Abstract
Cells sort into regions and groups in part by their selective surface expression of particular classic cadherins during development. In the nervous system, cadherin-based sorting can define axon tracts, restrict axonal and dendritic arbors to particular regions or layers, and may encode certain aspects of synapse specificity. The underlying model has been that afferents and their targets hold in common the expression of a particular cadherin, thereby providing a recognition code of homophilic cadherin binding. However, most neurons express multiple cadherins, and it is not clear whether multiple cadherins all act similarly in shaping neural circuitry. Here we asked how two such cadherins, cadherin-8 and N-cadherin, influence the guidance and differentiation of hippocampal mossy fibers. Using organotypic hippocampal cultures, we find that cadherin-8 regulates mossy fiber fasciculation and targeting, but has little effect on CA3 dendrites. In contrast, N-cadherin regulates mossy fiber fasciculation, but has little impact on axonal growth and targeting. However, N-cadherin is essential for CA3 dendrite arborization. Both cadherins are required for formation of proper numbers of presynaptic terminals. Mechanistically, such differential actions of these two cadherins could, in theory, reflect coupling to distinct intracellular binding partners. However, we find that both cadherins bind b-catenin in dentate gyrus (DG). This suggests that cadherins may engage different intracellular signaling cascades downstream of b-catenin, coopt different extracellular binding partners, or target distinct subcellular domains. Together our findings demonstrate that cadherin-8 and N-cadherin are critical for generating the mossy fiber pathway, but that each contributes differentially to afferent and target differentiation, thereby complementing one another in the assembly of a synaptic circuit.
Keywords: axon guidance, synaptogenesis, DG, dendritic arborization, hippocampus
Introduction
Axons project accurately to their targets by sensing and responding to a variety of chemical cues. Diffusible and standing gradients of cues can guide axons over relatively long distances, while regionally restricted, more homogeneously distributed cues appear to more strongly influence guidance at intermediate “choice points” and to help stop growth within a target. Such regionally restricted cues can influence dendritic differentiation, highlighting the collaboration that has to occur between ingrowing axons and their simultaneously differentiating targets.
Classic cadherins show strict regional and laminar distribution patterns (Redies et al., 1993; Yamagata et al., 1995; Suzuki et al., 1997a; Huntley and Benson, 1999; Bekirov et al., 2002). Homophilic binding between cadherins generates strong adhesion that sorts cells into regions and groups early in development (Hatta and Takeichi, 1986; Espeseth et al., 1998; Price et al., 2002). As neurons differentiate and send axons to distant targets, it has been proposed that homophilic cadherin adhesion between source and target neurons may be important for ensuring accuracy in the development of connectivity. Cadherins can promote axon extension (Neugebauer et al., 1988; Tomaselli et al., 1988; Friedlander et al., 1989; Balsamo and Lilien, 1990; Drazba and Lemmon, 1990; Doherty et al., 1991; Shimoyama et al., 2000; Utton et al., 2001; Andrews and Mastick, 2003; Marthiens et al., 2005) and selective fasciculation (Wöhrn et al., 1999; Treubert-Zimmermann et al., 2002), and can provide short-range instructional cues (Andrews and Mastick, 2003). Within a target, cadherins can influence dendritic differentiation (Zhu and Luo, 2004). Additionally, cadherin binding across the synaptic cleft is essential for stabilizing young synapses (Togashi et al., 2002; Bozdagi et al., 2004; Elste and Benson, 2006)—an action that would be expected to prevent further extension. Consistent with this idea, N-cadherin adhesion retains retinal-collicular and thalamocortical axons within their target layers (Inoue and Sanes, 1997; Poskanzer et al., 2003), and in Drosophila, appropriate photoreceptor-target interactions require DN-cadherin (Lee et al., 2001). However, individual neurons express several cadherins, and many of these are shared between source and target (Suzuki et al., 1997b; Miskevich et al., 1998; Wöhrn et al., 1999; Bekirov et al., 2002; Gil et al., 2002). It remains an unanswered question as to whether, and how, such multiply expressed cadherins function in the same pathway. The intracellular domains of classic cadherins are highly conserved and are presumed to engage the same intracellular binding partners, making it difficult to understand how they might achieve a coherent functional output. Here, we sought to determine the individual actions of coexpressed cadherins on the generation of a well-defined anatomical pathway-the hippocampal mossy fiber pathway leading from dentate gyrus (DG) to the CA3 region of hippocampus. Cadherin-8 and N-cadherin are two prototypical, classic cadherins expressed early in development by DG granule neurons and their target, area CA3 neurons (Redies and Takeichi, 1993; Korematsu and Redies, 1997; Bekirov, 2004). We find that cadherin-8 and N-cadherin are both essential for the development of the mossy fiber projection. However, each cadherin contributes differently to establishing axon trajectory and target region, thus complementing one another in the formation of this synaptic circuit.
Materials and Methods
Animals
Sprague Dawley rat pups were used for all of the experiments. The day of birth was designated as postnatal day (P) 0. The care and treatment of the rats adhered strictly to guidelines established by both the National Institutes of Health and Mount Sinai's Institutional Animal Care and Use Committee.
Organotypic Hippocampal Cultures
Hippocampal slices were cultured on membranes as described previously (Stoppini et al., 1991). After inducing anesthesia by hypothermia, 5- to 6-day-old rat pups were decapitated and the hippocampus was dissected in sterile, ice cold d-(1)-glucose-enriched (6.5 g/L) Gey's Balanced Salt Solution. Four-hundred micron thick slices were cut using a McIIwain Tissue Chopper (Surrey, England), and those from the midseptotemporal level of the hippocampus were transferred to membranes in culture inserts (0.4 lm pores; Millipore, MA) and placed in 6 or 12-well plates containing a mixture of Eagle's Basal Medium with Earle's Salts (50%), Earle's Balanced Salt Solution (25%), horse serum (25%), 50% d-(1)-glucose (1.0%), and 200 mM l-glutamine (0.5%). Slices were cultured at 378C, 5% CO2. After 24 h, cultures were placed on a rocking platform for the remainder of the culture period. A mixture of antimitotic inhibitors (1026 M of cytosine arabinoside, uridine, and fluorodeoxyuridine, Sigma, St. Louis, MO) was added after 2 days, and media was changed every 2–3 days.
Cadherin Reagents
Synthetic peptides
The following peptide sequences, HLRAHAVDINGNQVEN (HAV), TLTAQAVDDWETNKPLE (QAV), and ARLQHDVNANVHEING (scrambled control) were purchased from Mimotopes (Raleigh, NC). The HAV sequence was chosen because several studies have demonstrated that the tripeptide sequence, HAV, is contained within a region important for mediating adhesion (Blaschuk et al., 1990; Nose et al., 1990). Structural data demonstrate that at least four residues in the peptide sequence are part of the adhesive interface (Patel et al., 2006), and the flanking regions are sufficient to confer specificity (Blaschuk et al., 1990; Noe et al., 1999; Williams et al., 2000). The HAV motif in type I cadherins is replaced with QAV in cadherin-8 and several other type II cadherins (Shimoyama et al., 2000). Structural data indicate that the adhesive interface residues contained within this region are common to type I and type II cadherins (Patel et al., 2006). The flanking sequences correspond to rat cadherin-8 (Williams et al., 2000). Cadherin peptides or a scrambled control peptide (SCR) were added to culture media starting at 0 days in vitro (div) at a concentration of 200 lg/mL unless indicated otherwise (Doherty et al., 1991). Peptide was replenished when media was changed, and slices were fixed after 5 days in culture by immersion in 4% paraformaldehyde in phosphate buffered saline (PBS). Effects on the mossy fiber pathway were evaluated using DiI labeling or immunostaining as described later.
Cadherin-Fc chimeras
A cadherin-8-Fc chimeric DNA construct was generated by joining the extracellular domain spanning EC1 through EC4 of rat cadherin-8 to the Fc region of human IgG. The extracellular domain of cadherin-8 was generated by PCR from pBS SK containing full-length cadherin-8 [a kind gift from Dr. S.T. Suzuki, Kido et al., 1998)] as a 1,657 bp XbaI-NotI restriction fragment. This fragment was then inserted into a modified pCR3.1 vector [a kind gift from Dr. Martin Grumet, (Haspel et al., 2001)] cut with XbaI and NotI. The DNA was transformed into Top10F' E. coli cells (Invitrogen, Carlsbad, CA). Plasmid DNA was purified from colonies using a standard plasmid isolation protocol (Qiagen, Valencia, CA) and presence of insert was verified by restriction analysis and sequencing. Cadherin-8-Fc plasmid DNA was transiently transfected in COS-7 cells using Polyfect (Qiagen, Valencia, CA), and after 5 days the supernatant was collected and concentrated. N-cadherin-Fc was similarly isolated from either HEK 293 cells that were stably expressing N-cadherin-Fc (a gift from Dr. Takeshi Sakurai) or COS-7 cells that were transiently transfected. The identity and size of both Fc proteins was verified by Western blotting (data not shown). The Fc modification at the C-terminus does not affect adhesive function (Chappuis-Flament et al., 2001).
