Abstract
Background
To date, it has been widely assumed that malaria is a common cause of morbidity and mortality in children with sickle cell disease (SCD) in malaria-endemic countries, and as a result, malarial prophylaxis is commonly recommended. Nevertheless, few data are available that support this practice.
Methods
We conducted a retrospective analysis of the data collected prospectively from children aged 0-13 years who were admitted to Kilifi District Hospital during the period from July 1998 through June 2005. We studied the prevalence, clinical features, and outcome of malarial infections in these children, stratified by SCD status.
Results
Although we estimated the prevalence of SCD in children to be only 0.8% (71 of 8531 children) during the period from August 2006 through September 2008 in the community surrounding the hospital, 555 (1.6%) of 34,529 children admitted to the hospital during the study period (i.e., from July 1998 through June 2005) were children with SCD; in fact, a total of 309 children with SCD were admitted 555 times. The prevalence of Plasmodium falciparum parasitemia was lower among children with SCD than it was among children without SCD (86 [15.6%] of 551 children vs. 13,835 [41.3%] of 33,500 children; P < .001). Similarly, among those infected with P. falciparum parasites, the mean parasite density was significantly lower among children with SCD than it was among children without SCD (2205 vs. 23,878 parasites/μL; P < .001). Fourteen (16.3%) of 86 parasitemic patients with SCD had features consistent with severe malaria, compared with 3424 (24.7%) of 13,835 parasitemic patients without SCD (odds ratio, 0.59; P = .07). We found no association between malarial parasitemia and death.
Conclusions
We found no evidence to support the conclusion that the risk of malaria is higher among children with SCD than it is among children without SCD in a rural area on the coast of Kenya. Further studies should be undertaken to help policy makers develop appropriate guidelines regarding malarial prophylaxis for patients with SCD in malaria-endemic regions.
Globally, some 300,000 children are born with sickle cell disease (SCD) every year [1]. More than three-quarters of these children are born in sub-Saharan Africa, where, in the absence of interventions, their mortality remains high [2, 3]. Although the sickle cell trait (i.e., hemoglobin genotype AS [HbAS]) is known to protect against clinical malaria, it is widely believed that malaria is a major risk factor for death among children with SCD who are born in malaria-endemic areas [4-6]. Nevertheless, the data supporting this assumption are largely inconclusive. Although there is no doubt that individuals with SCD can develop severe and fatal malaria, there is no direct evidence that they are at greater risk from the disease than individuals without SCD. Although a number of studies appear to support this conclusion [7-9], the evidence presented has been largely circumstantial, and such studies are balanced by others that have concluded that individuals with SCD are at equal [10] or even lower risk [11, 12]. We have studied the relationship between malaria and SCD in the context of a long-standing clinical surveillance study conducted at a rural district hospital in a malaria-endemic region on the coast of Kenya with a view to clarifying this question.
PATIENTS AND METHODS
Study area
Our study was conducted in Kilifi District Hospital, which is situated 60 km to the north of Mombasa on the Kenyan coast [13]. Kilifi District Hospital provides primary care for the local community, serving a population of ∼100,000 children <14 years old, and acts as a first-referral center for health care facilities throughout Kilifi District. Kilifi District Hospital is the only health care facility in Kilifi District providing specialized care for children with SCD. Ninety percent of the children admitted to this hospital live less than 6.5 h away on foot or 2 h by vehicle. Approximately one-third of all child deaths in the area served by the hospital occur at Kilifi District Hospital. In an ongoing cohort study conducted in the community surrounding the hospital, we estimated the prevalence of the sickle cell trait (i.e., HbAS) in this population to be 14.9% (1267 of 8531 children) and the prevalence of SCD to be 0.8% (71 of 8531 children) during the period from August 2006 through September 2008 (T.W., unpublished data). At present, we have no data from which to judge the survival of children with SCD in our study area.
Study population and data collection
The current study included all children <14 years who were admitted to Kilifi District Hospital during the period from 1 July 1998 through 31 June 2005. During this period, the details of a standard clinical history and physical examination were recorded on all admissions, along with admission and discharge diagnoses. Routine blood tests included a full blood count, venous blood gas analysis, and a blood film examination for malaria. Further investigations were conducted as clinically indicated. Details regarding inpatient course of treatment were recorded in hand-written hospital notes.
