Summary
Prolonged inactivity leads to disuse atrophy, a loss of muscle and bone mass. Hibernating mammals are inactive for 6–9 months per year but must return to full activity immediately after completing hibernation. This necessity for immediate recovery presents an intriguing conundrum, as many mammals require two to three times the period of inactivity to recover full bone strength. Therefore, if hibernators experience typical levels of bone disuse atrophy during hibernation, there would be inadequate time available to recover during the summer active season. We examined whether there were mechanical consequences as a result of the extended inactivity of hibernation. We dissected femur and tibia bones from squirrels in various stages of the annual hibernation cycle and measured the amount of force required to fracture these bones. Three groups were investigated; summer active animals were captured during the summer and immediately killed, animals in the 1 month detraining group were captured in the summer and killed following a 1-month period of restricted mobility, hibernating animals were killed after 8 months of inactivity. A three-point bend test was employed to measure the force required to break the bones. Apparent flexural strength and apparent flexural modulus (material stiffness) were calculated for femurs. There were no differences between groups for femur fracture force, tibia fracture force, or femur flexural strength. Femur flexural modulus was significantly less for the 1 month detraining group than for the hibernation and summer active groups. Thus, hibernators seem resistant to the deleterious effects of prolonged inactivity during the winter. However, they may be susceptible to immobilization-induced bone loss during the summer.
Keywords: disuse atrophy, torpor, flexural strength, flexural modulus
INTRODUCTION
Metabolic depressions are a common physiological response to challenging environmental conditions including freezing temperatures, lack of oxygen and limited food supplies. There are many variations upon the theme of hypometabolism including aestivation, daily torpor, denning, and seasonal hibernation (Storey and Storey, 1990). Hibernating ground squirrels repeatedly experience extended periods of inactivity ranging from 6 to 9 months (i.e. Carey et al., 2003).
Although there may be differences in the parameters affected, there is a vast literature supporting immobilization-induced bone loss in numerous species of non-hibernating mammals. Various outcomes have been documented following rat hindlimb immobilizations of as little as 6 weeks; these immobilization responses include significant decreases in femur and tibia bone mineral density, bone formation rate, osteoblast surface, and femoral bone mass (reviewed by Giangregorio and Blimkie, 2002). Limb immobilization in dogs resulted in a bone volume loss of 32–54%, depending on age (Jaworski and Uhthoff, 1986). The lost bone volume was partially recoverable; maximal values of recovery were 70%, even after an extensive 7 month recovery period (Jaworski and Uhthoff, 1986). Even in humans, an extended inactivity of 4 months may result in almost a 30% loss of femur bone mass (reviewed by Giangregorio and Blimkie, 2002). Many mammals require two to three times the period of immobility to achieve maximum recovery, and in some cases complete recovery is unachievable (e.g. Leblanc et al., 1990; Jaworski and Uhthoff, 1986; Trebacz, 2001).
Ground squirrels are exceptional hibernators. During torpor these animals experience metabolic rates as low as 1% of active rates, and body temperatures as low as –2.9°C have been recorded (reviewed by Wang and Lee, 2000; Barnes, 1989). Hibernating squirrels face an intriguing conundrum. The use of hibernation restricts an animal to the confines of a burrow for the entire winter season. However in spring, these same squirrels emerge from the burrow, establish territories, and quickly increase their movements. Golden-mantled ground squirrels run about 3.5 km per day, and when the walking gait is included the total distance traveled is approximately 5 km per day (Kenagy and Hoyt, 1989). One might predict that prolonged immobility in winter would result in significant bone disuse atrophy. However, the requirement for extensive locomotion immediately upon emergence might mitigate this disuse atrophy. Common approaches for investigating bone loss during hibernation have included radiography, histology, microscopy, morphology, and biochemical analysis of mineral content. The effects of bone disuse during true mammalian hibernation are unclear. Some studies report signs of bone loss such as decreased cortical width, enlarged lacunae, and decreased osteoblast activity (e.g. Steinberg et al., 1981; Whalen et al., 1972; Steinberg et al., 1986). Other investigations suggest that bone is maintained during hibernation as evidenced by maintenance of bone calcium content, bone phosphorus content, and unchanged histological appearance (Zimmerman et al., 1976).
Of note is whether any of these observed effects might impact on the ecology of these animals: does this extended inactivity affect bone strength in a meaningful way? We directly measured bone strength by employing three-point bending tests, which yield information about the strength and flexibility of a material. This technique has previously been used to successfully evaluate changes in bone strength for many species including rats, bears and frogs (e.g. Inman et al., 1999; Harvey and Donahue, 2004; Harvey et al., 2005; McGee et al., 2008; Hudson et al., 2004). As might be expected given the need for a high level of activity following hibernation, our results indicate a profound resistance to bone disuse atrophy despite an 8 month period of inactivity.
MATERIALS AND METHODS
Animals
Golden-mantled ground squirrels (Spermophilus lateralis Say) were live-trapped from the Southern Nevada/Southern California area then maintained in the University Animal Care Facility on a diet of rat chow supplemented with sunflower seeds. Since all animals were wild-caught, precise age determination was not possible. However, all animals used were adults. Based on size at capture, squirrels were at least 1 year old and epiphyseal plates had fused. Three groups of animals were investigated: summer active (SA) animals were captured during the summer and immediately killed; animals in the 1 month detraining group (DT) were captured in the summer and killed following a 1 month period of limited mobility by cage restriction, and hibernating (H) animals were captured in the summer and killed at the end of the hibernation season. Hibernating animals experienced a total of 8 months of inactivity: ∼3 months of limited mobility by cage restriction before the onset of torpor and ∼5 months of hibernation. Both the SA and H groups contained five male and five female animals; the DT group contained two males and one female. Femur and tibia bones were dissected then stored at –20°C until mechanical testing. The University of Nevada, Las Vegas Institutional Animal Care and Use Committee approved all procedures, and animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals.