Immunohistochemistry
Cultures were fixed as described earlier and immunolabeled for either MAP2 [monoclonal antibody AP-14; (Stearns and Binder, 1987)], b-III tubulin (monoclonal; Covance, Berkeley, CA) or SV2 (Developmental Studies Hybridoma Bank). Antibody binding was visualized by incubation in fluorescently conjugated secondary antibodies (Southern Biotech, Birmingham, AL). Laminar and regional borders were confirmed with the fluorescent Nissl stain TOTO-3 iodide (Molecular Probes, Eugene, OR). Images were acquired using a Zeiss LSM 510 META confocal microscope (Zeiss, Thornwood, NY) with Argon and k633 He-Ne lasers. To assay cell death, slice cultures were maintained in a CO2 incubator for 30 min in culture media containing 1 lM of the membrane-impermeant dead-cell marker Sytox Green (Molecular Probes, Eugene, OR). Fluorescence of living slices was visualized directly through the culture plate using an inverted fluorescence microscope (Nikon Diaphot) and imaged using the same exposure parameters with a Hamamatsu ORCA-ER digital camera. Fluorescence intensity was quantified in areas CA3 and CA1 using MetaMorph (Universal Imaging, Dowlington, PA). To quantify SV2 labeling, images were acquired using a 1003/1.5 N.A. oil immersion objective and a zoom factor of 2 on a Zeiss LSM 410 confocal microscope (Zeiss, Thornwood, NY) with an Ar/Kr laser and filters k515–540 and k670–810. Twelve confocal images per slice (four images/lamina) were taken in strata lucidum, pyramidale, and oriens and at least five slices were imaged per treatment condition. All images were obtained using the same brightness and contrast settings. Puncta number, area, and intensity were determined using MetaMorph, and the data were exported to Excel. Differences between groups were assessed using unpaired t-tests; alpha was corrected for the parameter being compared and the peptide used (3 groups) (JMP software, SAS Institute, Cary, NC), and the data were graphed in Excel.
DiI Labeling
To assess mossy fiber trajectories in organotypic slices a pulled glass capillary filament was used to place small crystals of DiI (DiIC18(3); Molecular Probes, Eugene, OR) taken from a 0.5% DiI solution diluted in dimethylformamide according to previously described procedures (Poskanzer et al., 2003). Slice cultures were fixed for 12 h in 4% paraformaldehyde and crystals were deposited under microscope guidance into the hilar region, where fibers converge as they exit the DG. Slices were then placed in PBS containing 0.2% sodium azide and incubated at 378C in the dark for 2–4 days. Labeling was visualized by using a Zeiss LSM 410 and a 403/0.9 N.A. oil objective and photomontages were generated using Adobe Photoshop (Mountain View, CA). Maps of the trajectories were drawn using a Zeiss Axiophot equipped with Neurolucida (MicroBrightField, Colchester, VT).
Dissociated Granule Cell Cutures
Postnatal day 6 (P6) rat pups were deeply anesthetized by hypothermia, and the hippocampi were dissected. A cut was made along the hippocampal fissure and the DG was excised. Dentate tissue was placed in balanced salt solution (BSS: 13 Hank's BSS; 0.3 M HEPES, pH 5 7.3; 13 penicillin/streptomycin), cut into small pieces and incubated in 0.25% trypsin and 0.1% DNase I at 378C for 30 min. Dissociated cells were filtered through nylon mesh into a sterile conical tube containing 3 mL 10% horse serum, pelleted, and resuspended in BSS. Sixty-three hundred granule cells per well were plated in 12-well cell culture plates (Costar, Corning, Corning, NY) containing Neurobasal medium with 0.4 mM l-glutamine and B-27 supplement (Gibco-Invitrogen Corp., Carlsbad, CA) on glass coverslips coated with N-cadherin-Fc, cadherin-8 Fc, or having a confluent layer of L cells, N-cadherin L cells or cadherin-8 L cells. To coat coverslips with Fc fusion proteins, poly-l-lysine coated coverslips were incubated with antihuman IgG antibody (Fc-specific monoclonal; Sigma, St. Louis, MO), followed by incubation with cadherin-8-Fc or N-cadherin-Fc. L-cells were plated directly on glass. Three hours after plating, synthetic peptides (QAV, HAV, or scrambled peptide) were added at a final concentration of 200 lg/mL (unless noted otherwise). Cultures were incubated overnight at 378C/5% CO2, rinsed in 0.01 M PBS and then fixed in 4% paraformaldehyde. Neurons were visualized under phase optics as well as fluorescence microscopy using a Zeiss Axiophot microscope with a 403/0.75 N.A. objective. Neurite lengths were traced using Neurolucida software and analyzed using NeuroExplorer. All individual tree length data was imported into JMP for statistical analysis. Means were compared using unpaired Student's t-test or analysis of variance (ANOVA) and a post test (level of significance, P < 0.05). P-values and details are provided in the figure legends. Results were then graphed using Prism (GraphPad software).
Coimmunoprecipitations
Dentate gyri from postnatal day 6 rat pups were isolated and homogenized in extraction buffer [1% digitonin in HEPES buffer (25 mM HEPES, pH 7.4; 150 mM NaCl)] containing 13 Complete protease inhibitor cocktail (Roche, Indianapolis, IN). One milligram of protein was preabsorbed with Protein G Agarose (Invitrogen) for 2 h at 48C. The spun-down supernatant was incubated overnight at 48C with N-cadherin (BD Transduction Labs, San Jose, CA) or cadherin-8 (Santa Cruz Biotechnology, Santa Cruz, CA). After adding beads, samples were incubated for 2 h at 48C. All IPs were washed twice in 1% digitonin/HEPES, once in HEPES, and then analyzed by 10% SDS-PAGE followed by Western blotting with anti-b-catenin antibody (monoclonal; BD Transduction Labs). Labeling was visualized using ECL (Pierce, Rockland, IL).
Results
Classic cadherins generally bind homophilically. In the first part of this study, we exploited this property to assess functional activity of N-cadherin and cadherin-8 in granule cell axon outgrowth by culturing dissociated granule cells on cadherin substrates. Additionally, this strategy also allowed us to determine the effectiveness and specificity of peptide blocking reagents in a controlled environment. However, the development of connectivity in vivo occurs in a more complex, molecular environment. Thus, in the second part of this study, we assessed outgrowth and synapse formation in organotypic hippocampal slice cultures in order to determine the actions of cadherins in the assembly of a circuit in situ.
N-Cadherin, But Not Cadherin-8, Promotes Mossy Fiber Extension On Cadherin Substrates
DG granule cells express both N-cadherin and cadherin-8 during the first postnatal weeks when their axons are extending rapidly (Amaral and Dent, 1981; Korematsu and Redies, 1997; Bekirov et al., 2002). To determine whether N-cadherin and cadherin-8 are equally capable of promoting mossy fiber outgrowth, dentate granule neurons were dissociated at P5 or P6, plated on immobilized N-cadherin or cadherin-8 substrates, and after 24 h, were fixed and immunostained for bIII-tubulin to label neuronal processes. Labeled neurites were traced using Neurolucida, and axons were defined conventionally as processes that exceed the others in length by at least 5 lm (Goslin and Banker, 1989b). At this stage of development, minor processes are commonly quite short (20 lm or less) and the differences between the lengths of minor processes and axons are usually tens of microns (Goslin and Banker, 1989a; Goslin et al., 1990; Esch et al., 1999) (Fig. 1). On an N-cadherin substrate, the average axon length is 90.7 lm (65.7), while when grown on cadherin-8, axons are on average 34% shorter (60.1 6 5) lm (Figs. 1A,C and 2A). The reduced axon outgrowth on cadherin-8 did not result from poor attachment, since cell counts indicate that similar numbers of neurons attached to cadherin-8 and N-cadherin substrates (data not shown). Additionally, all of the neurites appear to firmly adhere to cadherin-8 and do not float free. Thus, while both cadherin-8 and N-cadherin support cell binding and axon extension, it is possible that cadherin-8 binding may be more strongly adhesive than N-cadherin, attenuating the rate axon extension. Alternatively, its actions may be modified by a coreceptor or by the differential activation of a signaling pathway (e.g., Rhee et al., 2002).
FIGURE 1.
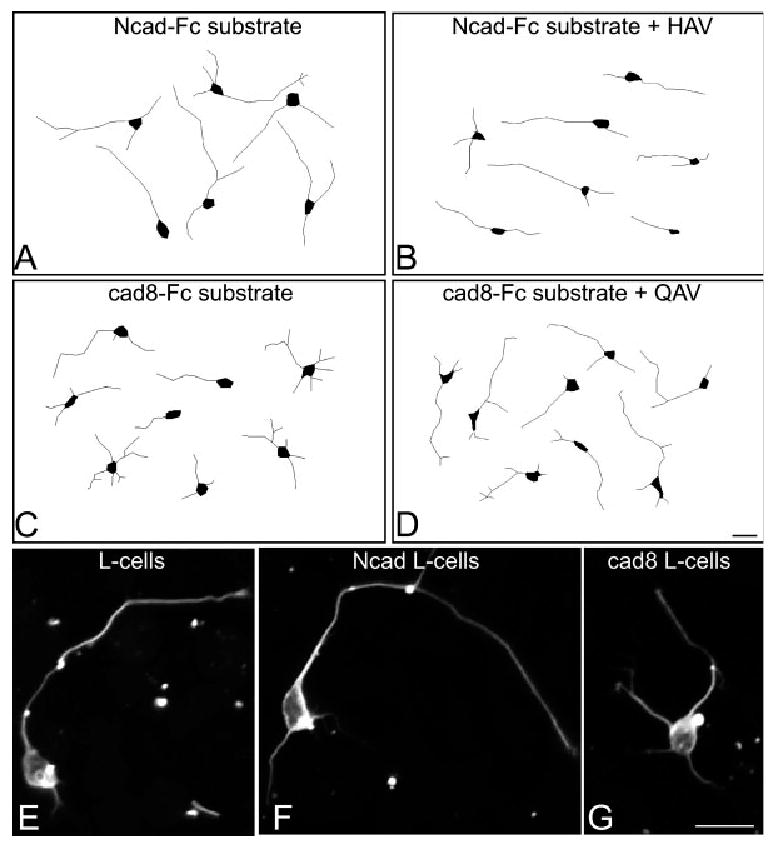
N-cadherin and cadherin-8 differentially promote outgrowth. Representative examples of Neurolucida drawings (A–D) or photomicrographs (E–G) of DG granule cells that were dissociated and plated on cadherin-Fc substrates (A–D) or cadherin-expressing L cells (E–G). The longest process was defined as the axon according to convention (see text). N-cadherin promotes axonal growth (A) and this is reduced by HAV peptides (B). In contrast, neurons grown on cadherin-8 substrate have shorter axons (C) in comparison with those grown on N-cadherin, and axons grow longer in the presence of QAV peptides (D). On L cells, cadherin-8-mediated outgrowth (G) is similar to L-cells alone (E), but axons are shorter than those on N-cadherin L cells (F). Magnification bars 5 10 lm. Ncad, N-cadherin; cad8, cadherin-8.