We applied the following definitions for the purposes of our analysis. Fever was defined as an axillary temperature of >37.5°C or a history of fever as given by the parent or caretaker on hospital admission. Malaria was considered the primary diagnosis if Plasmodium falciparum parasites were found in the peripheral blood and if clinical or laboratory features did not support an alternative diagnosis. Malarial episodes were further categorized on the basis of the following clinical features: coma (the inability to localize a painful stimulus or a Blantyre coma score of ≤2); prostration (a Blantyre coma score of 2-4); or acidosis (a base deficit of >8). Severe malarial anemia (SMA) was classified according to 2 separate definitions: an Hb concentration of <5 g/dL in association with P. falciparum malaria at a parasite density of >10,000 parasites/μL (hereafter referred to as SMA1) and an Hb concentration of <5 g/dL in association with malarial parasites at any density (hereafter referred to as SMA2). Hyperparasitemia was also classified according to 2 separate definitions: a P. falciparum parasite density of >500,000 or >250,000 parasites/μL. The definitions for SMA and hyperparasitemia are consistent with criteria for severe and complicated malaria that are commonly recognized by the World Health Organization [14]. Anemia was classified as mild (Hb concentration, 7-10 g/dL), moderate (Hb concentration, 5-6.9 g/dL), or severe (Hb concentration, <5 g/dL). Acute lower respiratory tract infection was defined as a history of cough or difficulty breathing in association with fast breathing, lower chest indrawing, central cyanosis, inability to drink, or an altered level of consciousness. Severe malnutrition was defined as a weight-for-height z score of <−3 standard deviations or clinical signs of kwashiorkor, and gastroenteritis was defined as ≥3 watery stools in the preceding 24 h and/or vomiting (≥3 episodes in the preceding 24 h). Dactylitis was diagnosed in children with SCD presenting with painful inflammation of the small bones of the hands and feet. Other diagnoses were made at the discretion of the consulting physician.
Classification of patients
Patients admitted to the hospital were classified retrospectively as either patients with SCD or patients without SCD. Patients were classified as having SCD if their hospital admission or discharge diagnoses included reference to a diagnosis of SCD or if they were already known to have the disease on the basis of their attending the sickle cell clinic. For the purposes of some analyses, we further categorized the patients with SCD into 2 subgroups: patients with an existing diagnosis of SCD who had visited a clinic for treatment on at least 1 occasion (hereafter referred to as clinic attenders) and previously undiagnosed patients who had not visited a clinic (hereafter referred to as clinic nonattenders). Because the hospital admission process did not include routine screening for SCD, it is likely that a number of patients with SCD were falsely classified as patients without SCD; however, because the prevalence of SCD in this community was estimated to be 0.8% (71 of 8531 children) during the period from August 2006 through September 2008, we assumed the rate of false classification would be too low to make a material difference to our subsequent analyses.
Laboratory investigations
Complete hemograms were performed using an automated cell counter (Beckman Coulter). Blood films were examined for P. falciparum parasites as described elsewhere [15]. Parasite densities were calculated from the ratio of parasites to white blood cells or from the ratio of parasites to red blood cells for more severe infections. Because white blood cells could not be differentiated from erythroblasts using our cell counter, we used an arbitrary white blood cell count of 12.1 × 106 cells/mL as the basis for these calculations (the mean white blood cell count at hospital admission) for all patients. Based on the group-specific geometric mean red blood cell counts of patients at hospital admission, if blood count data at hospital admission were missing, we assumed a red blood cell count of 2.4 × 1012 cells/L in patients with SCD and 4.0 × 1012 cells/L in patients without SCD. All cases of SCD were confirmed by hemoglobin electrophoresis or by polymerase chain reaction (PCR) [16].
Data processing and statistical analysis
Our principle analyses compared data from patients with SCD at hospital admission with data from patients without SCD at hospital admission. We compared the proportion of patients with and without SCD using the χ2 test and mean number of patients with and without SCD using the Student’s t test. Because some patients with SCD were admitted to the hospital more than once, we used generalized estimating equation modeling, a method that takes account of the intrasubject correlation between repeated observations of the same subject, to compute odds ratios (ORs) for the prevalence of malaria, severe malaria, and other diagnoses by category of SCD. Although it is probable that a number of children without SCD were also admitted to the hospital more than once, we were unable to quantify this from the data available. All other data were analyzed using Stata, version 10.0 (StataCorp). Ethical permission for the study was granted by the Kenya Medical Research Institute and the National Ethical Review Committee.