Material testing
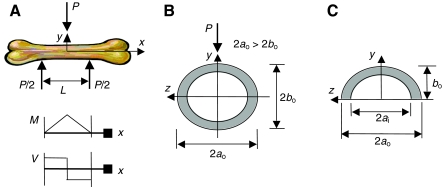
Apparent flexural strength and flexural modulus of the bones were determined using a three-point bending test (Fig. 1). The bones had a fairly uniform cross section over the span length, L, of 12.7 mm. For the sake of estimating cross-sectional area (A) and second area moment of inertia (I), the cross-sectional shape of the bones was idealized as a uniform hollow ellipse over the span length. The major axis, 2ao, was measured on average to be 8% larger than the minor axis, 2bo. The wall thickness, tw, was measured at many locations after the bones fractured during testing. The loading force during the experiments was applied perpendicular to the major axis of the ellipse (Fig. 2A,B). Bone mass, length and average mid-point diameter (Table 1) were measured in triplicate using a microbalance and a calibrated caliper. Volume and apparent bone density (mass/volume) were calculated in triplicate using water displacement.
Fig. 1.
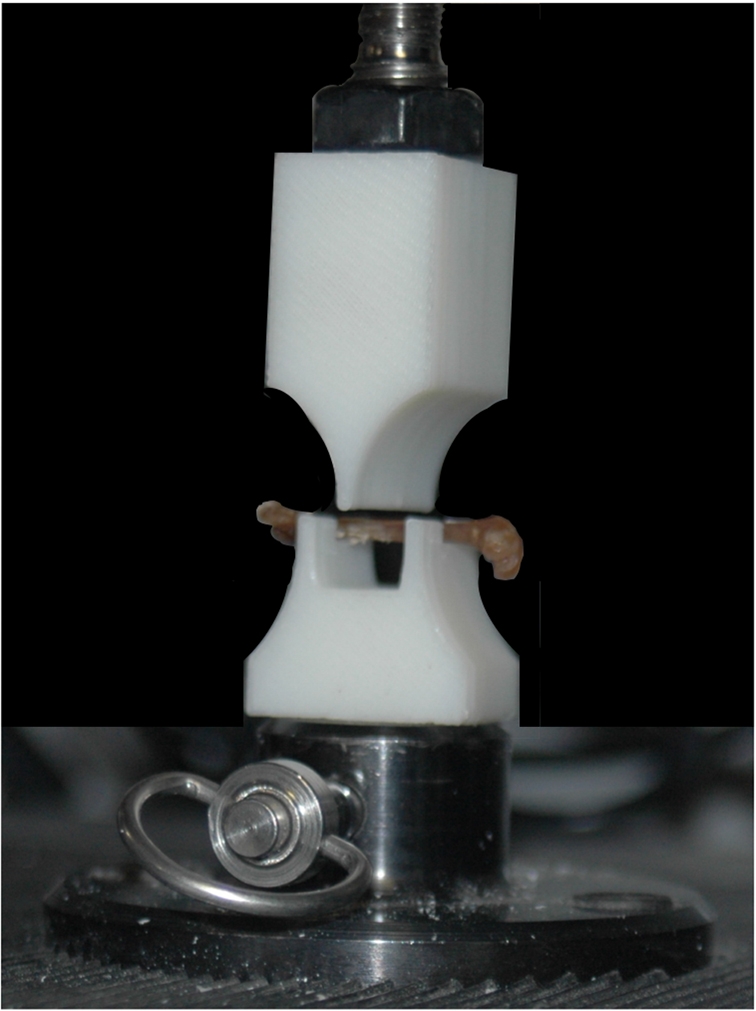
Photograph of a femur loaded in a United SSTM-1 testing machine just prior to application of breaking force. The area behind the testing fixture is blacked out to enhance visibility. The fixture was designed to maximize the span, hold the bone in one position, and resist deformation during breaking. All bones were loaded in the position shown.
Fig. 2.
(A) Schematic drawing showing the three-point bend test configuration with the corresponding bending moment and shear force diagrams. (B) The loading direction relative to the hollow elliptical bone cross section. (C) The hollow elliptical area above the neutral axis used to find the shear stress shape correction factor. All data reduction assumes an idealized elliptical cross section with a constant wall thickness. Measurements showed that the outer diameter in the major axis direction was 8% larger than the outer diameter in the minor axis direction.
Table 1.