FIGURE 2.
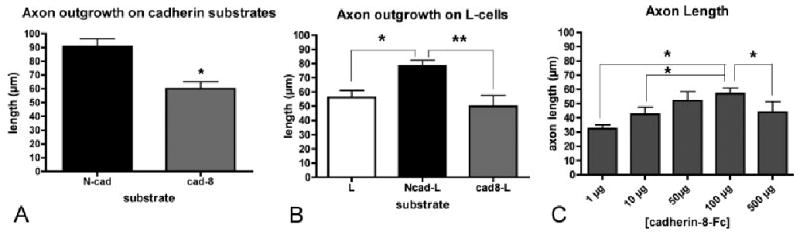
Quantitative differences between N-cadherin- and cadherin-8-mediated outgrowth. DG granule cells were dissociated and plated on purified cadherin-Fc substrates (A, C), or on plain or transfected L-cells (B). (A) Total axon length for neurons growing on cadherin-8 (gray) is significantly shorter than on N-cadherin (black) (90.7 6 5.7 lm, n 5 40 vs. 60.1 6 5 lm, n 5 43, *P < 0.0,001, t-test). (B) On L-cells expressing cadherin-8 (gray), axonal growth is similar to untransfected L-cells (white) (49.9 6 6.1 lm, n 5 21 vs. 53.15 6 6 lm, n 5 40), while cells expressing N-cadherin (black) support more outgrowth (78.4 6 6.1 lm, n 5 21) (ANOVA, P 5 0.023; Tukey's post test, *P < 0.05, **P < 0.002). Granule cells plated on a range of concentrations of cadherin-8, show increased axon outgrowth up to 100 lg/mL (ANOVA, P 5 0.0,001; Tukey's post test, *P < 0.05) (C). Ncad, N-cadherin-Fc substrate; cad-8, cadherin-8-Fc substrate; L, L cell; Ncad-L, N-cadherin expressing L cells; cad8-L, cadherin-8 expressing L cells.
Although our data indicate that N-cadherin is a better substrate for granule cell axon extension than cadherin-8, it is unclear whether such rates of extension are faster or slower in conjunction with those on other substrates. To fully understand the effects of these cadherins on outgrowth, we cultured dissociated granule cells on a more complex, cellular substrate. We compared axon extension on mouse L-cells [fibroblasts that express fibronectin (Toda et al., 1994), but no native cadherins (Nose et al., 1988)] with that on L-cells stably expressing N-cadherin or cadherin-8. Axons growing on N-cadherin L-cells are significantly longer than those on untransfected L-cells (Figs. 1E,F and 2B), while axons extending on cadherin-8 L-cells are slightly, but not significantly shorter than those growing on untransfected L-cells (Figs. 1E,G and 2B).
If axon extension on cadherin-8 substrates reflects a stronger adhesive interaction, we would expect that increasing concentrations of cadherin-8 would decrease growth. However, when granule cells were cultured on cadherin-8 concentrations ranging from 1 to 500 lg/mL, axon length gradually, albeit modestly, increased up to 100 lg/mL and decreased at 500 lg/mL (Fig. 2C). Thus, cadherin-8 supports slow mossy fiber extension at low concentrations and less extension at high concentrations, contrary to the accelerated rates seen with other classic cadherins (Fig. 2A).
Peptides Interfere With Cadherin-Dependent Axon Extension On Cadherin Substrates
The EC1 domain of classical cadherins is critical for mediating homophilic binding specificity (Nose et al., 1990) and adhesion (Nose et al., 1990; Shapiro et al., 1995; Patel et al., 2006). A His-Ala-Val (HAV) sequence within EC1 is common to Type I classical cadherins like N-cadherin, and several lines of experimental data show that exogenous peptides containing this sequence flanked by amino acids specific for N-cadherin are highly effective at blocking N-cadherin mediated neurite outgrowth (Blaschuk et al., 1990; Williams et al., 2000). Consistent with this, we find that an HAV peptide specifically inhibits granule cell axon extension on an N-cadherin substrate (Figs. 1B vs A and black bars in 3A).
FIGURE 3.
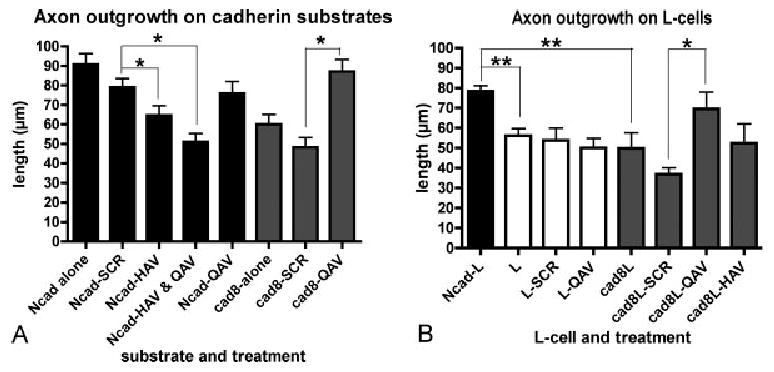
Effects of blocking reagents on cadherin-mediated outgrowth. Dentate granule neurons were grown on purified N-cadherin-Fc (black bars, A), cadherin-8-Fc (gray bars, A), or on L cells expressing N-cadherin (black bars), cadherin-8 (gray bars), or no cadherins (white bars) (B, C) and the effects of the blocking reagents were measured. (A) HAV reduces axon extension on N-cadherin, and QAV increases axon extension on cadherin-8 compared with scrambled peptide (SCR) controls (for A: ANOVA, P < 0.0,001; Tukey's post test, *P < 0.05). The effects of QAV are specific to cadherin-8: QAV has no effect on axon extension on N-cadherin (A), untransfected L cells (B), and similar to the effects observed purified cadherin-8 (A), QAV increases axon extension on cadherin-8 expressing L cells compared with SCR-treated or untreated cadherin-8 L-cells (B). The magnitude of the QAV-mediated increase is similar to that observed on N-cad expressing L-cells (black, B). (for B: ANOVA, P 5 0.0,019; Tukey's post test, *P < 0.05, **P < 0.01). Abbreviations: as in figure 2; SCR, scrambled peptide; QAV, QAV peptide; HAV, HAV peptide.
Type II cadherins-including cadherin-8-contain a glutamine (Q) in lieu of histadine in the homologous region. Thus, because HAV peptides interfere with N-cadherin mediated outgrowth, we designed a QAV peptide that covers the homologous region in cadherin-8, and similar to HAV peptides, covers several residues involved in the cadherin-8 adhesive interface (Patel et al., 2006). In contrast to the inhibitory effects on axon outgrowth observed with HAV peptides, we found that neurons exposed to the QAV peptide show increased axon extension on both purified cadherin-8 substrates and cadherin-8 expressing L cells, suggesting that cadherin-8 negatively regulates granule cell axon extension (Figs. 1D vs. C and gray bars in 3A,B). We verified that the QAV peptide does not simply stimulate axon extension nonspecifically, since outgrowth on purified N-cadherin or on untransfected L cells is not altered by exposure to QAV (black bars in Fig. 3A and white bars in 3B). Taken together, these data indicate that regardless of whether extension is on purified cadherin substrates or on cadherin expressing L-cells, blocking N-cadherin with HAV peptide reduces axon growth while blocking cadherin-8 with QAV peptide enhances axon growth.
N-Cadherin and Cadherin-8 Are Required for Mossy Fiber Laminar and Regional Specificity
To determine the contributions of N-cadherin and cadherin-8 to mossy fiber targeting, we examined mossy fiber outgrowth in organotypic cultures of hippocampal slices. In this ex vivo environment, mossy fibers extend from granule cells and innervate the CA3 pyramidal cell layer much as they do in vivo, indicating that the cues required to target this pathway remain intact (Gahwiler, 1981, 1984a,b; Frotscher and Heimrich, 1995). We cultured slices from P5/6 hippocampus for 5 days in the presence of blocking or control peptides and labeled mossy fibers with DiI.
In organotypic hippocampal slice cultures exposed to scrambled peptides, the majority of DiI-labeled mossy fibers travel in a well-defined, fasciculated bundle that runs immediately superficial to the CA3 pyramidal cell layer and ends at or near the CA1 border (Figs. 4A,B). The length of the pathway varies depending on the size of DG relative to CA3, but it never extends into CA1 (Zimmer and Gahwiler, 1984) (Figs. 4A,B). Occasionally, axons are observed in the pyramidal cell layer (Figs. 4A–C). Mossy fibers also innervate the DG molecular layer (not shown) and sometimes form an infrapyramidal tract (Fig. 4B). This pattern is indistinguishable from untreated cultures (not shown) and similar to what has been described in previous work (Zimmer and Gahwiler, 1984; Dailey et al., 1994; Robain et al., 1994).