RESULTS
Characteristics of the study patients
A total of 34,529 children were admitted to the hospital during the period of observation (i.e., from July 1998 through June 2005). Of these 34,529 children, 555 (1.6%) were children with SCD; in fact, a total of 309 children with SCD were admitted 555 times. The characteristics of these patients are summarized in table 1.
Table 1. Characteristics of patients with and without sickle cell disease (SCD) who were admitted to Kilifi District Hospital during the period 1 July 1998 through 31 June 2005.
| Baseline characteristic | Patients with SCD (n = 555) |
Patients without SCD (n = 33,974) |
P a |
|---|---|---|---|
| No. (%) of male patients | 315 (56.8) | 18,724 (55.1) | .66 |
| No. (%) of female patients | 240 (43.2) | 15,250 (44.9) | .66 |
| Median age (IQR), months | 58.3 (29.3-90.9) | 17.3 (6.7-90.8) | <.001 |
| Proportion (%) of patients with severe malnutritionb | 77/555 (13.9) | 6491/33,042 (19.6) | .001 |
| Mean Hb concentration (95% CI), g/dL | 6.5 (6.1-6.5) | 9.0 (9.01-9.08) | <.001 |
| Proportion (%) of patients with Hb concentration <5 g/dL | 160/535 (29.9) | 3169/30,655 (10.3) | <.001 |
| Proportion (%) of patients with Hb concentration 5-7 g/dL | 152/535 (28.4) | 4303/30,655 (14.0) | <.001 |
| Proportion (%) of patients with Hb concentration >7 g/dL | 223/535 (41.7) | 23,183/30,655 (75.6) | <.001 |
| Geometric MCV (95% CI), fL | 82.4 (81.3-83.5) | 73.7 (73.5-73.8) | <.001 |
| Mean WBC count (95% CI), 106 cells/mL | 22.2 (20.8-23.6) | 12.0 (11.9-12.1) | <.001 |
| Mean platelet count (95% CI), 109 platelets/L | 254.3 (232.9-277.8) | 217.1 (214.7-219.6) | <.001 |
| No. (%) of patients with malariac | 59 (10.6) | 13,032 (38.4) | <.001 |
| Proportion (%) of patients infected with malarial parasites | 86/551 (15.6) | 13,835/33,500 (41.3) | <.001 |
| Geometric mean parasite density (95% CI), parasites/μL | 2205 (1384-3513) | 23,878 (22,907-24,891) | <.001 |
NOTE. Data were not available for some children; some patients were admitted to the hospital more than once. CI, confidence interval; Hb, hemoglobin; IQR, interquartile range; MCV, mean corpuscular volume; WBC, white blood cell.
Determined by use of χ2 test, Mann-Whitney test, or Student’s t test, as appropriate.
Defined as a weight-for-height z score of <−3 standard deviations or clinical signs of kwashiorkor.
Malaria was recorded as a primary or secondary diagnosis at hospital discharge.
Clinical and hematological characteristics at hospital admission
Among the 555 children with SCD, the most common symptoms at hospital admission were fever (414 children [74.6%]) and pain (355 children [64.0%]). Primary diagnoses included acute lower respiratory tract infection (40 children [7.2%]), dactylitis (31 children [5.6%]), and diarrhea (15 children [2.7%]). The mean Hb concentration was statistically lower among patients with SCD at hospital admission than among patients without SCD at hospital admission (6.5 vs. 9.0 g/dL; P < .001). The mean corpuscular volume was statistically significantly higher among patients with SCD at hospital admission than among patients without SCD at hospital admission (82.4 vs. 73.7 fL; P < .001). The mean platelet count was statistically significantly higher among patients with SCD at hospital admission than among patients without SCD at hospital admission (254.3 × 109 vs. 217.1 × 109 platelets/L; P < .001). The mean white blood cell count was statistically significantly higher among patients with SCD at hospital admission than among patients without SCD at hospital admission (22.2 × 106 vs. 12.0 × 106 cells/mL; P < .001). Of the 555 patients with SCD at hospital admission, Hb values were available for 535 (96.4%). Of these 535 patients, 152 (28.4%) had moderate anemia, and 160 (29.9%) had severe anemia. Of the 33,974 patients without SCD at hospital admission, Hb values were available for 30,655 (90.2%). Of these 30,655 patients, 4303 (14.0%) had moderate anemia, and 3169 (10.3%) had severe anemia (table 1). At hospital admission, 153 (32.6%) of 469 patients with SCD and 3581 (13.4%) of 26,749 patients without SCD received a blood transfusion.