Bone morphometry measurements for golden-mantled ground squirrel femur and tibia bones during the summer active season, the hibernation season and following 1 month of inactivity during the summer
| Group | Length (mm) | Mass (mg) | Diameter (mm) | Volume (μl) | Apparent density (gl–1) | |
|---|---|---|---|---|---|---|
| Femur | H | 32.22±0.23 | 328.16±7.83 | 2.67±0.03 | 279.16±5.70 | 1.18±0.02 |
| SA | 32.52±0.26 | 324.63±9.47 | 2.76±0.03 | 264.06±6.47 | 1.23±0.03 | |
| DT | 31.98±0.49 | 352.54±16.67 | 2.85±0.06* | 292.65±25.10 | 1.17±0.04 | |
| Tibia | H | 34.77±0.30 | 247.44±6.45 | 2.23±0.03 | 197.83±5.37 | 1.25±0.02 |
| SA | 34.39±0.24 | 241.07±5.67 | 2.29±0.03 | 190.65±3.46 | 1.27±0.02 | |
| DT | 34.59±0.52 | 251.13±11.40 | 2.19±0.06 | 210.07±8.65** | 1.20±0.01 |
Values are means ± s.e.m. Hibernating animals (H; N=10) were sampled following 8 months of inactivity including ∼3 months of cage restriction and ∼5 months of hibernation, summer active animals (SA; N=10) were sampled immediately after capture from the wild, and 1 month detrained (DT; N=3) animals were sampled after 1 month of cage restriction during the summer active season. *Value is statistically different from value for H animals (P<0.05; ANOVA). **Value is statistically different from value for SA animals (P<0.05; ANOVA)
Bones were loaded into a United SSTM-1 testing machine (United Calibration Corporation, Huntington Beach, CA, USA) with a 445N load cell. All tests were conducted under displacement control of the crosshead at a displacement rate of 4 mm min–1. Instantaneous load and crosshead displacement data were recorded. All specimens were tested to the point of catastrophic failure. The recorded load and displacement data were used to determine the apparent flexural strength and apparent flexural modulus. The measured displacement included contributions from the deflection of the specimen caused by bending and transverse shear deformation of the bone, as well as deflection caused by the compliance of the fixture and test machine.
Support and loading fixtures were made from acrylonitrile butadiene styrene (ABS) and were visually inspected during testing; no wearing or deformation was observed. The compliance of the entire test system was measured by conducting experiments in the same fixtures with a steel rectangular bar that was 3 mm wide, 25.4 mm high and 20 mm long. The flexural stiffness, EI, of the steel compliance specimen was approximately 25,000 times the flexural stiffness, EI, of the bone specimens. A compliance curve, deflection/applied force, was determined for the test system. The system compliance was measured to be 0.00245 mm N–1. The deflection due to the test system compliance was subtracted from the measured mid-span deflection of the bone specimens.
The compliance-adjusted mid-span deflection is due to both the bending and shear deformation of the bones. The span length to outer diameter ratio for the bones was 4.5 so the shear deformation effects must be included in the data reduction. The internal strain energy, U, in the three-point bending specimens can be found by integrating over the volume of the specimen. The volume integral simplifies to a line integral along the longitudinal axis (x) of the bone as shown in Eqn 1 (Boresi et al., 1993). The bending moment squared, M2, and the shear force squared, V2 are symmetrical about the center of the beam (Fig. 2A) so it is simpler to double the integral from zero to L/2 as shown below:
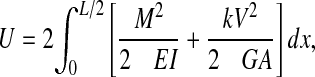 |
(1) |
where M is the bending moment, M=Px/2, E is the modulus of elasticity, I is the second area moment of inertia, V is the transverse shear force, V=P/2, k is a shape correction factor, G is the shear modulus of rigidity, A is the cross-sectional area, L is the support span, and the longitudinal position, x, varies from 0 to L/2. The second area moment of inertia, I, is taken about the z-axis of the hollow ellipse as shown in Fig. 2B and can be expressed as:
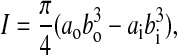 |
(2) |
where ao is the outer semi-major axis, bo is the outer semi-minor axis, ai is the inner semi-major axis, and bi is the inner semi-minor axis of the hollow ellipse. The cross-sectional area, A, of the hollow ellipse is:
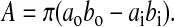 |
(3) |
The shape correction factor, k, is estimated as the ratio of the maximum shear stress to the average shear stress, k=τmax/τave, at a given cross section (Boresi et al., 1993). The maximum shear stress occurs at the neutral axis for the hollow elliptical cross section, which corresponds to the z-axis in Fig. 2B and is defined as:
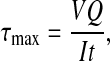 |
(4) |
where V is the transverse shear force, I is the second area moment of inertia defined in Eqn 2, t is the length of the cross section along the neutral axis, t=2tw=2(ao–ai), and Q is the first moment of area of the shaded region above the neutral axis in the hollow ellipse as shown in Fig. 2C:
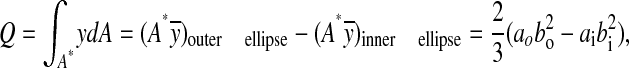 |
(5) |
where y is the vertical position measured from the neutral axis of the cross section, A* is the area of a half ellipse, A*=πab/2, and ȳ is the centroid of a half ellipse, ȳ=4b/3π. The average shear stress is defined as τave=V/A, where V and A are the same as previously defined. The shape correction factor, k, now becomes:
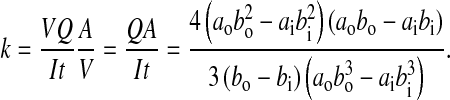 |
(6) |
Eqn 6 was verified by substituting values of the inner and outer diameters corresponding to a solid circular cross section, k=1.33, and a thin walled circular cross section, k=2.00 (Boresi et al., 1993). The shape correction factor, k, for the elliptical bone cross sections was, k=2.02, a value very close to that of a thin-walled circular cross section.
