intermediate as a colorless oil: [α]D20 = −4.6 ° (C =0.62, CHCl3); IR (neat) 1726 cm−1; 1H NMR δ 7.26 (m, 4 H, Ph), 7.56 (q, J = 2.1 Hz, 1 H, H-6), 7.39 (m, 6 H, Ph), 5.23 (m, 1 H, H-6a), 4.50 (d, J = 5.4 Hz, 1 H, H-3a), 4.41 (mAB, 2 H, -CH2OTBDPS), 1.42 (s, 3 H, -CH3), 1.40 (s, 3 H, -CH3), 1.09 (s, 9 H, -C(CH3)3); 13C NMR: δ 201.4 (C-4), 152.5 (C-6), 147.0 (C-5), 135.4 (Ph), 132.8 (Ph), 132.7 (Ph), 129.9 (Ph), 127.8 (Ph), 115.4 (-C(CH3)2), 78.0 (C-3a), 77.0 (C-6a), 58.8 (-CH2OTBDPS), 27.6 (-CH3), 26.8 (-C(CH3)3), 26.2 (-CH3), 19.2 (-C(CH3)3); FAB MS m/z (relative intensity): 423 (5), 365 (100), 345 (86). Anal. Calcd. for C25H30O4Si: %C, 71.05; %H, 7.16. Found: %C, 71.09; %H, 7.23.
A solution of this intermediate (3.81 g, 9.02 mmol) in MeOH:CH2Cl2 (2:1) (150 mL) was treated with CeCl3.7H2O (1.70 g, 4.51 mmol) and NaBH4 (170 mg, 4.51 mmol) at 0 °C. The reaction mixture was stirred at that temperature for 15 min. The pH was adjusted to 7 with acetic acid and the solution was concentrated in vacuo. The residue was dissolved in CHCl3 (3 × 50 mL) and the combined organic layers were washed with brine (2 × 20 mL), dried (MgSO4) and concentrated. The crude product was purified by combi-flash chromatography (silica gel) employing hexanes:ethyl acetate (19:1) as eluant to afford 3.78 g (90% yield) of 6 as a colorless oil: [α]D20 = −6.1 ° (C =1.80, CHCl3); IR (neat) 3418 cm−1; 1H NMR δ 7.67 (m, 4 H, Ph), 7.38 (m, 6 H, Ph), 5.89 (m, 1 H, H-6), 5.02 (m, 1 H, H-6a), 4.75 (t, J = 5.7 Hz, 1 H, H-3a), 4.36 (m, H, H-4, -CH2OTBDPS), 1.44 (s, 3 H, -CH3), 1.41 (s, 3 H, -CH3), 1.08 (s, 9 H, -C(CH3)3); 13C NMR: δ 149.2 (C-5), 135.5 (Ph), 133.4 (Ph), 129.7 (Ph), 127.7 (Ph), 125.0 (C-6), 112.2 (-C(CH3)2), 82.4 (C-6a), 77.6 (C-3a), 61.1 (-CH2OTBDPS), 27.7 (-CH3), 26.8 (-C(CH3)3), 26.6 (-CH3), 19.3 (-C(CH3)3); FAB MS m/z (relative intensity): 425 (12), 367 (47), 309 (47). Anal. Calcd. for C25H32O4Si: %C, 70.72; %H, 7.60. Found: %C, 70.78; %H, 7.41.