Malaria
Malaria was recorded as a primary or secondary diagnosis at hospital discharge for 59 (10.6%) of the 555 patients with SCD at hospital admission, compared with 13,032 (38.4%) of the 33,974 patients without SCD at hospital admission (OR, 0.19; P < .001). P. falciparum parasites were detected in a significantly lower proportion of patients with SCD (86 [15.6%] of 551), compared with patients without SCD (13,835 [41.3%] of 33,500) (OR, 0.27; 95% confidence interval, 0.21-0.34; P < .001) (table 1). Furthermore, among these patients infected with P. falciparum parasites, the mean parasite density was significantly lower among patients with SCD than it was among patients without SCD (2205 vs. 23,878 parasites/μL; P < .001) (table 1). Since the mean age at hospital admission was significantly older for patients with SCD than it was for patients without SCD and since malaria transmission also decreased during the course of the study [17], we further analyzed the data by age (figure 1) and year of hospital admission (figure 2). The reduced parasite densities seen in patients with SCD were consistent throughout the study period (figure 2) and were significant at all ages (figures 1 and 3). Recognizing that clinic attenders might well have been receiving antimalarial chemoprophylaxis, we also analyzed the data according to the 2 subgroups of patients with SCD (i.e., clinic attenders and clinic nonattenders). Although malarial parasitemia was significantly less frequent in clinic attenders than it was in nonattenders (table 2), all analyses remained significant after adjustments for clinic-attender status as well as age and year of admission.
Figure 1.
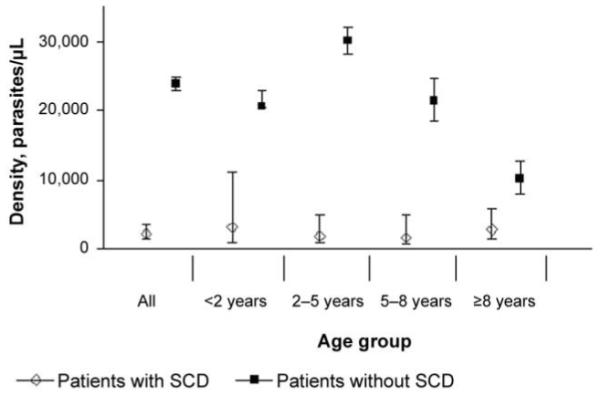
Parasite densities among patients with and without sickle cell disease (SCD), by age group. Densities were calculated from the ratio of parasites to white blood cells or from the ratio of parasites to red blood cells for more severe infections.
Figure 2.
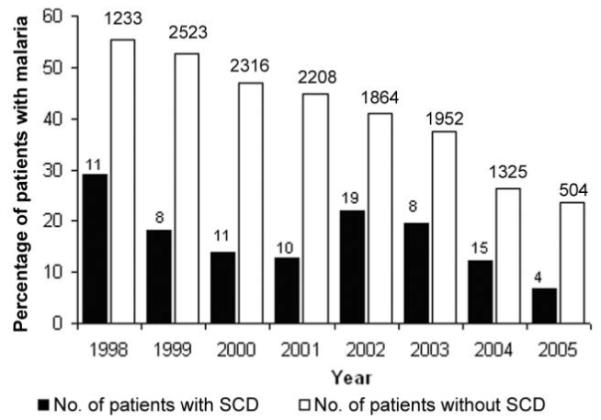
Prevalence of malaria among patients with and without sickle cell disease (SCD), by year of hospital admission.
Figure 3.