Integration of Eqn 1 results in the following expression for the strain energy, U:
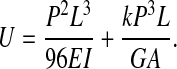 |
(7) |
The mid point deflection, ymax, directly under the applied load P can be found according to Castigliano's theorem (Boresi et al., 1993) where ymax is equal to the partial derivative of the strain energy with respect to the applied load, P:
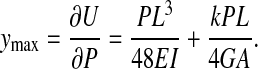 |
(8) |
The first term in Eqn 8 is the deflection caused by bending of the bone and the second term is deflection caused by transverse shear. The ratio of Young's modulus to shear modulus, E/G, is reported to be on the order of 20:1 for a variety of bone types (Spatz et al., 1996). The ratio of E/G for human and bovine cortical bones is also reported to be in the range of 3:6 (Martin et al., 1998). We used a rough average of these values and approximated the ratio of E/G to be 12 for this study. The shear modulus can be written as G=nE, where n=0.0833. Eqn 8 can be rearranged to solve for the apparent flexural modulus:
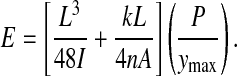 |
(9) |
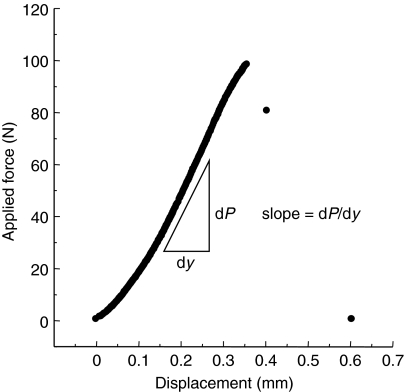
The slope, P/ymax, of the load-deflection curve is taken from the linear portion of the data before yielding (Fig. 3). We use the term apparent flexural modulus because of the assumptions used to reduce the data, namely that we assume the cross section to be a perfect ellipse and we assume the ratio of E/G to be 12.
Fig. 3.
Typical applied load versus mid-span displacement curve for a three-point bending test of the squirrel bones. The slope, dP/dy, which was used for the calculation of apparent flexural modulus was taken from the linear portion of the curve before any yielding occurred.
The apparent flexural strength was determined by using the peak applied force, P, in the following bending stress equation:
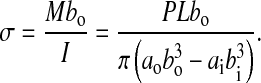 |
(10) |
Calculated values for the apparent flexural modulus from our experimental data were in the range of 9 to 24 GPa, whereas the strength values were in the range of 110 to 257 MPa. These values fall within the expected range for bone properties (Currey, 1999).
Statistical analyses
Data were analyzed in Statview 5.0; P<0.05 was considered significant for all analyses. All data are presented as means ± s.e.m. (standard error of the mean). Although our preliminary analyses revealed a significant effect of length on measured parameters, there was no bias for any group in respect to bone length. As a result, all data were subjected to ANOVA rather than ANCOVA as appropriate. The summer active group included squirrels captured across four months; we subjected the data on bone strength parameters to a regression analysis, using month of capture as the abscissa.
RESULTS
Bone morphometry
Femur and tibia bones were dissected from golden-mantled ground squirrels collected during the summer active season (SA), following 1 month of restricted mobility during the summer (DT), or following 8 months of inactivity at the end of the hibernation season (H). Bone length, mass, mid-point diameter, and volume were measured; apparent bone density (mass/volume) was calculated (Table 1). There were no significant differences between the summer active and hibernation groups for any femur or tibia parameter (Table 1). There was, however, a statistically significant but modest 6.3±2.3% difference in femur diameter between the DT and H groups and a 9.5±4.4% difference in tibia volume between the DT and SA groups (Table 1).
Force required to induce fracture
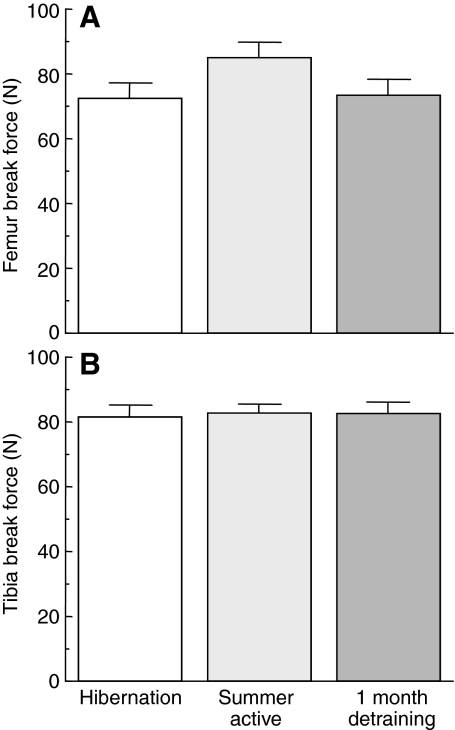
Bones were loaded to catastrophic failure in three-point bending tests on a United SSTM-1 testing machine (Fig. 1). There was no difference between groups for the amount of force required to fracture femurs (Fig. 4A) nor is there a difference between groups for the amount of force required to fracture tibias (Fig. 4B).
Fig. 4.
(A) Femur break force as a function of state. Values are means ± s.e.m. Hibernating animals (N=10) were sampled after 8 months of inactivity, summer active animals (N=10) were sampled immediately after capture from the wild, and 1 month detrained (N=3) animals were sampled after 1 month of cage restriction during the summer season. There is no difference between groups for femur break force (P>0.05, ANOVA). (B) Tibia break force as a function of state; details as in A. There is no difference between groups for tibia break force (P>0.05, ANOVA).