(4S,5S,6S,1R,2R)-4-[(2,2-dimethyl-1,1-diphenyl-1-silapropoxy)methyl]-8,8-dimethyl-7,9-dioxatricyclo[4.3.0.0<2,4>]nonan-5-ol (7)
To a solution of 6 (2.62 g, 6.17 mmol) in dichloromethane (80 mL) at 0 °C under argon, diethylzinc (8.65 mL, 8.64 mmol) and diiodomethane (0.7 mL, 8.64 mmol) were added. The mixture was allowed to reach room temperature overnight. Brine (30 mL) was added and the mixture was extracted with dichloromethane (3 × 50 mL). The organic phase was dried (MgSO4) and concentrated in vacuo. The crude product was purified by combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (19:1) as eluant to afford 2.00 g (74% yield) of 7 as a colorless oil: [α]D20 = −18.8 ° (C =0.90, CHCl3); IR (neat) 3484 cm−1; 1H NMR δ 7.64 (m, 4 H, Ph), 7.38 (m, 6 H, Ph), 4.79 (pseudo t, J = 6.0 Hz, 1 H, H-1), 4.66 (bs, 1 H, H-5), 4.55 (t, J = 7.0 Hz, 1 H, H-6), 4.06 (d, J = 11.0 Hz, 1 H, -CHaHOTBDPS), 3.32 (d, J = 11.0 Hz, 1 H, -CHHbOTBDPS), 1.54 (s, 3 H, -CH3), 1.40 (m, 1 H, H-2), 1.30 (s, 3 H, -CH3), 1.09 (dd, J = 5.2, 4.0 Hz, 1 H, H-3a), 1.05 (s, 9 H, -C(CH3)3), 0.55 (m, 1 H, H-3b); 13C NMR: δ 135.6 (Ph), 135.5 (Ph), 133.6 (Ph), 129.7 (Ph), 127.7 (Ph), 127.6 (Ph), 112.4 (-C(CH3)2), 80.3 (C-1)*, 79.3 (C-6)*, 71.5 (C-5), 65.3 (-CH2OTBDPS), 41.4 (C-4), 26.9 (-C(CH3)3), 26.3 (-CH3), 26.0 (-CH3), 24.6 (-CH3), 19.3 (-C(CH3)3), 10.3 (C-3); FAB MS m/z (relative intensity): 439 (3), 381 (9), 363 (30). Anal. Calcd. for C26H34O4Si: %C, 71.19; %H, 7.81. Found: %C, 71.16; %H, 7.63.
1-[((4S,6S,1R,2R)-8,8-dimethyl-7,9-dioxatricyclo[4.3.0.0<2,4>]non-4-yl)methoxy]-2,2-dimethyl-1,1-diphenyl-1-silapropane (8)
Alcohol 7 (3.70 g, 8.44 mmol) was dissolved in dichloroethane (100 mL), and pyridine (10 mL), dimethylaminopyridine (cat) and p-toluenesulfonyl chloride (4.80 g, 25.18 mmol) were added successively. The reaction mixture was stirred at 40 °C for 72 h. NH4Cl (satd. solution, 50 mL) was added and the aqueous phase was extracted with dichloromethane (2 × 50 mL). The combined organic phases were washed with NaHCO3 (satd. solution, 30 mL) and brine (30 mL), dried (MgSO4) and concentrated in vacuo. The residue was co-evaporated with toluene (3 × 30 mL) and purified by combi-flash column chromatography (silica gel) employing hexanes:ethyl acetate (9:1) as eluant to give 4.91 g (98% yield) of the tosylate ester as a colorless oil. This tosylate intermediate was unstable on standing so it was used immediately in the next step. Thus, a solution of LiEt3BH (1.0 M in THF, 57.0 mL) was added dropwise to a solution of the tosyl ester (3.35 g, 5.65 mmol) in anhydrous THF (50 mL) at 0 °C. The reaction mixture was stirred at rt overnight. Water (30 mL) was slowly added and the volatiles were eliminated. The residue was extracted with dichloromethane (3 × 50 mL). The organic phase was dried and concentrated in vacuo. The residue was purified by combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (9:1) as eluant to give 2.87g (93% yield) of 8 as a colorless oil: [α]D20 = −8.9 ° (C = 1.17, CHCl3); IR (neat) 1107, 1077, 1065 cm−1; 1H NMR δ 7.64 (m, 4 H, Ph), 7.39 (m, 6 H, Ph), 4.86 (dist t, J = 7.0 Hz, 1 H, H-1), 4.71 (dd, J = 7.3, 6.8 Hz, 1 H, H-6), 3.73 (d, J = 10.8 Hz, 1 H, –CHaHOTBDPS), 3.43 (d, J = 10.8 Hz, 1 H, –CHHbOTBDPS), 2.27 (ddd, J = 14.6, 7.3, 1.9 Hz, 1 H, H-5a), 2.01 (d, J = 14.6 Hz, 1 H, H-5b), 1.49 (s, 3 H, -CH3), 1.36 (m, 1 H, H-2), 1.27 (s, 3 H, -CH3), 1.05 (s, 9 H, -C(CH3)3), 0.94 (m, 1 H, H-3a), 0.65 (m, 1 H, H-3b); 13C NMR: δ 135.6 (Ph), 133.8 (Ph), 133.7 (Ph), 129.7 (Ph), 127.6 (Ph), 110.5 (-C(CH3)2), 82.5 (C-1)*, 82.1 (C-6)*, 67.2 (-CH2OTBDPS), 35.4 (C-5)*, 33.9 (C-4)*, 27.7 (CH3), 26.9 (C(CH3)3), 26.3 (C-2), 24.4 (CH3), 19.3 (C(CH3)3), 14.5 (C-3); FAB MS m/z (relative intensity): 421 (7), 365 (13). Anal. Calcd. for C26H34O3Si: %C, 73.89; %H, 8.11. Found: %C, 73.59; %H, 8.21.
(1S,3S,4R,5R)-3,4-dihydroxybicyclo[3.1.0]hexyl)methyl benzoate (9)
A solution of 8 (2.87 g, 6.79 mmol) in THF (20 ml) was treated dropwise with a solution of TBAF (1.0 M, 8.2 mL, 8.15 mmol) at rt for 1 h. The solvent was removed and the residue was purified by combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (3:7) as eluant to give 1.25 g (100% yield) of ((4S,6S,1R,2R)-8,8-dimethyl-7,9-dioxatricyclo[4.3.0.0<2,4>]non-4-yl)methan-1-ol as a colorless oil: [α]D20 = 0° (C = 0.44, CHCl3); IR (neat) 3422 cm−1; 1H NMR δ 4.85 (dist t, 1 H, H-1), 4.66 (t, J = 7.0 Hz, 1 H, H-6), 3.55 (d, J = 11.5 Hz, 1 H, –CHaHOH), 3.35 (d, J = 11.5 Hz, 1 H, –CHHbOH), 2.19 (ddd, J = 14.6, 7.3, 1.9 Hz, 1 H, H-5a), 2.00 (d, J = 14.6 Hz, 1 H, H-5b), 1.86 (bs, 1 H, -OH), 1.45 (m, 1 H, H-2), 1.43 (s, 3 H, -CH3), 1.20 (s, 3 H, -CH3), 0.96 (t, J = 4.4 Hz, 1 H, H-3a), 0.66 (m, 1 H, H-6b); 13C NMR: δ 110.6 (-C(CH3)2), 82.2 (C-1)*, 82.1 (C-6)*, 67.2 (-CH2OH), 35.7 (C-5)*, 33.9 (C-4)*, 27.7 (CH3), 26.3 (C-2), 24.3 (CH3), 15.0 (C-3). Anal. Calcd. for C10H16O3.0.1H2O: %C, 64.60; %H, 8.78. Found: %C, 64.42; %H, 8.81.