FIGURE 4.
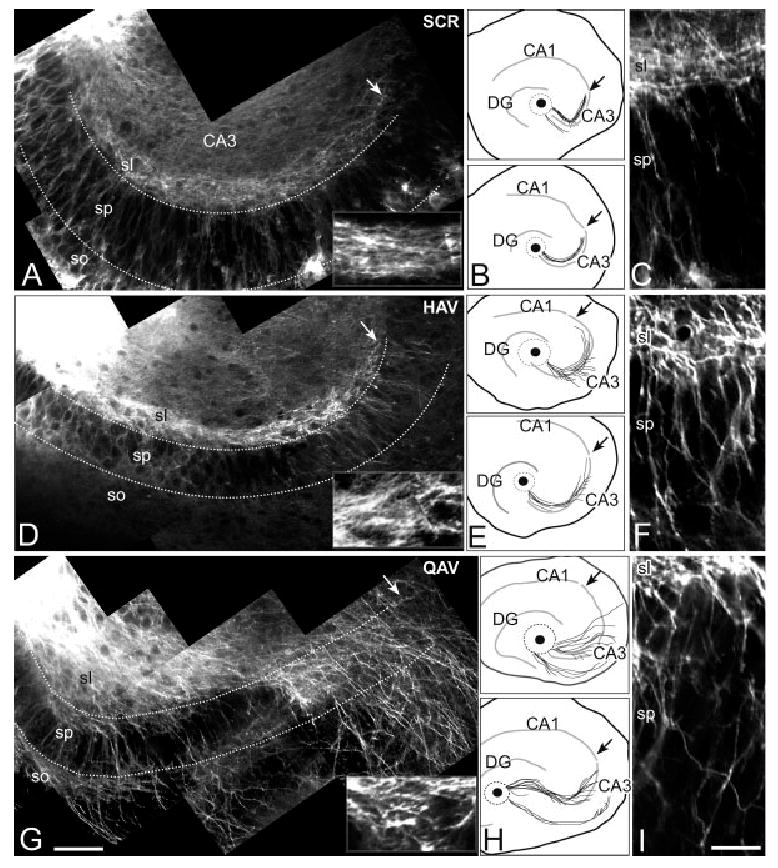
Cadherin-8 and N-cadherin differentially affect mossy fiber outgrowth in situ. Confocal images and Neurolucida generated maps of DiI-labeled mossy fibers in P5/P6 hippocampal organotypic slices that were cultured for 5 days. Low-magnification images are shown at left with higher magnification images of stratum lucidum as insets (A, D, G). Maps of additional cultures under similar conditions are shown in the middle column (B, E, H), and high-magnification images focused on the pyramidal cell layer (sp) are shown in the right hand column (C, F, I). Cultures treated with scrambled peptides (SCR) show hippocampal mossy fibers concentrated in stratum lucidum (sl) with only a few fibers invading the pyramidal cell layer (sp, A–C). Higher magnification inset of a more heavily labeled preparation more clearly reveals the tight, parallel organization of the fibers. Dotted lines delineate layers, arrows indicate the CA3/CA1 border, and the injection site in the dentate hilus is just out of the field of view in the upper left (A). In the maps (B, E, H) a black dot indicates the injection site and the dotted circle, the intense glow surrounding the site. The principal cell layers are indicated by thick lines. Cultures incubated with HAV (D–F) show mossy fibers that more frequently invade the pyramidal cell layer (sp). The inset in D shows the mossy fibers to be more splayed than in A. Following exposure to QAV peptide (G–I) mossy fibers show an even greater degree of disorganization and loss of laminar specificity. Fibers frequently appear overgrown and extend into stratum oriens (so). sl, stratum lucidum, sp, stratum pyramidale, so, stratum oriens. Magnification bars 5 82 lm (A, D, G); 24.6 lm (C, F, I); 41 lm (insets).
Hippocampi exposed to HAV for 5 days show poorly fasciculated mossy fibers that cover a broader swath (Figs. 4D–F). Decreased fasciculation has also observed following antibody-induced N-cadherin blockade in chick retinal ganglion cells grown on laminin (Drazba and Lemmon, 1990) and in a population of longitudinal axon tracts in Drosophila embryos lacking functional DN-cadherin (Iwai et al., 1997). Mossy fibers commonly invade the CA3 pyramidal cell layer. Usually this is observed toward the end of the trajectory, but can also occur more proximally (Figs. 4E,F). However, fibers do not extend into CA1 (Figs. 4D–F). Thus, while HAV inhibits axon extension in dissociated granule cells grown on N-cadherin substrates, it does not prevent the formation of the mossy fiber trajectory in organotypic slices. These data underscore the importance of comparing outcomes on defined substrates, where the actions of a peptide can be more precisely isolated, with those derived from more complex, in vivo-like contexts, where it is clear that the substrate for extension is not N-cadherin. Rather, it appears that mossy fibers in vivo are more likely to engage N-cadherin through interactions with other mossy fibers.
Cultures incubated with QAV show exuberant mossy fiber growth (Figs. 4G,H). Axons extend up to, but not beyond the CA1 border and then turn toward stratum oriens. All along the pathway, axons are poorly fasciculated and send branches into the CA3 pyramidal layer and stratum oriens (Figs. 4G–I). The increase in outgrowth observed is similar to what we see in dissociated granule cell axons grown on cadherin-8 and suggests that homophilic cadherin-8 interactions negatively regulate mossy fiber extension both on defined cadherin-8 substrates and in the more molecularly complex environment of organotypic cultures.
Interfering With N-Cadherin, But Not Cadherin-8, Affects Postsynaptic CA3 Dendrites
Dendritic arborization is profoundly affected by innervating axons (Rakic, 1975; Cline, 2001) and both axons and dendrites express classic cadherins (Yamagata et al., 1995; Benson and Tanaka, 1998; Gil et al., 2002; Elste and Benson, 2006). Thus, the peptides we employed in the organotypic cultures are likely to have had effects on postsynaptic target dendrites as well as on presynaptic axons. To assess dendritic differentiation in CA3 in situ, hippocampal slices that were cultured in the presence or absence of the cadherin peptides were immunostained for MAP2, a dendrite-specific marker. In comparison with untreated slices (Fig. 5A), those exposed for 5 days to HAV (Fig. 5B) exhibited little MAP2-labeling in CA3 dendrites compared with controls or to CA1 and the DG (Figs. 5A,B), but Nissl-stained cell bodies were still detected in the pyramidal cell layer (Fig. 5C).
FIGURE 5.
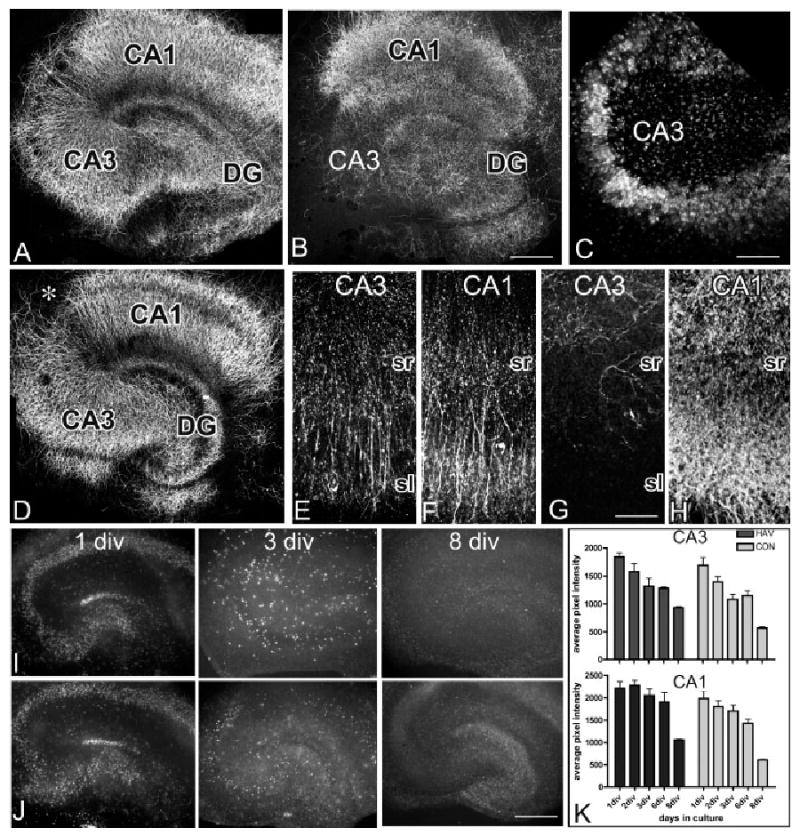
Impeding N-cadherin, but not cadherin-8, leads to a loss of CA3 dendrites. Confocal image of MAP2 immunostaining shows the normal morphology of hippocampal dendrites at 5 days in culture (A). Following 5 days of HAV exposure (B, C), there is a dramatic loss of MAP2 immunostained dendrites in the CA3 region of hippocampus, but not elsewhere in the slice. Fluorescent Nissl staining using TOTO-3 shows an intact cell body layer in CA3 (C). There are no notable changes in dendritic morphology after 5 days exposure to QAV (D). The break seen between CA3 and CA1 (*) is the normal site of attachment for the fimbria (D). It is commonly seen in these types of organotypic slice cultures and is not a consequence of the treatment (Zimmer and Gahwiler, 1984). Higher magnification images show that dendritic morphology in CA3 (E) is similar to that of CA1 (F) after one day exposure to HAV peptide. After 5 days of HAV treatment (G,H), it is clear that CA1 dendrites have continued to grow and arborize (H), while the CA3 dendrites present on the first day are lost and are not replaced (G). In E–H, the pyramidal cell layer is at the bottom of the field. Fluorescence images of Sytox labeling reveals compromised neurons in both untreated (row I) and HAV-treated (row J) slice cultures. In both conditions, labeling is abundant and evenly distributed at one div (I,J; left column), substantially reduced by three div (I, J; middle column) and near absent by eight div (I, J; right column). Quantitative analysis of Sytox labeling in CA3 and CA1 (K). Bars show the mean fluorescence intensity 6 standard error of mean (SEM) (2-way ANOVA shows no interaction between peptide treatments and days in vitro, P 5 0.7,717, F 5 0.45 (CA3) and P 5 0.9,016, F 5 0.26 (CA1), but the peptide treatment significantly influences outcomes in both CA3 (P 5 0.001, F 5 11.64) and CA1 (P < 0.001, F 5 17.4). However, Bonferroni post tests comparing peptide treatments in either CA3 or CA1 revealed no significant differences between groups of the same age (div), n 5 5–10 slices per time point). Magnification bars 5 246 lm (A–C), 61.5 lm (D), 82.5 lm (E, F), 363 lm (G, H). DG, dentate gyrus; sr, stratum radiatum; sl, stratum lucidum.