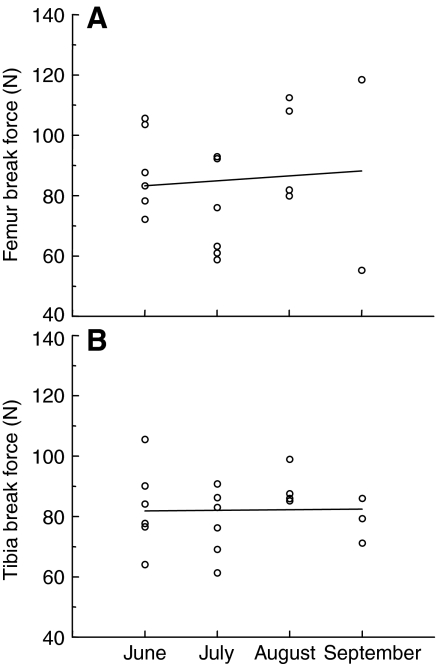
If ground squirrels were losing bone strength as a result of the inactivity of hibernation, it would be expected that some strength would be regained upon entering the summer active period when squirrels travel approximately 5 km per day (Kenagy and Hoyt, 1989). In order to address potential changes in bone strength across the summer active season, break force was regressed against summer month. Regression analyses indicate no change in bone strength across the summer active period for either femur (Fig. 5A) or tibia bones (Fig. 5B).
Fig. 5.
(A) Femur break force during the summer as a function of the date of sampling. Symbols represent individual bone measurements; N=11 animals. Not all bones were available from each squirrel. The date of sampling during the summer has no effect on femur break force (r2=0.09; P>0.05, ANOVA). (B) Tibia break force during the summer as a function of the date of sampling; details as in A. The date of sampling has no effect on tibia break force (r2=0.02; P>0.05, ANOVA).
Femur material properties
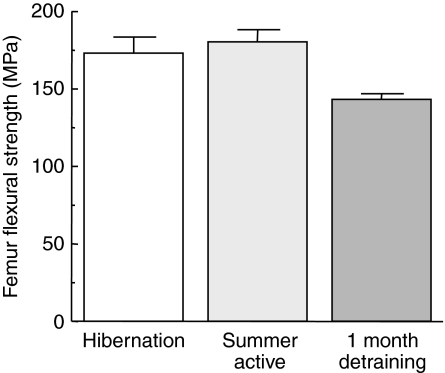
Although the amount of force required to fracture a given length of bone is physiologically relevant to an organism, analysis of the fracture force does not account for the amount of bone material present in the test. Therefore bone wall thickness was measured from the femur test fragments, allowing determination of the cross sectional area. Wall thickness measurements for tibia fragments were not available because of technical restrictions, therefore apparent flexural strength and apparent flexural modulus could only be determined for femurs. Flexural strength measurements indicate the highest stress experienced within a material at its moment of fracture. Surprisingly, we found no difference for femur apparent flexural strength despite 8 months of inactivity (Fig. 6). It is remarkable that there was no loss of apparent flexural strength for the hibernation group. It should be noted that following a mere 6 weeks of immobilization, nearly a 20% decrease in rat tibia strength was found (Inman et al., 1999). The error associated with our measurements would have allowed us to reliably detect as little as a 10% change in apparent flexural strength.
Fig. 6.
Apparent femur flexural strength as a function of state. Values are means ± s.e.m.; N=10 squirrels for both the hibernation and summer active groups, N=3 squirrels for the 1 month detraining group. There is no statistical difference (P>0.05, ANOVA) between groups for femur flexural strength.
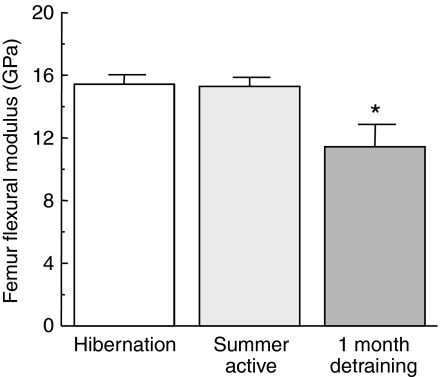
Flexural modulus measurements indicate the stiffness of a material i.e. its resistance to deflection by an applied force. Again, there was no change in bone stiffness following the prolonged inactivity of hibernation (Fig. 7). However, femur apparent flexural modulus for the DT group was significantly less than in both the H and SA groups (Fig. 7). One month of inactivity resulted in a 25.1±9.4% loss in apparent flexural modulus as compared to summer active controls (Fig. 7).
Fig. 7.
Apparent femur flexural modulus as a function of state. Values are means ± s.e.m.; N=10 squirrels for both the hibernation and summer active groups, N=3 squirrels for the 1 month detraining group. Femur flexural modulus for the 1 month detraining group is significantly less than both the hibernation and the summer active groups (*P<0.05, ANOVA).
DISCUSSION
We present evidence that bone strength is maintained despite the extensive inactivity of hibernation in ground squirrels. No differences were found between the summer active and hibernation groups for bone length, mass, diameter, volume or apparent density (mass/volume); this maintenance of bone morphometry was present for both femur and tibia bones (Table 1). Additionally, no difference was found in the amount of force required to break tibia or femur bones when comparing the summer active and hibernation groups (Fig. 4A,B). We note that our analyses employed three-point bending tests; it is possible that other analytical methods might reveal differences between these groups. If animals were losing bone strength during hibernation, they should be recovering during the summer active season. The lack of change in break force across the summer months further supports the notion that significant bone loss does not occur during the winter season (Fig. 5A,B). Analysis of femur material properties also failed to reveal any changes following the extensive inactivity of hibernation. There was no difference between the SA and H groups for apparent flexural strength (Fig. 6) or apparent flexural modulus (Fig. 7). There were, however, some intriguing differences between the DT and SA groups. Interestingly, in the summer squirrels appear to be susceptible to the effects of disuse as evidenced by reduced femur apparent flexural modulus (Figs 6 and 7). The data presented here support a seasonal shift in the response to inactivity. Squirrels seem protected against bone disuse atrophy during the winter hibernation season and susceptible during the summer active season. It should be noted that animals in the hibernation group were captured at the same time as animals in the 1 month detraining group, raising the possibility that some bone may have been recovered during the winter season.