A solution of this alcohol (1.25 g, 6.79 mmol) in dichloromethane (20 mL) was treated with pyridine (0.7 mL, 8.16 mmol) and BzCl (1.3 mL, 11.21 mmol) at 0 °C. The reaction mixture was stirred for 1 h at the same temperature. Brine (20 mL) was added and the aqueous phase was extracted with dichloromethane (3 × 30 mL). The organic phase was dried (MgSO4) and concentrated in vacuo. The residue was purified by combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (9:1) as eluant to give 1.90 mg (97% yield) of the corresponding benzoate ester as a colorless oil: [α]D20 = −8.3 ° (C = 0.91, CHCl3); IR (neat) 1717 cm−1; 1H NMR δ 8.02 (m, 2 H, Ph), 7.55 (m, 1 H, Ph), 7.44 (m, 2 H, Ph), 4.94 (dist t, 1 H, H-1), 4.74 (t, J = 6.9 Hz, 1 H, H-6), 4.30 (d, J = 11.7 Hz, 1 H, –CHaHOBz), 4.20 (d, J = 11.7 Hz, 1 H, –CHHbOBz), 2.27 (ddd, J = 14.7, 7.1, 1.8 Hz, 1 H, H-5a), 2.18 (d, J = 14.7 Hz, 1 H, H-5b), 1.68 (m, 1 H, H-2), 1.50 (s, 3 H, -CH3), 1.26 (s, 3 H, -CH3), 1.13 (t, J = 4.6 Hz, 1 H, H-3a), 0.88 (m, 1 H, H-6b); 13C NMR: δ 166.5 (COOPh), 132.9 (Ph), 130.2 (Ph), 129.5 (Ph), 128.3 (Ph), 110.7 (-C(CH3)2), 82.0 (C-1)*, 82.0 (C-6)*, 69.1 (-CH2OBz), 34.6 (C-5), 32.5 (C-4), 28.4 (C-2), 26.2 (CH3), 24.3 (CH3), 15.5 (C-3); FAB MS m/z (relative intensity): 289 (47), 231 (13). Anal. Calcd. for C17H20O4: %C, 70.81; %H, 6.99. Found: %C, 71.12; %H, 7.06.
The benzoate ester (113 mg, 0.39 mmol) was treated with 60% HOAc at 70 °C for 3 h. The volatiles were eliminated and the residue was purified by combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (3:7) as eluant to give 92 mg (97% yield) of 9 as a white solid: mp 59-58 °C, [α]D20 = −42.4 ° (C = 0.74, CHCl3); 1H NMR δ 8.04 (m, 2 H, Ph), 7.57 (m, 1 H, Ph), 7.45 (m, 2 H, Ph), 4.54 (t, J = 5.1 Hz, 1 H, H-4), 4.29 (ABq, J = 11.6 Hz, 2 H, -CH2OBz), 4.16 (t, J = 6.2 Hz, 1 H, H-3), 2.26 (ddd, J = 14.2, 6.2, 1.5 Hz, 1 H, H-2a),2.23 (bs, 2 H, -OH), 2.10 (d, J = 14.2 Hz, 1 H, H-2b), 1.65 (m, 1 H, H-5), 1.36 (t, J = 4.3 Hz, 1 H, H-6a), 0.68 (m, 1 H, H-6b). This 1H NMR was identical to that of the racemic compound reported before.[2]
(1S,2S,4R,5R,6R)-8,8-dimethyl-7,9-dioxa-4[(phenylmethoxy)methyl]tricyclo- [4.3.0.0<2,4>]nonan-5-ol (12)
A solution of known compound 11[6] (3.64 g, 12.54 mmol) and p-TsOH (600 mg, 3.14 mmol) in acetone (70 mL) was refluxed for 2 h. Et3N (1 mL) was added and the volatiles were eliminated under vacuum. The residue was purified by combi-flash chromatography (silica gel) employing hexanes:ethyl acetate (3:2) as eluant to give 2.38 g of pure 12 as a colorless oil and 1.22 g of recovered starting material (99% yield based on recovered starting material). Compound 12: [α]D20 = 8.2 ° (C = 0.67, CHCl3); IR (neat) 3453 cm−1; 1H NMR (CD2Cl2) δ 7.31 (m, 5 H, Ph), 4.81 (pseudo t, 1 H, H-1), 4.54-4.45 (m, 4 H, H-5, H-6, -OCH2Ph), 3.75 (d, J = 10.4 Hz, 1 H, -CHaHOBn), 3.15 (d, J = 10.4 Hz, 1 H, -CHHbOBn), 2.30 (br s, 1 H, OH), 1.56 (m, 1 H, H-2), 1.49 (s, 3 H, -CH3), 1.25 (s, 3 H, -CH3), 1.10 (dd, J = 5.2, 4.1 Hz, 1 H, H-3a), 0.57 (m, 1 H, H-3b); 13C NMR: δ 138.6, 128.2, 127.6, 127.5, 112.2, 80.2, 79.3, 72.8, 72.6, 71.9, 39.4, 26.5, 25.8, 24.3, 10.6; FAB MS m/z (relative intensity): 291 (14), 215 (4). Anal. Calcd. for C17H22O4: %C, 70.32; %H, 7.64. Found: %C, 70.64; %H, 7.64.