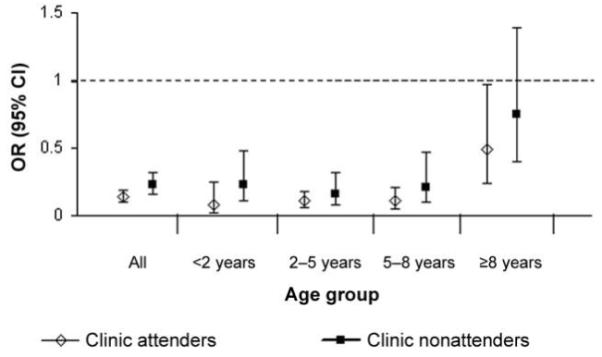
Odds ratios (ORs) and 95% confidence intervals (CIs) for malarial parasitemia among 2 subgroups of patients with sickle cell disease (SCD; i.e., clinic attender and clinic nonattenders), by age group. ORs were derived by generalized estimating equation analysis.
Table 2. Characteristics of patients with sickle cell disease (SCD) who were admitted to Kilifi District Hospital during the period 1 July 1998 through 31 June 2005, by clinic attendance.
| Baseline characteristic | Clinic attenders (n = 326) | Clinic nonattenders (n = 229) | Total | P a |
|---|---|---|---|---|
| Mean Hb concentration (95% CI), g/dL | 6.7 (6.5-6.9) | 5.8 (5.5-6.2) | 6.5 (6.1-6.5) | <.001 |
| Proportion (%) of patients with P. falciparum parasitemia | 42/321 (13.1) | 44/223 (19.7) | 86/544 (15.8) | .037 |
| Geometric mean parasite density (95% CI), parasites/μL | 1532 (775-3026) | 3098 (1622-5917) | 2205 (1384-3513) | .1 |
NOTE. Patients with an existing diagnosis of SCD who had visited a clinic for treatment on at least 1 occasion were referred to as clinic attenders, and previously undiagnosed patients who had not visited a clinic were referred to as clinic nonattenders. CI, confidence interval; Hb, hemoglobin; P. falciparum, Plasmodium falciparum.
Determined by use of χ2 test, Mann-Whitney test, or Student’s t test, as appropriate.
Severe P. falciparum malaria
Of the 13,921 children admitted to the hospital with P. falciparum parasitemia, 14 (16.3%) of 86 children with SCD and 3424 (24.7%) of 13,835 children without SCD (OR, 0.59; P = .07) had at least 1 of 5 conditions that were consistent with the World Health Organization criteria for severe and complicated malaria (table 3). Prostration and hyperparasitemia were both significantly less frequent among patients with SCD at hospital admission than they were among patients without SCD at hospital admission, whereas we found no significant difference between patients with and without SCD in the prevalence of coma or acidosis. We also found no significant difference in the prevalence of SMA1; however, we did find a higher prevalence of SMA2. Only 4 parasitemic children with SCD died.
Table 3. Criteria for severe and complicated malaria among Plasmodium falciparum parasite-positive children with and without sickle cell disease (SCD) who were admitted to Kilifi District Hospital during the period 1 July 1998 through 31 June 2005.
| Children who were admitted (n = 13,921) |
Children who died (n = 490) |
|||||
|---|---|---|---|---|---|---|
| Criteria, condition | With SCD (n = 86) | Without SCD (n = 13,835) | P | With SCD (n = 4) | Without SCD (n = 486) | P |
| WHO criteria | ||||||
| Prostration | 2 (2.3) | 1188 (8.6) | .04 | ... | 88 (18.1) | .34 |
| Coma | 4 (4.7) | 404 (2.9) | .34 | 1 (25) | 74 (15.2) | .58 |
| Acidosis | 5 (5.8) | 1225 (8.9) | .32 | ... | 209 (43) | .08 |
| Hyperparasitemiaa | 1 (1.2) | 1198 (8.7) | .014 | 1 (25) | 51 (10.5) | .35 |
| Severe malarial anemiab | 6 (7.0) | 1208 (8.7) | .56 | ... | 65 (13.4) | .37 |
| Non-WHO criteria | ||||||
| Hyperparasitemiac | 2 (2.3) | 2967 (21.4) | <.001 | 1 (25) | 92 (18.9) | .57 |
| Severe malarial anemiad | 38 (44.2) | 2099 (15.2) | <.001 | ... | 132 (27.2) | .19 |
Data are no. (%) of children. WHO, World Health Organization.
A P. falciparum parasite density of >500,000 parasites/μL.