The HAV-induced loss of MAP2 staining could reflect several possibilities: HAV treatment could prevent dendrite outgrowth, promote dendrite retraction, or initiate neuronal cell death. The decreased labeling could also reflect a selective loss of MAP2 in otherwise intact dendrites, but this is unlikely as staining for tubulin also decreases in CA3 (data not shown). To distinguish between an effect on dendritic outgrowth or retraction, we monitored dendritic arbors over the course of 5 days of HAV treatment. On day one, burgeoning dendritic arbors cover the full extent of stratum radiatum in CA3 and CA1 in control and treated cultures (Figs. 5E,F). By the end of 3 days, labeled dendrites are reduced in CA3, and by day 5, nearly absent in HAV-treated cultures (Fig. 5G, and data not shown), a time at which DiI labeled mossy fibers show a relatively normal trajectory (Figs. 4D,E). Over the same time course, CA3 arbors in control cultures, and CA1 arbors in all cultures, show a dramatic increase in density (Figs. 5A,B,E,F,H). These data suggest that HAV treatment prevents further dendritic elaboration and leads to dendritic retraction in CA3.
To determine whether this is a selective effect on dendrites or a cytotoxic effect on CA3 pyramidal neurons, we labeled HAV-treated and control cultures with Sytox, a high affinity, DNA binding stain that readily crosses compromised plasma membrane in late apoptotic and necrotic cells and is excluded from healthy cells (Petersen and Dailey, 2004; Evans and Cousin, 2006). At one day in culture, both control and HAV-treated cultures show extensive labeling (Figs. 5I–K) consistent with the trauma that occurs on the surface during slicing (Pozzo Miller et al., 1994; Laake et al., 1999; Petersen and Dailey, 2004). There is culture-to-culture variability in the regional intensity of the labeling patterns, but all principal layers are labeled consistently in both groups. By 3 days, Sytox labeling is clearly decreased in control and HAV-treated cultures (Figs. 5I–K). Very little labeling is observed by 8 days in any culture (Figs. 5I–K). Thus, some cell death occurs throughout the hippocampus similar to what has been reported previously (Pozzo Miller et al., 1994; Laake et al., 1999; Petersen and Dailey, 2004), but the vast majority is early (1–2 days in culture) and transient, similar between treatment groups, and shows no bias toward CA3 (Fig. 5K). Additionally, survival in dissociated hippocampal pyramidal neurons exposed to HAV for 2–7 days is indistinguishable from controls (DLB, unpublished data). Together these data suggest that N-cadherin adhesion is required to support and maintain the integrity of dendrites in CA3 and that the mistargeting we observe in the mossy fibers (Figs. 4D–F) can be attributed, either partly or in whole, to a loss of the target structure to which the axons normally grow. Given the dramatic changes observed in CA3, mossy fiber targeting remains remarkably intact in the presence of HAV peptide.
In contrast to the effects of HAV peptide on CA3 pyramidal neuron dendrites, MAP2 labeled dendrites in organotypic slices that have been exposed for 5 days to QAV appear very similar to untreated slices (Figs. 5A,D).
Cadherin-8 and N-Cadherin Are Required for the Development of Presynaptic Terminals
Since mossy fiber targeting and CA3 pyramidal cell dendritic arbors are greatly changed following exposure to QAV or HAV peptides, respectively, we asked how exposure to the peptides impacts the generation of synapses in CA3. To monitor synapses, cultures were immunostained for an integral synaptic vesicle protein, SV2 and the distribution of labeled clusters was assessed (Voigt et al., 1993; Bözdagi et al., 2000). In HAV-treated cultures the numbers of SV2-labeled clusters are reduced in stratum lucidum as well as in the pyramidal cell layer and stratum oriens (Figs. 6A,B). In addition, size and intensity of individual clusters are reduced in stratum lucidum and the pyramidal cell layer as might be expected for vesicles that fail to form presynaptic aggregates and remain below the limit of resolution. These data are consistent with a dramatically reduced target in CA3.
FIGURE 6.
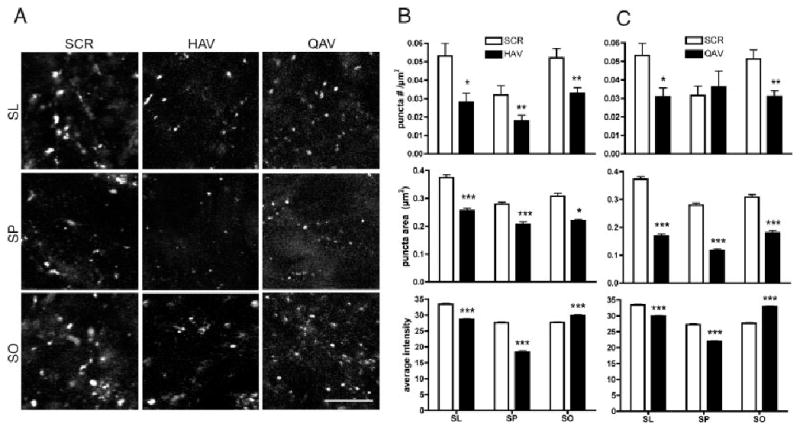
Synaptogenesis is inhibited when either N-cadherin or cadherin-8 function is impeded. Confocal images of P5/6 slices that were cultured for 5 days in the presence of the peptides indicated and then fixed and immunostained for SV2 (A). The hippocampal layers from which the images were taken are indicated by row labels: SL (stratum lucidum), SP (stratum pyramidale), and SO (stratum oriens). Images from HAV- and QAV-treated cultures show decreased labeling for SV2 throughout CA3 relative to SCR control. The source of these differences was determined using a morphometric analysis, and the data are summarized in the bar graphs shown in B (HAV) and C (QAV). The number of SV2-labeled puncta decreases after HAV treatment in all lamina compared with control (B). The size and intensity of the puncta also decrease significantly in SL and SP. Following QAV treatment (C), number, size, and intensity of labeled puncta are decreased in SL. Puncta area and intensity are reduced in SP, and while puncta number and area are decreased in SO, intensity is slightly increased. Comparisons were made using unpaired t-tests and an alpha value that was corrected for layer and peptide. *P < 0.05, **P < 0.005, ***P < 0.0,001. Magnification bar 5 12.3 lm.
Exposure to QAV also reduces the number, size, and intensity of SV2-labeled clusters throughout most of CA3 (Figs. 6A,C). Although pyramidal cell dendritic arbors remain intact in QAV-treated cultures, mossy fibers defasciculate and invade strata pyramidale and oriens. Thus, QAV appears to prevent the assembly of normal presynaptic vesicle aggregates in stratum lucidum despite multiple opportunities for en passant contact. This suggests that cadherin-8 binding or activation may be important to stabilize developing synaptic contacts in stratum lucidum as well as in the pyramidal cell layer and stratum oriens, which also show a reduced cluster size (Figs. 6A,C).
Cadherin-8 and N-Cadherin Both Bind b-Catenin in DG Granule Cells
Interfering with either cadherin-8 or N-cadherin adhesion ultimately reduces the number of presynaptic terminals forming in CA3. However, each cadherin appears to influence differently either the pre- or the postsynaptic component of the mossy fiber—CA3 synaptic circuit. In the organotypic cultures, interfering with N-cadherin binding primarily alters the organization of the target CA3 pyramidal cell dendrites while interfering with cadherin-8 binding alters the innervating mossy fiber trajectory. Since both cadherins are expressed by granule cells, we asked whether they might differentially recruit b-catenin. The highly conserved intracellular domains of all classic cadherins would be predicted to bind to b-catenin, which via a-catenin can firmly anchor cadherins to F-actin (Ozawa et al., 1989; Knudsen et al., 1995).
Immunoblots for cadherin-8 and N-cadherin indicate that both proteins are expressed in DG, and cadherin-8 levels are enriched relative to the cerebral cortex (Fig. 7A). Both cadherin-8 and N-cadherin can efficiently pull down b-catenin from DG lysates (Fig. 7B). Cadherin-8 precipitates more b-catenin in the DG than in cerebral cortex, consistent with the relatively greater levels of cadherin-8 in DG (Figs. 7A,B). In contrast, N-cadherin pulls down far more b-catenin in the DG than in the cerebral cortex where expression levels are roughly equivalent (Figs. 7A,B). This suggests that N-cadherin has a stronger, more stable interaction with b-catenin in the DG than in cerebral cortex, but could also reflect the increased levels of b-catenin in the DG. Although it is not possible to compare the relative levels of proteins pulled down in separate immunoprecipitates, the results show clearly that b-catenin can interact with both N-cadherin and cadherin-8 in DG. These data suggest that the different functional outcomes we observe for N-cadherin and cadherin-8 may result from differences in their subcellular distribution or from the recruitment and activation of cis-binding partners that could activate different signaling cascades.