((1S,2S,4R,6R)-8,8-dimethyl-7,9-dioxatricyclo[4.3.0.0<2,4>]non-4-yl)(phenylmethoxy)methane (13)
Alcohol 12 (1.73 g, 5.96 mmol) was dissolved in dichloromethane (30 mL), and pyridine (3 mL), dimethylaminopyridine (cat) and p-toluenesulfonyl chloride (7.0 g, 35.7 mmol) were successively added. The reaction mixture was stirred at 40 °C for 72 h. NH4Cl (satd. solution, 30 mL) was added and the aqueous phase was extracted with dichloromethane (3 × 30 mL). The combined organic phases were washed with NaHCO3 (satd. solution, 30 mL) and brine (30 mL), dried (MgSO4) and concentrated in vacuo. The residue was co-evaporated with toluene (3 × 30 mL) and purified by Combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (9:1) as eluant to give 2.5 g (96 % yield) of the tosylate ester as a colorless oil. This intermediate was unstable on standing and was used immediately in the next step. Thus, a solution of LiEt3BH (1.0 M in THF, 52.0 mL) was added dropwise to a solution of the tosylate (2.3 g, 5.17 mmol) in anhydrous THF (30 ml) at 0 °C. The reaction mixture was stirred at rt overnight. Water (30 mL) was slowly added and the volatiles were eliminated. The residue was extracted with dichloromethane (3 × 50 mL). The organic phase was dried (MgSO4) and concentrated in vacuo. The residue was purified by Combi-flash column chromatography (silica gel) employing hexanes: ethyl acetate (9:1) as eluant to give 1.26 g (90 % yield) of 13 as a colorless oil: [α]D20 = 7.9 ° (C = 1.37, CHCl3); IR (neat) 1207, 1066 cm−1; 1H NMR δ 7.30 (m, 5 H, Ph), 4.89 (pseudo t, 1 H, H-1), 4.68 (t, J = 7.1 Hz, 1 H, H-6), 4.48 (ABm, 2 H, -OCH2Ph), 3.46 (d, J = 10.4 Hz, 1 H, -CHaHOBn), 3.23 (d, J = 10.4 Hz, 1 H, -CHHbOBn), 2.25 (ddd, J = 14.6, 7.1, 1.9 Hz, 1 H, H-5a), 2.01 (d, J = 14.6 Hz, 1 H, H-5b), 1.48 (m, 4 H, H-2, -CH3), 1.23 (s, 3 H, -CH3), 1.00 (t, J = 4.5 Hz, 1 H, H-3a), 0.67 (m, 1 H, H-3b); 13C NMR: δ 138.5, 128.4, 127.6, 127.5, 110.6, 82.3, 82.2, 74.5, 72.7, 34.4, 33.4, 28.1, 26.3, 24.3, 15.2; FAB MS m/z (relative intensity): 275 (3), 217 (7). Anal. Calcd. for C17H22O3.0.2H2O: %C, 73.51; %H, 8.13. Found: %C, 73.51; %H, 7.97.