A hemoglobin (Hb) concentration of <5 g/dL in association with P. falciparum malaria at a parasite density of >10,000 parasites/μL.
A P. falciparum parasite density of >250,000 parasites/μL.
An Hb concentration of <5 g/dL in association with malarial parasites at any density.
Mortality in patients with SCD
Twenty-five (4.5%) of the 555 children with SCD at hospital admission died. Of these 25 children who died, 12 (48.0%) were <5 years of age, 4 (16.0%) had malarial parasitemia, and 9 (36.0%) had severe anemia (Hb concentration, <5 g/dL). There was no statistically significant association between malarial parasitemia and death among patients with SCD (OR, 0.92; 95% confidence interval, 0.31-2.7; P = .87). In this analysis, adjustments for age, Hb concentration, clinic attendance, and nutritional status made no statistically significant difference.
DISCUSSION
To date, it has been widely assumed that children with SCD in sub-Saharan Africa are at increased risk of morbidity and mortality from malaria. A study undertaken in the Garki District of Abuja, Nigeria, by Molineaux et al. [7]—in which it was found that survival among children with SCD improved after implementation of intensive malaria control measures—has often been used as supportive evidence, and numerous case and series reports have highlighted the fact that severe and fatal malaria can occur in patients with SCD [8, 9, 18, 19]. To our knowledge, however, the studies conducted to date have not provided conclusive evidence for the conclusion that the risks or consequences of malaria are higher in patients with SCD. For example, although a recent study from Nigeria [8] found that malaria was the most common infection among patients with SCD, it contains no comparative data regarding patients without SCD.
Although few studies have reported the relative risk of malaria among children with SCD, on balance, these studies suggest that such children may even enjoy a degree of protection. For example, in a case-control study of febrile children that was conducted in Nigeria, Okuonghae et al. [19] found that P. falciparum parasites were detectable in only 9% of patients with SCD, compared with 29% of patients without SCD. Similarly, a number of studies have found either a lower prevalence or a lower density of parasitemia among children with SCD, compared with children without SCD [12, 18]. The fact that many patients with SCD have received malarial prophylaxis has made some studies difficult to interpret. However, at the conclusion of a cohort study conducted in Western Kenya by Aidoo et al. [11], the authors used PCR methods to test participants for SCD, making it unlikely that their status was known at the time of observation. Although the subjects with SCD were few in number, Aidoo et al. [11] found that the incidence of both severe malarial anemia and high-density parasitemia were both significantly lower among subjects with SCD, providing some of the best evidence to date for a protective effect of SCD.
In the present study, we used the framework of a well-established surveillance study to explore the relative risk of malaria among children with SCD in coastal Kenya. We found that malaria was diagnosed less than half as often in children with SCD than it was in children without SCD, an observation supported by the prevalence of P. falciparum parasitemia at hospital admission. We wondered whether this might be explained by the age difference between the patients with SCD and the patients without SCD but found that the observation was true at all ages. Similarly, we could not explain our observation on the basis of temporal patterns of malaria transmission or other confounding factors that we could identify. In particular, we wondered whether this observation might have been attributable to antimalarial chemoprophylaxis. However, 233 (42.0%) of the 555 patients with SCD at hospital admission were among those who were previously undiagnosed, and the reduced prevalence of malaria was significant for both the clinic attenders and the clinic nonattenders, individually and in all patients when adjusting for clinic-attender status. As a result, we believe it unlikely that the decrease in parasite prevalence could be entirely explained by behavioral effects.
At the same time that we found a decrease in parasite prevalence among children with SCD, we also found that the mean parasite density was 10-fold lower among patients with SCD at hospital admission than among patients without SCD at hospital admission. This observation was similarly robust enough to take into account adjustment for known confounders. Finally, among the subgroup of patients with malarial parasitaemia at hospital admission, we compared the prevalence of conditions of severe and complicated malaria among those with and without SCD. We found no significant differences in the prevalence of coma, acidosis, or severe anemia, whereas the prevalence of both prostration and hyperparasitemia were significantly lower among children with SCD. By contrast, the prevalence of anemia in association with lower parasite densities (<10,000 parasites/μL; i.e., SMA2) was significantly higher among children with SCD than it was among children without SCD. Although, in our study, this complication was not associated with any deaths, it might be in a health care setting with limited resources.