FIGURE 7.
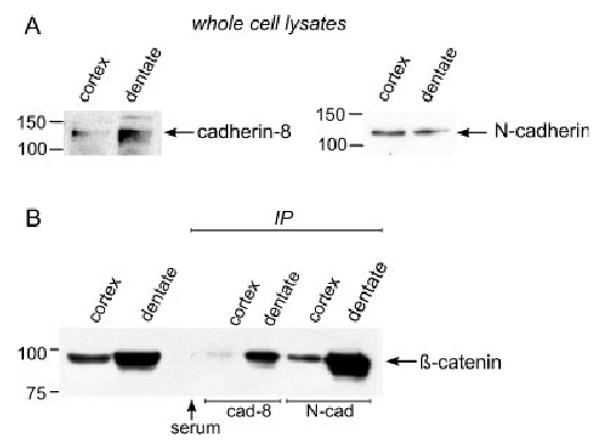
Cadherin-8 and N-cadherin bind b-catenin in DG granule cells. (A) Whole cell lysates from P5/6 cerebral cortex and DG contain both cadherin-8 and N-cadherin. Cadherin-8 is more abundant in DG and N-cadherin levels are equivalent in both regions. (B) Immunoprecipitates of both cadherin-8 and N-cadherin pull down b-catenin. Less b-catenin is pulled down in cerebral cortex lysates than in DG by both cadherin-8 and N-cadherin. For cadherin-8, this likely reflects the different levels of cadherin-8 in the lysates, but for N-cadherin, the interaction in DG appears to be particularly strong. At the same time, b-catenin is expressed at higher levels in the DG than in cortex. Lysates incubated with equivalent levels of serum failed to show any specific reaction with b-catenin.
Discussion
The selective expression of particular cadherins can sort cells, including neurons, into tissues, areas, or subregional domains during development. As neurons differentiate and form connections, most express several cadherins simultaneously. How individual neurons utilize this diversity is poorly understood. Here we asked how N-cadherin and cadherin-8, both coexpressed by DG granule cells and their targets, area CA3 neurons, influence the development and targeting of the hippocampal mossy fiber pathway. Using organotypic hippocampal slice cultures, we find that cadherin-8 is important for regulating mossy fiber growth and targeting, while N-cadherin is essential for maintaining the integrity of CA3 pyramidal neurons. Both cadherins, however, contribute to stabilization of presynaptic boutons. Thus, each cadherin appears to be recruited for distinct functions that differentially influence proper development of the presynaptic innervating pathway or the postsynaptic target dendrites, thereby acting complementing each other in the establishment of a neural circuit.
Role of N-Cadherin in Mossy Fiber Targeting
In hippocampus, N-cadherin is distributed broadly in neurons and astrocytes (Redies and Takeichi, 1993; Wilby et al., 1999; Bekirov et al., 2002). Extending mossy fibers would be expected to partner with N-cadherin on adjacent axons as well as on astrocytes and dendrites in the neuropil. Thus, as expected, HAV defasciculated mossy fibers in organotypic cultures. Premature defasciculation exposes fibers to inappropriate cues, and this is likely to be the cause of the increase in fibers invading the pyramidal cell layer. Nevertheless, the effects on outgrowth are modest, and the basic trajectory taken by the fibers is relatively normal. Fibers do not invade area CA3 stratum radiatum, rarely invade CA3 stratum oriens, and were never observed anywhere in CA1. This is surprising, since HAV destroys the target CA3 dendrites in organotypic cultures, which are a likely source of homophilic binding interactions. Thus, mossy fiber guidance does not require interaction between appropriate pre- and postsynaptic targets. Although a similar molecular disassociation has been observed in the developing Drosophila olfactory system where N-cadherin influences axon and dendrite arborization independently (Hummel and Zipursky, 2004; Zhu and Luo, 2004), our data show that no physical association is required. The findings indicate that for at least this pathway, guidance and synaptogenesis are separable events.
Role of Cadherin-8 in Mossy Fiber Targeting
N-cadherin's ability to promote axon extension has been well-documented in vitro for a variety of neuron types (Neugebauer et al., 1988; Tomaselli et al., 1988; Friedlander et al., 1989; Bixby and Zhang, 1990; Drazba and Lemmon, 1990; Doherty et al., 1991; Shimoyama et al., 2000; Utton et al., 2001). R-cadherin and cadherin-11 can also promote axon extension in vitro (Andrews and Mastick, 2003; Marthiens et al., 2005). The high degree of structural similarity between classic cadherins suggests that cadherin-8 should also promote growth. But we find that while dissociated granule cells adhere and extend axons on cadherin-8 substrates, axonal growth is not enhanced as it is on N-cadherin. Exposure to QAV peptides, covering the domain analogous to the HAV domain in N-cadherin, increases mossy fiber growth on defined cadherin-8 substrates and in organotypic cultures, but not when dissociated cells are plated on N-cadherin or untransfected L-cells. The simplest interpretation of these findings is that cadherin-8 promotes stronger adhesion than N-cadherin in growing axons and that QAV attenuates this, producing less controlled extension. This idea is consistent with the distribution of cadherin-8 in hippocampus: within CA3, cadherin-8 mRNA is expressed at low levels in proximal CA3, increases gradually, and reaches a peak near the CA1 border where mossy fibers end (Korematsu and Redies, 1997; Bekirov et al., 2002). However, dissociated granule cells show a slight increase in average axon length as substrate concentrations are increased from 1 to 100 lg/mL, suggesting that concentration dependent changes in adhesive strength are insufficient to account for our findings. Additionally, in vitro binding data suggest that cadherin-8 mediated adhesion may be weaker than that mediated by N-cadherin (Kido et al., 1998). Although it is possible that the concentrations tested are saturating and that the range observed in vivo is far below 1 lg/mL, it appears more likely that cadherin-8 binding provokes a response via signaling intermediates, perhaps by recruiting a stop signal via b-catenin (Figs. 4 and 7). Interestingly, b-catenin binding may be more important for stopping than for promoting growth as it also appears to be dispensable for N-cadherin mediated outgrowth in Xenopus retinal ganglion cells in vivo (Riehl et al., 1996).
Given that both N-cadherin and cadherin-8 bind b-catenin in DG, how might they differentially affect the mossy fiber trajectory? Several studies suggest that changes in additional juxtamembrane cadherin binding proteins can greatly alter strength of adhesion by regulating lateral clustering (Yap et al., 1997, 1998). Additionally, juxtamembrane-binding proteins, such as p120 catenin, delta catenin, and presenilin can bind and signal via distinct sets of signaling partners (Hirano et al., 2003). However, it is not clear how cadherin-8 and N-cadherin, with their highly similar intracellular domains, could recruit different juxtamembrane partners. One possibility is that the cadherins are signaling via distinct coreceptors or signaling complexes. The idea of a coreceptor is particularly appealing because cadherin ectodomains are more likely to show binding characteristics specific to particular cadherins and could be used to distinguish cadherin-8 and N-cadherin. In endothelial cells, interactions between the ectodomains of cadherin-5 and a coreceptor, the receptor protein tyrosine phosphatase (RPTP) VE-PTP mediate dephosphorylation of cadherin-5 and enhance the barrier integrity of adherens junctions (Nawroth et al., 2002). In retinal ganglion cells, binding of the RPTP, PTPl, to N-cadherin regulates axon extension on an N-cadherin substrate (Burden-Gulley and Brady-Kalnay, 1999). Cadherin interaction with either of these RPTP partners is associated with stronger adhesion, and consistent with this, PTPl can coimmunoprecipitate with an N-cadherin-b-catenin complex (Brady-Kalnay et al., 1995).
Proteoglycans may also regulate cadherin actions in mossy fibers (Balsamo and Lilien, 1990). Immunostaining for neurocan reveals a sharp border of expression with high levels in CA3 stratum radiatum and low levels within stratum lucidum (Kurazono et al., 2001; Butler et al., 2004). Neurocan stimulation of retinal ganglion cells disassociates N-cadherin from actin, presumably by binding a GPI-linked cell surface glycosyltransferase that can form a complex with N-cadherin (Li et al., 2000). The phosphacan splice variant, RPTPb/f, may also regulate N-cadherin adhesion (Meng et al., 2000), but it is largely restricted to astrocytes (Snyder et al., 1996; Butler et al., 2004), making it difficult to model a direct action with the neuronally restricted cadherin-8.
Recent work also suggests that N-cadherin can form a coreceptor complex with Robo and that this inhibits N-cadherin dependent axon extension in the presence of the Robo ligand, Slit (Rhee et al., 2002). The proposed N-cadherin-Robo complex provides an attractive mechanism to couple the mechanics of outgrowth with axon guidance. Furthermore, one would anticipate such interactions to be regulated locally in a cell type specific or regionally restricted manner. However, we have thus far been unable to detect interactions between Robo family members and cadherins in DG (Bekirov, Svoronos, Benson, Kang, unpublished data).