(4S,5S,1R,3R,)-3,4-dihydroxybicyclo[3.1.0]hexyl)methyl benzoate (14)
A solution of 13 (1.24 g, 4.52 mmol) in THF (10 mL) was treated with liquid ammonia at −78 °C until the blue color persisted. NH4Cl (satd. solution, 5 mL) was slowly added and the mixture was allowed to reach rt. The mixture was extracted with dichloromethane (3 × 20 mL). The organic phase was dried (MgSO4) and concentrated in vacuo. The crude product was treated as described in the synthesis of 9 to give 1.0 g of compound 14 (90% yield for three steps) as a clear oil: [α]D20 = 42.2 ° (C = 1.01, CHCl3). The 1H NMR was identical to that of the racemic compound[2] and enantiomeric compound 9 (vide supra).
Syntheses of (−)-3 [L-iso-(−)-MCT] and (+)-3 [D-iso-(+)-MCT]
These compounds and their intermediates were synthesized in the same manner as described for the racemate (±)-3.[2] All the spectral data, physical properties, and combustion analyses matched those obtained for the corresponding racemates, except for optical rotations.
[(1S,3S,4S,5R)-4-azido-3-hydroxybicyclo[3.1.0]hexyl]methyl benzoate (15)
[α]D20 = 125.4 ° (C = 0.90, CHCl3); 1H NMR δ 8.03 (m, 2 H, Ph), 7.53 (m, 1 H, Ph), 7.41 (m, 2 H, Ph), 4.35 (ABq, J = 11.7 Hz, 2 H, -CH2OBz), 4.24 (d, J = 6.8 Hz, 1 H, H-3), 3.64 (s, 1 H, H-4), 2.35 (ddd, J = 14.2, 7.0, 2.2 Hz, 1 H, H-2a), 1.91 (d, J = 14.2 Hz, 1 H, H-2b), 1.72 (bs, 1 H, -OH), 1.60 (ddd, J = 9.2, 4.1, 1.6 Hz, 1 H, H-5), 1.23 (dd, J = 5.5, 4.5 Hz, 1 H, H-6a), 0.87 (ddd, J = 9.2, 5.3, 2.0 Hz, 1 H, H-6b).
(1S,3S,4S,5R)-4-azido-3-(1,1,2,2-tetramethyl-1-silapropoxy)bicyclo[3.1.0]hexyl] methyl benzoate (16)
[α]D20 = 89.0 ° (C = 1.30, CHCl3); 1H NMR δ 8.07 (m, 2 H, Ph), 7.56 (m, 1 H, Ph), 7.45 (m, 2 H, Ph), 4.47 (d, J = 11.7 Hz, 1 H, –CHaHOBz), 4.30 (d, J = 11.7 Hz, 1 H, –CHHbOBz), 4.16 (dd, J = 6.5, 1.5 Hz, 1 H, H-3), 3.57 (s, 1 H, H-4), 2.31 (ddd, J = 13.8, 6.6, 2.0 Hz, 1 H, H-2a), 1.85 (d, J = 13.8 Hz, 1 H, H-2b), 1.57 (ddd, J = 9.1, 4.1, 1.5 Hz, 1 H, H-5), 1.30 (m, 1 H, H-6a), 0.87 (s, 9 H, -C(CH3)3), 0.85 (m, 1 H, H-6b), 0.05 (s, 3 H, -CH3), 0.05 (s, 3 H, -CH3).
1-[(2S,3S,5S,1R)-3-hydroxy-5-(hydroxymethyl)bicyclo[3.1.0]hex-2-yl]-5-methyl-1,3-dihydropyrimidine-2,4-dione [(−)-3]
[α]D20 = −23.9 ° (C = 1.05, CHCl3); 1H NMR (CD3OD) δ 7.93 (d, J = 1.2 Hz, 1 H, H-6), 4.69 (s, 1 H, H-2′), 4.04 (d, J = 7.3 Hz, 1 H, H-3′), 4.02 (d, J = 11.7 Hz, 1 H, –CHaHOH), 3.34 (d, J = 11.7 Hz, 1 H, –CHHbOH), 2.44 (ddd, J = 14.3, 7.3, 2.0 Hz, 1 H, H-4′a), 1.86 (d, J = 1,2 Hz, 3 H, -CH3), 1.71 (d, J = 14.3 Hz, 1 H, H-4′b), 1.32 (ddd, J = 9.2, 4.0, 1.4 Hz, 1 H, H-1′), 1.16 (dd, J = 4.9, 4.0 Hz, 1 H, H-6′a), 0.77 (ddd, J = 9.2, 4.9, 2.0 Hz, 1 H, H-6b).