Although it is widely accepted in non-hibernators that inactivity as a result of injury, immobilization or microgravity results in significant bone atrophy, the effects of disuse in the context of hibernation have been unclear. Relatively few studies have examined bone disuse atrophy in true hibernators, and the results have been disparate. Zimmerman et al. investigated the effects of disuse on bone composition in both active and hibernating thirteen-lined ground squirrels. Active animals were immobilized by subjecting one hindlimb to sciatic nerve section; the immobilized limbs had significantly lower muscle mass, bone mass, total bone calcium, percentage calcium and percentage phosphorus than those of active control animals (Zimmerman et al., 1976). After 30 days of hibernation, bone calcium and phosphorus content were comparable with values from the active control group; histological analysis of bone from the hibernation group also supported the maintenance of bone tissue. Additionally, this study showed that limb immobilization by sciatic nerve section in hibernating animals did not lead to bone atrophy. Of course, 30 days is a relatively short period in the context of a 6–9 month hibernation season. Zimmerman et al. also investigated long-term effects. Following 150 days of hibernation, a few enlarged lacunae were observed and there were still no changes in phosphorus or calcium content (Zimmerman et al., 1976). Conversely, when comparing interradicular alveolar bone between nonhibernating and hibernating ground squirrels, hibernating animals had an increased number of lacunae and an overall `foamy' appearance, consistent with osteocytic osteolysis (Haller and Zimny, 1977).
A few investigations utilizing hamsters have indicated bone disuse atrophy during hibernation. Procion Red dye was used as a marker of bone matrix, and dye visibility progressively decreased with hibernation season duration (Steinberg et al., 1979). The effects of cold stress and hibernation on hamster bone morphology were also studied. After 83 days of cold stress and 7 days of hibernation, femur cortical width significantly increased; by contrast, after 40 days of cold stress and 50 days of hibernation, cortical width was significantly reduced (Steinberg et al., 1981). Their conclusion was that hibernation resulted in bone atrophy. However, the results of this study must be interpreted with caution, as neither treatment could be statistically evaluated independently of the other, i.e. only combined treatments were evaluated, the effects of solely hibernation or solely cold stress could not be determined. A study following the uptake of tritiated proline by osteoblasts during hibernation in hamsters found a marked drop in the percentage of labeled cells following 12 h of hibernation (Steinberg et al., 1986). The conclusion drawn from these data was reduced osteoblast activity during hibernation. However, these data must be interpreted cautiously as injection with the radiolabel might have caused partial arousal from torpor. Heart rate is reduced to 20% of euthermic values during daily torpor in Djungarian hamsters (Mertens et al., 2008). Factors such as proline delivery by the cardiovascular system, activity of plasma membrane proline transport channels during hibernation, and specific radioactivity may confound the interpretation of these proline uptake data. Additionally, hamsters may not be an ideal model for hibernation, since they are not obligate hibernators and may only spend about half of their time during the hibernation season actually in torpor (i.e. Ueda and Ibuka, 1995; Wassmer, 2004).
Bats as a hibernation model are phylogenetically distant from rodents. Bats appear to experience bone loss during hibernation. Femur radiographs indicate progressive cortical thinning and development of enlarged lacunae during hibernation (Whalen et al., 1972). However, cortical thickness appears to return to normal following spring emergence. When hibernating bats are injected with calcitonin, the nonhibernating bone morphology re-emerges (Krook et al., 1977). However, it should be noted that osteoclasts and osteoblasts are absent from long bone surfaces during hibernation in bats (Doty and Nunez, 1985).
Interestingly bears seem resistant to bone disuse atrophy during denning (i.e. Donahue et al., 2006b). However, we note that bear denning is a distinct physiological phenomenon from true hibernation. Reductions in body temperature during denning are typically 3–4°C, and reductions in metabolic rate are equally modest in comparison to true hibernators (Hellgren, 1998). Serum markers indicate increased resorption and an increase or no change in bone formation during denning (Donahue et al., 2003a; Donahue et al., 2003b). However, the bone formation marker increases by several fold following spring remobilization, and it appears that there is no net bone loss or loss of bone strength as animals age (Donahue et al., 2003a; Donahue et al., 2003b; Harvey and Donahue, 2004; McGee et al., 2007).
In a disuse atrophy study using a non-mammalian vertebrate, the green-striped burrowing frog, 9 months of aestivation resulted in no changes in long bone size, anatomy, and bending strength (Hudson et al., 2004). However, the natural history of these animals would suggest a limited amount of locomotion even during the active period.