Cadherins and Dendritic Arbors
Dendritic arbors of a variety of neurons require N-cadherin for distinct stages of development. Xenopus retinal ganglion cells expressing a dominant negative N-cadherin fail to generate dendrites (Riehl et al., 1996), and chick horizontal cells show reduced arbor size (Tanabe et al., 2006). N-cadherin promotes dendritic outgrowth in hippocampal neurons (Esch et al., 2000), zebrafish amacrine cells require N-cadherin to arborize appropriately (Masai et al., 2003), and DN-cadherin restricts dendritic arbors that are generated by olfactory projection neurons in Drosophila (Zhu and Luo, 2004). Here we find that in CA3 of slice cultures, interfering with N-cadherin binding decreases MAP2-labeling over time suggesting that existing arbors retract and new growth is prevented, while MAP2-labeled dendrites in CA1 stratum radiatum and DG molecular layer appear comparatively normal. Thus, it appears as though interactions across the HAV interface trigger signals that are important for dendrite elaboration and maintenance and perhaps for CA3 pyramidal cell health in general, since it seems unlikely that neurons will survive long without their dendrites. Since N-cadherin adhesion appears to be important for stabilizing synapses in developing hippocampal neurons (Togashi et al., 2002; Bozdagi et al., 2004), and recent work supports a strong interrelationship between arbor and synapse stability (Meyer and Smith, 2006; Ruthazer et al., 2006), it may be that dendrites retract in the absence of the stabilizing influence of synapses. Consistent with this, overall number and size of presynaptic terminal puncta are reduced in CA3 in HAV-treated slices. However, QAV treatment produces similar changes in presynaptic terminal clusters without the devastating effect on CA3 neurons suggesting that synapse stability alone does not fully account for our observations.
Cadherins and Mossy Fiber Guidance
A variety of cell surface and cell adhesion proteins appears to collectively promote mossy fiber fasciculation, as disruption of any single one produces some defasciculation. This is particularly notable in proximal CA3. Mice deficient in CHL1 (close homolog of L1) or NCAM, or the introduction of reagents interfering with LAMP or cadherin-8 adhesion produce defasciculation in proximal CA3 (Pimenta et al., 1995; Cremer et al., 1997; Seki and Rutishauser, 1998; Montag-Sallaz et al., 2002). The defasciculated fibers inappropriately target the CA3 pyramidal cell layer. Some innervation of the pyramidal cell layer occurs normally over the course of development, but typically not to the degree observed following the manipulations described (Amaral, 1979; Amaral and Dent, 1981). The consistent direction of the misguided trajectories is likely to be driven by the intact inhibitory extracellular matrix and repulsive Sema6A activity in stratum radiatum (Wilson and Snow, 2000; Butler et al., 2004; Suto et al., 2007), coupled with a permissive or even attractive environment generated locally by the CA3 pyramidal cell layer (Steup et al., 2000). Distal CA3 mistargeting occurs in mice lacking NCAM (Cremer et al., 1997; Seki and Rutishauser, 1998), following enzymatic removal of keratan sulfate glycosaminoglycans, or following exposure to anti-LAMP antibodies, HAV or QAV peptides (Piment et al., 1995; Butler et al., 2004; present study). The targeting defects suggest that keratan sulfate proteoglycans prevent mossy fibers from invading stratum radiatum (Butler et al., 2004), that LAMP and NCAM may be particularly important for promoting mossy fiber fasciculation or the retraction of aberrant projections (Pimenta et al., 1995; Cremer et al., 1997; Seki and Rutishauser, 1998), and cadherin-8 for stopping mossy fiber extension (Fig. 4). Together, these molecules help account for the high fidelity of mossy fiber guidance.
Acknowledgments
Grant Sponsors: NINDS, NIMH, NIDA; Grant numbers: NS37731, NS34659, NS43083 (predoctoral training), MH075783, DA07135 (postdoctoral training); Grant sponsor: Irma T. Hirschl Career Scientist Award. Iddil H. Bekirov is currently at Section on Fundamental Neuroscience, NIMH, Bldg. 35/Rm1B-213, [35 Convent Dr., MSC 3728 (US mail)], Bethesda, MD 20892-3728, USA.
The authors thank Dr. S. T. Suzuki for generously providing his cadherin-8 construct and stable cadherin-8 L-cell line, Dr. Ioana Carcea for her help with substrate strategies, and Dr. Caitlin Trasande for her work on the cell survival experiments.
References
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol (Berl) 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Andrews GL, Mastick GS. R-cadherin is a Pax6-regulated, growth-promoting cue for pioneer axons. J Neurosci. 2003;23:9873–9880. doi: 10.1523/JNEUROSCI.23-30-09873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J, Lilien J. N-cadherin is stably associated with and is an acceptor for a cell surface N-acetylgalactosaminylphosphotrans-ferase. J Biol Chem. 1990;265:2923–2928. [PubMed] [Google Scholar]
- Bekirov IH. Ph D. New York: Mount Sinai School of Medicine of New York University; 2004. The role of classic cadherins in the development of hippocampal circuitry; p. 107. [Google Scholar]
- Bekirov IH, Needleman LA, Zhang W, Benson DL. Identification and localization of multiple classic cadherins in developing rat limbic system. Neuroscience. 2002;115:213–227. doi: 10.1016/s0306-4522(02)00375-5. [DOI] [PubMed] [Google Scholar]
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- Bözdagi O, Shan W, Tanaka H, Benson DL, Huntley GW. Increasing numbers of synaptic puncta during late-phase LTP: N-cadherin is synthesized, recruited to synaptic sites and required for potentiation. Neuron. 2000;28:245–259. doi: 10.1016/s0896-6273(00)00100-8. [DOI] [PubMed] [Google Scholar]
- Bozdagi O, Valcin M, Poskanzer K, Tanaka H, Benson DL. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol Cell Neurosci. 2004;27:509–521. doi: 10.1016/j.mcn.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden-Gulley SM, Brady-Kalnay SM. PTPmu regulates N-cadherin-dependent neurite outgrowth. J Cell Biol. 1999;144:1323–1336. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler CD, Schnetz SA, Yu EY, Davis JB, Temple K, Silver J, Malouf AT. Keratan sulfate proteoglycan phosphacan regulates mossy fiber outgrowth and regeneration. J Neurosci. 2004;24:462–473. doi: 10.1523/JNEUROSCI.3040-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol. 2001;154:231–243. doi: 10.1083/jcb.200103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- Dailey ME, Buchanan J, Bergles DE, Smith SJ. Mossy fiber growth and synaptogenesis in rat hippocampal slices in vitro. J Neurosci. 1994;14(3 Part 1):1060–1078. doi: 10.1523/JNEUROSCI.14-03-01060.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Rowett LH, Moore SE, Mann DA, Walsh FS. Neurite outgrowth in response to transfected N-CAM and N-cadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 1991;6:247–258. doi: 10.1016/0896-6273(91)90360-c. [DOI] [PubMed] [Google Scholar]
- Drazba J, Lemmon V. The role of cell adhesion molecules in neurite outgrowth on Muller cells. Dev Biol. 1990;138:82–93. doi: 10.1016/0012-1606(90)90178-l. [DOI] [PubMed] [Google Scholar]
- Elste AM, Benson DL. Structural basis for developmentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495:324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Local presentation of substrate molecules directs axon specification by cultured hippocampal neurons. J Neurosci. 1999;19:6417–6426. doi: 10.1523/JNEUROSCI.19-15-06417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol. 2000;29:215–223. doi: 10.1023/a:1026515426303. [DOI] [PubMed] [Google Scholar]
- Espeseth A, Marnellos G, Kintner C. The role of F-cadherin in localizing cells during neural tube formation in Xenopus embryos. Development. 1998;125:301–312. doi: 10.1242/dev.125.2.301. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Simultaneous monitoring of three key neuronal functions in primary neuronal cultures. J Neurosci Methods. 2007;160:197–205. doi: 10.1016/j.jneumeth.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Mege RM, Cunningham BA, Edelman GM. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proc Natl Acad Sci USA. 1989;86:7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M, Heimrich B. Lamina-specific synaptic connections of hippocampal neurons in vitro. J Neurobiol. 1995;26:350–359. doi: 10.1002/neu.480260307. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Development of the hippocampus in vitro: Cell types, synapses and receptors. Neuroscience. 1984a;11:751–760. doi: 10.1016/0306-4522(84)90192-1. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Slice cultures of cerebellar, hippocampal and hypothalamic tissue. Experientia. 1984b;40:235–243. doi: 10.1007/BF01947561. [DOI] [PubMed] [Google Scholar]
- Gil OD, Needleman L, Huntley GW. Developmental patterns of cadherin expression and localization in relation to compartmentalized thalamocortical terminations in rat barrel cortex. J Comp Neurol. 2002;453:372–388. doi: 10.1002/cne.10424. [DOI] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989a;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989b;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslin K, Schreyer DJ, Skene JHP, Banker G. Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci. 1990;10:588–602. doi: 10.1523/JNEUROSCI.10-02-00588.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel J, Blanco C, Jacob J, Grumet M. System for cleavable Fc fusion proteins using tobacco etch virus (TEV) protease. Bio-techniques. 2001;30:60–61. 64–66. doi: 10.2144/01301st01. [DOI] [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hirano S, Suzuki ST, Redies CM. The cadherin superfamily in neural development: Diversity, function and interaction with other molecules. Front Biosci. 2003;8:D306–D355. doi: 10.2741/972. [DOI] [PubMed] [Google Scholar]