Our study has a number of limitations. The most important one being that our SCD group only included children with an existing diagnosis or in whom the diagnosis was made during the course of their hospitalization. As a result, we cannot exclude the possibility that some patients with SCD might have had malaria at hospital admission and that some might have died from malaria, without their SCD diagnosis being made. Moreover, we have no data regarding the survival of patients with SCD in our community from which to estimate their relative incidence of malaria and/or other diagnoses at hospital admission. Although approximately one-third of child deaths in the area served by the hospital occur at Kilifi District Hospital, a higher proportion of deaths occurs outside hospital, and it is therefore entirely conceivable that some children with SCD may die from malaria at home, undetected by the current surveillance system. Finally, in the present study, we used children with or without SCD at hospital admission as denominators for our prevalence estimates. Because children with SCD are likely to be admitted to a hospital more often than children without SCD, this method could underestimate the risk of malaria among these children. Studies that include a more inclusive screening policy and allow an estimate of the incidence of malaria among patients with and without SCD at hospital admission, such as case-control or cohort studies, would constitute a stronger study design—something we hope to explore in future studies. Nevertheless, we believe that both the size of our study and the systematic nature of our inpatient surveillance offer strong support for the conclusion that, rather than being at increased risk for malaria, children with SCD enjoy a significant degree of protection.
At a mechanistic level, this conclusion seems entirely plausible. Children with HbAS are strongly protected against malaria [11, 20-22], possibly because P. falciparum parasites are unable to thrive in red blood cells containing HbS or because infected erythrocytes are prematurely removed during an immune response [23]. It seems logical, therefore, that homozygosity, in which intracellular concentrations of HbS are considerably higher, might be associated with even greater protection. This is consistent with studies that have investigated the mechanisms of malaria protection by HbS [24, 25] and with the observation that the protective effects of HbC are greatest in homozygotes [26]. Moreover, the high concentrations of fetal hemoglobin in the erythrocytes of children with SCD, another factor thought to confer malaria protection [27], could also mediate additional protection. Nevertheless, it seems equally plausible that, among patients with SCD, severe malaria might also occur at lower parasite densities or could trigger important pathological processes that could lead to the sequestration of red blood cells or some other clinically important event.
It would be inappropriate to use our study as the basis for treatment recommendations. Rather, we hope our study will prompt interested scientists to revisit the issue of malarial prophylaxis for children with SCD and to conduct further studies with a view to developing evidence-based recommendations. A better understanding of the risks for malaria among patients with SCD will form an important initial step.
Acknowledgments
We thank Hussein Kivugo, Brett Lowe, Isiah Mwangi, Norbert Peshu, and the clinicians and nursing and laboratory staff at the Kenya Medical Research Institute’s Centre for Geographic Medicine Research-Coast for their help in conducting this study and Greg Fegan for statistical support. This study was published with the permission of the Director of the Kenya Medical Research Institute.
Financial support. This work was supported by funds from the Wellcome Trust of the United Kingdom (grant 076934 to T.N.W. and grant 077092 to K.M.) and from the European Union Network 6 BioMalpar initiative.