It is interesting to note that resistance to bone disuse atrophy has been documented for various classes of metabolic depression including hibernation, denning, and aestivation. Although the relationship between metabolic depressions, as in hibernation, and a resistance to disuse-induced bone loss is becoming apparent, the mechanisms that underlie this relationship require further elucidation. Calcium homeostasis as influenced by parathyroid hormone has been implicated as an important factor during bear denning, and calcitonin-mediated regulation has been implicated in bat hibernation (Donahue et al., 2006a; Krook et al., 1977). Our data demonstrate no loss of bone in hibernators; bone morphometry, bone strength and other mechanical properties were all maintained during hibernation. It appears that ground squirrels emerge from the burrow with adequate bone strength to support their locomotor activities.
Many processes are involved in the regulation of bone remodeling. Numerous questions require extensive investigation. For example, what mechanisms are employed in regulating calcium solubility and exchange, including mechanisms of non-osteoclastic requisition of bone calcium? Are there differences in the effects of hibernation on skeletal mass maintenance for large versus small animals (i.e. disuse in bears may elicit a different molecular response than disuse in hamsters or squirrels)? Do the effects of disuse differ between the axial and appendicular skeleton? Our future efforts will be directed towards understanding the regulatory mechanisms involved in preventing bone loss during hibernation in ground squirrels. We also plan to investigate the role of temperature in these processes.
LIST OF ABBREVIATIONS
- A
cross-sectional area
- A*
area of the section of the hollow ellipse above neutral axis
- ai
inner semi-major axis of a hollow ellipse
- ao
outer semi-major axis of a hollow ellipse
- bi
inner semi-minor axis of a hollow ellipse
- bo
outer semi-minor axis of a hollow ellipse
- DT
squirrels in the 1 month detraining group
- E
modulus of elasticity
- G
shear modulus
- H
squirrels in the hibernation group
- I
second moment of area or moment of inertia
- k
shape correction factor for transverse shear stress calculation
- L
length of support span
- M
bending moment in the bone (acting about the z-axis)
- n
ratio of shear modulus to modulus of elasticity
- P
applied force
- Q
first moment of area
- SA
squirrels in the summer active group
- s.e.m.
standard error of measurement
- t
length of bone cross section that lies on neutral axis
- tw
wall thickness of the bone
- U
strain energy
- V
transverse shear force
- x
longitudinal axis of bone
- y
vertical axis of bone cross section
- ȳ
centroid of the section of the hollow ellipse above neutral axis
- ymax
deflection at mid-span directly under the applied load
- z
horizontal axis of bone cross section (also the neutral axis)
- ∂
partial derivative symbol
- σ
flexural strength of bone
- τave
average shear stress at a particular cross section
- τmax
maximum shear stress at a particular cross section
We thank Anthony Marlon and Lori McFadden for assistance with bone dissections and Daniel Lowe for designing the testing fixtures. This study was supported by grants from the National Science Foundation (IOB 0448396) and the National Institutes of Health (2 P20 RR016464). J.C.U. is supported by a NSF Graduate Research Fellowship. Deposited in PMC for release after 12 months.
References
- Barnes, B. M. (1989). Freeze avoidance in a mammal: body temperatures below 0°C in an Arctic hibernator. Science 244, 1593-1595. [DOI] [PubMed] [Google Scholar]
- Boresi, A. P., Schmidt, R. J. and Sidebottom, O. M. (1993). Advanced Mechanics of Materials, 5th edn, pp. 175-178. New York, NY: John Wiley and Sons.
- Carey, H. V., Andrews, M. T. and Martin, S. L. (2003). Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153-1181. [DOI] [PubMed] [Google Scholar]
- Currey, J. D. (1999). What determines the bending strength of compact bone? J. Exp. Biol. 202, 2495-2503. [DOI] [PubMed] [Google Scholar]
- Donahue, S. W., Vaughan, M. R., Demers, L. M. and Donahue, H. J. (2003a). Bone formation is not impaired by hibernation (disuse) in black bears Ursus americanus. J. Exp. Biol. 206, 4233-4239. [DOI] [PubMed] [Google Scholar]
- Donahue, S. W., Vaughan, M. R., Demers, L. M. and Donahue, H. J. (2003b). Serum markers of bone metabolism show bone loss in hibernating bears. Clin. Orthop. Relat. Res. 408, 295-301. [DOI] [PubMed] [Google Scholar]
- Donahue, S. W., Galley, S. A., Vaughan, M. R., Patterson-Buckendahl, P., Demers, L. M., Vance, J. L. and McGee, M. E. (2006a). Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J. Exp. Biol. 209, 1630-1638. [DOI] [PubMed] [Google Scholar]
- Donahue, S. W., McGee, M. E., Harvey, K. B., Vaughan, M. R. and Robbins, C. T. (2006b). Hibernating bears as a model for preventing disuse osteoporosis. J. Biomech. 39, 1480-1488. [DOI] [PubMed] [Google Scholar]
- Doty, S. B. and Nunez, E. A. (1985). Activation of osteoclasts and the repopulation of bone surfaces following hibernation in the bat, Myotis lucifugus. Anat. Rec. 213, 481-495. [DOI] [PubMed] [Google Scholar]