- Hummel T, Zipursky SL. Afferent induction of olfactory glomeruli requires N-cadherin. Neuron. 2004;42:77–88. doi: 10.1016/s0896-6273(04)00158-8. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Benson DL. Neural (N)-cadherin at developing thalamocortical synapses provides an adhesion mechanism for the formation of somatopically organized connections. J Comp Neurol. 1999;407:453–471. [PubMed] [Google Scholar]
- Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Kido M, Obata S, Tanihara H, Rochelle JM, Seldin MF, Taketani S, Suzuki ST. Molecular properties and chromosomal location of cadherin-8. Genomics. 1998;48:186–194. doi: 10.1006/geno.1997.5152. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Peralta Soler A, Johnson KR, Wheelock MJ. Interaction of a-actinin with the cadherin/catenin cell-cell adhesion complex via a-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korematsu K, Redies C. Expression of cadherin-8 mRNA in the developing mouse central nervous system. J Comp Neurol. 1997;387:291–306. [PubMed] [Google Scholar]
- Kurazono S, Okamoto M, Sakiyama J, Mori S, Nakata Y, Fukuoka J, Amano S, Oohira A, Matsui H. Expression of brain specific chondroitin sulfate proteoglycans, neurocan and phosphacan, in the developing and adult hippocampus of Ihara's epileptic rats. Brain Res. 2001;898:36–48. doi: 10.1016/s0006-8993(01)02128-x. [DOI] [PubMed] [Google Scholar]
- Laake JH, Haug FM, Wieloch T, Ottersen OP. A simple in vitro model of ischemia based on hippocampal slice cultures and propidium iodide fluorescence. Brain Res Brain Res Protoc. 1999;4:173–184. doi: 10.1016/s1385-299x(99)00021-5. [DOI] [PubMed] [Google Scholar]
- Lee C, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- Li H, Leung TC, Hoffman S, Balsamo J, Lilien J. Coordinate regulation of cadherin and integrin function by the chondroitin sulfate proteoglycan neurocan. J Cell Biol. 2000;149:1275–1288. doi: 10.1083/jcb.149.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthiens V, Gavard J, Padilla F, Monnet C, Castellani V, Lambert M, Mege RM. A novel function for cadherin-11 in the regulation of motor axon elongation and fasciculation. Mol Cell Neurosci. 2005;28:715–726. doi: 10.1016/j.mcn.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Masai I, Lele Z, Yamaguchi M, Komori A, Nakata A, Nishiwaki Y, Wada H, Tanaka H, Nojima Y, Hammerschmidt M, Wilson SW, Okamoto H. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494. doi: 10.1242/dev.00465. [DOI] [PubMed] [Google Scholar]
- Meng K, Rodriguez-Pena A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF. Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta. Proc Natl Acad Sci USA. 2000;97:2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskevich F, Zhu Y, Ranscht B, Sanes JR. Expression of multiple cadherins and catenins in the chick optic tectum. Mol Cell Neurosci. 1998;12:240–255. doi: 10.1006/mcne.1998.0718. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol Cell Biol. 2002;22:7967–7981. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. Embo J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. N-cadherin, NCAM, and integrins promote retinal neurite outgrowth on astrocytes in vitro. J Cell Biol. 1988;107:1177–1187. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe V, Willems J, Vandekerckhove J, Roy FV, Bruyneel E, Mareel M. Inhibition of adhesion and induction of epithelial cell invasion by HAV-containing E-cadherin-specific peptides. J Cell Sci. 1999;112(Part 1):127–135. doi: 10.1242/jcs.112.1.127. [DOI] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Nose A, Tsuji K, Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990;61:147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. Embo J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type II cadherin ectodomain structures: Implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Petersen MA, Dailey ME. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia. 2004;46:195–206. doi: 10.1002/glia.10362. [DOI] [PubMed] [Google Scholar]
- Pimenta AF, Zhukareva V, Barbe MF, Reinoso BS, Grimley C, Henzel W, Fischer I, Levitt P. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron. 1995;15:287–297. doi: 10.1016/0896-6273(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzo Miller LD, Mahanty NK, Connor JA, Landis DM. Spontaneous pyramidal cell death in organotypic slice cultures from rat hippocampus is prevented by glutamate receptor antagonists. Neuroscience. 1994;63:471–487. doi: 10.1016/0306-4522(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Rakic P. Role of cell interaction in development of dendritic patterns. Adv Neurol. 1975;12:117–134. [PubMed] [Google Scholar]
- Redies C, Engelhart K, Takeichi M. Differential expression of N- and R-cadherin in functional neuronal systems and other structures of the developing chicken brain. J Comp Neurol. 1993;333:398–416. doi: 10.1002/cne.903330307. [DOI] [PubMed] [Google Scholar]
- Redies C, Takeichi M. Expression of N-cadherin mRNA during development of the mouse brain. Dev Dyn. 1993;197:26–39. doi: 10.1002/aja.1001970104. [DOI] [PubMed] [Google Scholar]
- Rhee J, Mahfooz NS, Arregui C, Lilien J, Balsamo J, VanBerkum MF. Activation of the repulsive receptor roundabout inhibits N-cadherin-mediated cell adhesion. Nat Cell Biol. 2002;4:798–805. doi: 10.1038/ncb858. [DOI] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Lilienbaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Robain O, Barbin G, Billette de Villemeur T, Jardin L, Jahchan T, Ben-Ari Y. Development of mossy fiber synapses in hippocampal slice culture. Brain Res Dev Brain Res. 1994;80:244–250. doi: 10.1016/0165-3806(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Tsujimoto G, Kitajima M, Natori M. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J. 2000;349(Part 1):159–167. doi: 10.1042/0264-6021:3490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SE, Li J, Schauwecker PE, McNeill TH, Salton SR. Comparison of RPTP zeta/beta, phosphacan, and trkB mRNA expression in the developing and adult rat nervous system and induction of RPTP zeta/beta and phosphacan mRNA following brain injury. Brain Res Mol Brain Res. 1996;40:79–96. doi: 10.1016/0169-328x(96)00039-3. [DOI] [PubMed] [Google Scholar]
- Stearns ME, Binder LI. Evidence that MAP-2 may be involved in pigment granule transport in squirrel fish erythrophores. Cell Motil Cytoskeleton. 1987;7:221–234. doi: 10.1002/cm.970070305. [DOI] [PubMed] [Google Scholar]
- Steup A, Lohrum M, Hamscho N, Savaskan NE, Ninnemann O, Nitsch R, Fujisawa H, Puschel AW, Skutella T. Sema3C and netrin-1 differentially affect axon growth in the hippocampal formation. Mol Cell Neurosci. 2000;15:141–155. doi: 10.1006/mcne.1999.0818. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, Chédotal A, Mitchell KJ, Manabe T, Fujisawa H. Interactions between Plexin-A2, Plexin-A4, and Semaphorin 6A control lamina-restricted projection of hippocampal mossy fibers. Neuron. 2007;53:535–547. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997a;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997b;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Takahashi Y, Sato Y, Kawakami K, Takeichi M, Nakagawa S. Cadherin is required for dendritic morphogenesis and synaptic terminal organization of retinal horizontal cells. Development. 2006;133:4085–4096. doi: 10.1242/dev.02566. [DOI] [PubMed] [Google Scholar]
- Toda M, Asou H, Miura M, Toya S, Uyemura K. GFAP transfected cells produce laminin, leading to neurite outgrowth promotion. Neuroreport. 1994;5:1969–1972. doi: 10.1097/00001756-199410000-00033. [DOI] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Tomaselli KJ, Neugebauer KM, Bixby JL, Lilien J, Reichardt LF. N-cadherin and integrins: Two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces. Neuron. 1988;1:33–43. doi: 10.1016/0896-6273(88)90207-3. [DOI] [PubMed] [Google Scholar]
- Treubert-Zimmermann U, Heyers D, Redies C. Targeting axons to specific fiber tracts in vivo by altering cadherin expression. J Neurosci. 2002;22:7617–7626. doi: 10.1523/JNEUROSCI.22-17-07617.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utton MA, Eickholt B, Howell FV, Wallis J, Doherty P. Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J Neurochem. 2001;76:1421–1430. doi: 10.1046/j.1471-4159.2001.00140.x. [DOI] [PubMed] [Google Scholar]
- Voigt T, De Lima AD, Beckmann M. Synaptophysin immunohistochemistry reveals inside-out pattern of early synaptogenesis in ferret cerebral cortex. J Comp Neurol. 1993;330:48–64. doi: 10.1002/cne.903300105. [DOI] [PubMed] [Google Scholar]
- Wilby MJ, Muir EM, Fok-Seang J, Gour BJ, Blaschuk OW, Fawcett JW. N-Cadherin inhibits Schwann cell migration on astrocytes. Mol Cell Neurosci. 1999;14:66–84. doi: 10.1006/mcne.1999.0766. [DOI] [PubMed] [Google Scholar]
- Williams E, Williams G, Gour BJ, Blaschuk OW, Doherty P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J Biol Chem. 2000;275:4007–4012. doi: 10.1074/jbc.275.6.4007. [DOI] [PubMed] [Google Scholar]
- Wilson MT, Snow DM. Chondroitin sulfate proteoglycan expression pattern in hippocampal development: Potential regulation of axon tract formation. J Comp Neurol. 2000;424:532–546. doi: 10.1002/1096-9861(20000828)424:3<532::aid-cne10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Wöhrn JC, Nakagawa S, Ast M, Takeichi M, Redies C. Combinatorial expression of cadherins in the tectum and the sorting of neurites in the tectofugal pathways of the chicken embryo. Neuroscience. 1999;90:985–1000. doi: 10.1016/s0306-4522(98)00526-0. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Herman JP, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. J Neurosci. 1995;15:4556–4571. doi: 10.1523/JNEUROSCI.15-06-04556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: A fundamental determinant of cadherin function. Curr Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Luo L. Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron. 2004;42:63–75. doi: 10.1016/s0896-6273(04)00142-4. [DOI] [PubMed] [Google Scholar]
- Zimmer J, Gahwiler BH. Cellular and connective organization of slice cultures of the rat hippocampus and fascia dentata. J Comp Neurol. 1984;228:432–446. doi: 10.1002/cne.902280310. [DOI] [PubMed] [Google Scholar]