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–7. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleming AF, et al. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979;73:161–72. doi: 10.1080/00034983.1979.11687243. [DOI] [PubMed] [Google Scholar]
- 3.Molineaux L, Gramiccia G. The Garki Project: research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. World Health Organization; Geneva: 1980. [Google Scholar]
- 4.Serjeant GR. Mortality from sickle cell disease in Africa. BMJ. 2005;330:432–3. doi: 10.1136/bmj.330.7489.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diallo D, Tchernia G. Sickle cell disease in Africa. Curr Opin Hematol. 2002;9:111–6. doi: 10.1097/00062752-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Fleming AF. The presentation, management and prevention of crisis in sickle cell disease in Africa. Blood Rev. 1989;3:18–28. doi: 10.1016/0268-960x(89)90022-2. [DOI] [PubMed] [Google Scholar]
- 7.Molineaux L, Fleming AF, Cornille-Brøgger R, Kagan I, Storey J. Abnormal haemoglobins in the Sudan savanna of Nigeria. III. Malaria, immunoglobulins and antimalarial antibodies in sickle cell disease. Ann Trop Med Parasitol. 1979;73:301–10. doi: 10.1080/00034983.1979.11687263. [DOI] [PubMed] [Google Scholar]
- 8.Ibidapo MO, Akinyanju OO. Acute sickle cell syndromes in Nigerian adults. Clin Lab Haematol. 2000;22:151–5. doi: 10.1046/j.1365-2257.2000.00292.x. [DOI] [PubMed] [Google Scholar]
- 9.Ambe JP, Fatunde JO, Sodeinde OO. Associated morbidities in children with sickle-cell anaemia presenting with severe anaemia in a malarious area. Trop Doct. 2001;31:26–7. doi: 10.1177/004947550103100109. [DOI] [PubMed] [Google Scholar]
- 10.Gellert S, Hassan BY, Meleh S, Hiesgen G. Malaria prevalence and outcome in the in-patients of the paediatric department of the State Specialists Hospital (SSH), Maiduguri, Nigeria. J Trop Pediatr. 1998;44:109–13. doi: 10.1093/tropej/44.2.109. [DOI] [PubMed] [Google Scholar]
- 11.Aidoo M, et al. Protective effects of the sickle cell gene against malaria morbidity and mortality. Lancet. 2002;359:1311–2. doi: 10.1016/S0140-6736(02)08273-9. [DOI] [PubMed] [Google Scholar]
- 12.Aluoch JR. Higher resistance to Plasmodium falciparum infection in patients with homozygous sickle cell disease in western Kenya. Trop Med Int Health. 1997;2:568–71. doi: 10.1046/j.1365-3156.1997.d01-322.x. [DOI] [PubMed] [Google Scholar]
- 13.Marsh K, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–1404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 14.WHO Expert Committee on Malaria World Health Organ Tech Rep Ser. 2000;892:1–74. [PubMed] [Google Scholar]
- 15.Maitland K, et al. Response to volume resuscitation in children with severe malaria. Pediatr Crit Care Med. 2003;4:426–31. doi: 10.1097/01.PCC.0000090293.32810.4E. [DOI] [PubMed] [Google Scholar]
- 16.Waterfall CM, Cobb BD. Single tube genotyping of sickle cell anaemia using PCR-based SNP analysis. Nucleic Acids Res. 2001;29:E119. doi: 10.1093/nar/29.23.e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Meara WP, et al. Effect of a fall in malaria transmission on morbidity and mortality in Kilifi, Kenya. Lancet. 2008;372:1555–62. doi: 10.1016/S0140-6736(08)61655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Awotua-Efebo O, Alikor EA, Nkanginieme KE. Malaria parasite density and splenic status by ultrasonography in stable sickle-cell anaemia (HbSS) children. Niger J Med. 2004;13:40–3. [PubMed] [Google Scholar]
- 19.Okuonghae HO, Nwankwo MU, Offor E. Malarial parasitaemia in febrile children with sickle cell anaemia. J Trop Pediatr. 1992;38:83–5. doi: 10.1093/tropej/38.2.83. [DOI] [PubMed] [Google Scholar]
- 20.Allison AC. Protection afforded by sickle cell trait against subtertian malarial infection. BMJ. 1954;1:290–5. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill AV, et al. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 22.Williams TN, Mwangi TW, Wambua S, et al. Sickle cell trait and the risk of Plasmodium falciparum malaria and other childhood diseases. J Infect Dis. 2005;192:178–86. doi: 10.1086/430744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams TN. Red blood cell defects and malaria. Mol Biochem Parasitol. 2006;149:121–7. doi: 10.1016/j.molbiopara.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Cholera R, Brittain NJ, Gillie MR, et al. Impaired cytoadherence of Plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc Natl Acad Sci U S A. 2008;105:991–6. doi: 10.1073/pnas.0711401105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasvol G, Weatherall DJ, Wilson RJ. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978;274:701–3. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- 26.Modiano D, Luoni G, Sirima BS, et al. Haemoglobin C protects against clinical Plasmodium falciparum malaria. Nature. 2001;414:305–8. doi: 10.1038/35104556. [DOI] [PubMed] [Google Scholar]
- 27.Pasvol G, Weatherall DJ, Wilson RJ. Effects of foetal haemoglobin on susceptibility of red cells to Plasmodium falciparum. Nature. 1977;270:171–3. doi: 10.1038/270171a0. [DOI] [PubMed] [Google Scholar]