[(1R,3R,4R,5S)-4-azido-3-hydroxybicyclo[3.1.0]hexyl]methyl benzoate (17)
[α]D20 = −124.0 ° (C = 0.68, CHCl3); The 1H NMR was identical to that of the racemic compound[2] and enantiomeric compound 15 (vide supra).
[(1R,3R,4R,5S)-4-azido-3-(1,1,2,2-tetramethyl-1-silapropoxy)bicyclo[3.1.0]hexyl] methyl benzoate (18)
[α]D20 = −91.5 ° (C = 0.85, CHCl3); The 1H NMR was identical to that of the racemic compound[2] and enantiomeric compound 16 (vide supra).
1-[(1S,2R,3R,5R)-3-hydroxy-5-(hydroxymethyl)bicyclo[3.1.0]hex-2-yl]-5-methyl-1,3-dihydropyrimidine-2,4-dione [(+)-3]
[α]D20 = 24.6 ° (C = 1.43, CHCl3); The 1H NMR was identical to that of the racemic compound[2] and enantiomeric compound (−)-3 (vide supra).
Supplementary Material
Acknowledgments
The authors thank Drs. James A. Kelley and Christopher Lai of the Laboratory of Medicinal Chemistry, NCI-Frederick, for mass spectral analyses. This research was supported in part by the Intramural Research Program of the NIH, Center for Cancer Research, NCI-Frederick.
Footnotes
Supporting Information Available: 1H and 13C NMR spectra of new compounds.
References
- 1.Marquez VE, Choi Y, Comin MJ, Russ P, George C, Huleihel M, BenKasus T, Agbaria R. J Am Chem Soc. 2005;127:15145–15150. doi: 10.1021/ja053789s. [DOI] [PubMed] [Google Scholar]
- 2.Comin MJ, Agbaria R, Ben-Kasus T, Huleihel M, Liao C, Sun G, Nicklaus MC, Deschamps JR, Parrish DA, Marquez VE. J Am Chem Soc. 2007;129:6216–6222. doi: 10.1021/ja0688732. [DOI] [PubMed] [Google Scholar]
- 3.Moon HR, Choi WJ, Kim HO, Jeong LS. Tetrahedron-Asymmetry. 2002;13:1189–1193. [Google Scholar]
- 4.Krishnamurthy S, Brown HC. J Org Chem. 1976;41:3064–3066. [Google Scholar]
- 5.Marquez VE, Lim M-I, Tseng CKH, Markovac A, Priest MA, Khan MS, Kaskar B. J Org Chem. 1988;53:5709–5714. [Google Scholar]
- 6.Jeong LS, Marquez VE, Yuan CS, Borchardt RT. Heterocycles. 1995;41:2651–2656. [Google Scholar]
- 7.Jeong LS, Buenger G, McCormack JJ, Cooney DA, Hao Z, Marquez VE. J Med Chem. 1998;41:2572–2578. doi: 10.1021/jm980111x. [DOI] [PubMed] [Google Scholar]
- 8.Delviks KA, Hu WS, Pathak VK. J Virol. 1997;71:6218–6224. doi: 10.1128/jvi.71.8.6218-6224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strehler DL, McElroy WD. In: Methods in Enzymology. Colowick P, Kaplan NO, editors. Vol. 3. Academic Press Inc; New York: 1957. pp. 871–873. [Google Scholar]
- 10.Boyer PL, Julias JG, Marquez VE, Hughes SH. J Mol Biol. 2005;345:441–450. doi: 10.1016/j.jmb.2004.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.