- Giangregorio, L. and Blimkie, C. J. (2002). Skeletal adaptations to alterations in weight-bearing activity: a comparison of models of disuse osteoporosis. Sports Med. 32, 459-476. [DOI] [PubMed] [Google Scholar]
- Haller, A. C. and Zimny, M. L. (1977). Effects of hibernation on interradicular alveolar bone. J. Dent. Res. 56, 1552-1557. [DOI] [PubMed] [Google Scholar]
- Harvey, K. B. and Donahue, S. W. (2004). Bending properties, porosity, and ash fraction of black bear (Ursus americanus) cortical bone are not compromised with aging despite annual periods of disuse. J. Biomech. 37, 1513-1520. [DOI] [PubMed] [Google Scholar]
- Harvey, K. B., Drummer, T. D. and Donahue, S. W. (2005). The tensile strength of black bear (Ursus americanus) cortical bone is not compromised with aging despite annual periods of hibernation. J. Biomech. 38, 2143-2150. [DOI] [PubMed] [Google Scholar]
- Hellgren, E. C. (1998). Physiology of hibernation in bears. Ursus 10, 467-477. [Google Scholar]
- Hudson, N. J., Bennett, M. B. and Franklin, C. E. (2004). Effect of aestivation on long bone mechanical properties in the green-striped burrowing frog, Cyclorana alboguttata. J. Exp. Biol. 207, 475-482. [DOI] [PubMed] [Google Scholar]
- Inman, C. L., Warren, G. L., Hogan, H. A. and Bloomfield, S. A. (1999). Mechanical loading attenuates bone loss due to immobilization and calcium deficiency. J. Appl. Physiol. 87, 189-195. [DOI] [PubMed] [Google Scholar]
- Jaworski, Z. F. and Uhthoff, H. K. (1986). Reversibility of nontraumatic disuse osteoporosis during its active phase. Bone 7, 431-439. [DOI] [PubMed] [Google Scholar]
- Kenagy, G. J. and Hoyt, D. F. (1989). Speed and time-energy budget for locomotion in golden-mantled ground squirrels. Ecology 70, 1834-1839. [Google Scholar]
- Krook, L., Wimsatt, W. A., Whalen, J. P., MacIntyre, I. and Nunez, E. A. (1977). Calcitonin and hibernation bone loss in the bat (Myotis lucifugus). Cornell Vet. 67, 265-271. [PubMed] [Google Scholar]
- Leblanc, A. D., Schneider, V. S., Evans, H. J., Engelbretson, D. A. and Krebs, J. M. (1990). Bone mineral loss and recovery after 17 weeks of bed rest. J. Bone Miner. Res. 5, 843-850. [DOI] [PubMed] [Google Scholar]
- Martin, R. B., Burr, D. B. and Sharkey, N. A. (1998). Skeletal Tissue Mechanics. New York: Springer.
- McGee, M. E., Miller, D. L., Auger, J., Black, H. L. and Donahue, S. W. (2007). Black bear femoral geometry and cortical porosity are not adversely affected by ageing despite annual periods of disuse (hibernation). J. Anat. 210, 160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee, M. E., Maki, A. J., Johnson, S. E., Nelson, O. L., Robbins, C. T. and Donahue, S. W. (2008). Decreased bone turnover with balanced resorption and formation prevent cortical bone loss during disuse (hibernation) in grizzly bears (Ursus arctos horribilis). Bone 42, 396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens, A., Stiedl, O., Steinlechner, S. and Meyer, M. (2008). Cardiac dynamics during daily torpor in the Djungarian hamster (Phodopus sungorus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R639-R650. [DOI] [PubMed] [Google Scholar]
- Spatz, H. C., O'Leary, E. J. and Vincent, J. F. (1996). Young's moduli and shear moduli in cortical bone. Proc. Biol. Sci. 263, 287-294. [DOI] [PubMed] [Google Scholar]
- Steinberg, B., Singh, I. J. and Mitchell, O. G. (1979). Changes observed in bone during hibernation using Procion red dye as a matrical marker. J. Exp. Zool. 210, 537-541. [DOI] [PubMed] [Google Scholar]
- Steinberg, B., Singh, I. J. and Mitchell, O. G. (1981). The effects of cold-stress, hibernation, and prolonged inactivity on bone dynamics in the golden hamster, Mesocricetus auratus. J. Morphol. 167, 43-51. [DOI] [PubMed] [Google Scholar]
- Steinberg, B., Singh, I. J. and Mitchell, O. G. (1986). An autoradiographic study of the uptake of tritiated proline by osteoblasts during hibernation. Histol. Histopathol. 1, 155-160. [PubMed] [Google Scholar]
- Storey, K. B. and Storey, J. M. (1990). Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q. Rev. Biol. 65, 145-174. [DOI] [PubMed] [Google Scholar]
- Trebacz, H. (2001). Disuse-induced deterioration of bone strength is not stopped after free remobilization in young adult rats. J. Biomech. 34, 1631-1636. [DOI] [PubMed] [Google Scholar]
- Ueda, S. and Ibuka, N. (1995). An analysis of factors that induce hibernation in Syrian hamsters. Physiol. Behav. 58, 653-657. [DOI] [PubMed] [Google Scholar]
- Wang, L. C. H. and Lee, T. F. (2000). Perspectives on metabolic suppression during mammalian hibernation and daily torpor. In Life in the Cold (ed. G. Heldmaier and M. Klingenspor), pp. 152-158. Berlin: Springer-Verlag.
- Wassmer, T. (2004). Body temperature and above-ground patterns during hibernation in European hamsters (Cricetus cricetus L.). J. Zool. 262, 281-288. [Google Scholar]
- Whalen, J. P., Krook, L. and Nunez, E. A. (1972). A radiographic and histologic study of bone in the active and hibernating bat (Myotis lucifugus). Anat. Rec. 172, 97-108. [DOI] [PubMed] [Google Scholar]
- Zimmerman, G. D., McKean, T. A. and Hardt, A. B. (1976). Hibernation and disuse osteoporosis. Cryobiology 13, 84-94. [DOI] [PubMed] [Google Scholar]