Abstract
Objective
To evaluate whether sugar sweetened beverage consumption, a significant source of dietary fructose, is associated with higher serum uric acid levels and blood pressure in adolescents.
Study design
We analyzed cross-sectional data from 4867 adolescents aged 12–18 years old in the National Health and Nutrition Examination Survey 1999–2004. Dietary data was assessed from 24-hour dietary recall interviews. Sugar sweetened beverages included fruit drinks, sports drinks, soda, and sweetened coffee or tea. We used multivariate linear regression to evaluate the association of sugar sweetened beverage consumption with serum uric acid and with blood pressure.
Results
Adolescents who drank more sugar sweetened beverages tended to be older and male. In the adjusted model, serum uric acid increased by 0.18 mg/dL and systolic blood pressure z-score increased by 0.17 from the lowest to the highest category of sugar sweetened beverage consumption (P for trend 0.01 and 0.03, respectively).
Conclusions
These results from a nationally representative sample of US adolescents indicate that higher sugar sweetened beverage consumption is associated with higher serum uric acid levels and systolic blood pressure, which may lead to downstream adverse health outcomes.
Keywords: Adolescents, obesity, blood pressure, uric acid, hypertension, epidemiology, NHANES, National Health and Nutrition Examination Survey, CDC, Centers for Disease Control and Prevention, sugar sweetened beverages
Since the introduction into the US food supply in the 1970s, high fructose corn syrup has become the most popular sweetener used in processed foods, especially in beverages such as sodas and fruit drinks.1 Fructose, unlike glucose or other monosaccharide sugars, is solely metabolized in the liver where it induces nucleotide catabolism, thereby producing uric acid.2, 3 Human studies confirm that experimental fructose feeding or intravenous fructose administration raises serum uric acid levels.4–7 This may be important because serum uric acid has been suggested to be a marker of cardiovascular disease risk8 and a potential intermediate step towards the development of hypertension.9
Sugar sweetened beverages are a significant source of calories for adolescents, contributing 301 kilocalories per day or 13% of total daily energy.10 Two previous epidemiologic studies in adults have shown that sugar sweetened beverage consumption is associated with higher serum uric acid levels.11, 12 Although sugar sweetened beverage consumption has been associated with obesity in children and adolescents,13 it is unknown how sugar sweetened beverage consumption is independently associated with serum uric acid levels in adolescents. In addition, even though uric acid has been positively associated with blood pressure elevation and primary hypertension in children,14–17 it is unknown how sugar sweetened beverage consumption is associated with blood pressure level in adolescents.
The goal of this study was to evaluate whether the consumption of sugar sweetened beverages is associated with higher serum uric acid levels, and with higher blood pressure, in a nationally representative sample of adolescents.
METHODS
This is a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) 1999–2004, a complex sample survey designed to collect data on the health and nutrition from a representative household population in the United States.18 Certain populations, such as adolescents and racial/ethnic minorities were over-sampled to improve estimates in these groups.
The NHANES protocol was reviewed and approved by the National Center for Health Statistic’s Institutional Review Board. Informed consent and assent were obtained, where appropriate, from all participants before any study procedure.
There were 4938 adolescents aged 12 to18 years with dietary intake data and serum uric acid levels measured in NHANES 1999–2004. We excluded pregnant adolescents (n= 71). Thus, there were 4867 adolescents available for this analysis.
In NHANES 1999–2004, information on race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), medical history, and medication usage was collected by self-report in a standardized in-home medical interview. Information on age, weight, height, body mass index (BMI), and blood pressure was determined at the time of the physical examination component of the survey. Blood pressure was measured using a mercury sphygmomanometer using the American Heart Association guidelines.19 Up to four blood pressure measurements were taken. When more than one blood pressure measurement was available, the average systolic and diastolic blood pressure measurements were calculated. Smoking status was determined by a self-reported history of smoking in the previous 5 days, or a serum cotinine level (a metabolite of nicotine) > 15 ng/mL.20
Twenty-four hour dietary recall interviews were administered at the time of the physical examination component of the survey using a computer-assisted dietary interview system with multiple-pass format and standardized probes. Both weekdays and weekend days were sampled. Dietary information was available from three data sets: the total nutrients file, the individual food files, and the pyramid servings intake data for NHANES. The NHANES total nutrient intake files provided dietary fiber intake, sodium intake, caffeine intake, and total calories. The individual food files provided alcohol intake and individual food items which were analyzed by their USDA food code. Sugar sweetened beverages included fruit drinks, soft drinks, sports drinks, reconstituted powders (e.g. Kool-aid®, Tang®), and sweetened coffee or tea. Juices included unsweetened fruit juice or nectar. Diet beverages included non-caloric beverages such as diet sodas, coffee or tea without sweeteners, and low calorie drinks. Milk beverages included: cows milk, buttermilk, goat’s milk, and reconstituted milk. The pyramid serving intake data provided information on the number of servings per day of fruit, vegetables, dairy, meat, and seafood.
Serum uric acid levels were measured by a colorimetric method whereby uric acid is oxidized by uricase to allantoin and hydrogen peroxide. Details about quality-control procedures have been published elsewhere.21 Values are reported in milligrams per deciliter (to convert to micromoles per liter, multiply by 59.48).
Beverage consumption was categorized based on readily understood serving sizes (12 ounces is equivalent to one can of soda and 8 ounces is equivalent to one serving size of milk or juice), distribution of the data, and to ensure sufficient sample size.18 Sugar sweetened beverage consumption was categorized by number of ounces consumed per day (0 oz/day, 1–12 oz/day, 13–24 oz/day, 25–36 oz/day, or >36 oz/day). Juices were categorized by number of ounces consumed per day (0 oz/day, 1–8 oz/day, 9–16 oz/day or >16 oz/day). Diet beverage consumption was categorized by number of ounces consumed per day (0 oz/day, 1–12 oz/day or > 12 oz/day). Milk beverage consumption was categorized by number of ounces consumed per day (0 oz/day, 1–8 oz/day, 9–16 oz/day, or >16oz/day).
All statistical analyses were completed using STATA (version 9.0 StataCorp, College Station, TX) taking into account samples’ weights, strata, and primary sampling units from NHANES 1999–2004 in order to adjust for unequal probabilities of selection and the multistage stratified sample design.22 The weighted proportions were used to extrapolate to the adolescent US population using 2000 US Census counts. Linear test for trend was reported for categories of beverage consumption.
We used linear regression models to evaluate the association of sugar sweetened beverage consumption categories with serum uric acid levels, and with blood pressure. We explored the following potential confounding variables: age (years), race/ethnicity, sex, BMI z-scores, height, z-scores, total calories (kcal/day), smoking, alcohol (≥ 50 gm/day), fruit (servings/day), dairy (servings/day), meat and seafood (servings/day), dietary fiber intake (gm/day), caffeine intake (mg/day), sodium intake (mg/day), milk consumption, and diet beverage consumption. Our initial linear regression models adjusted for basic clinical and demographic variables: age, race/ethnicity, sex, BMI z-score, and total calories simultaneously. Confounding variables chosen for our final linear regression model met at least one of the following criteria: variables with P < 0.20 or those that changed the coefficient > 10%. The final model for serum uric acid and sugar sweetened beverage consumption simultaneously adjusted for age, race/ethnicity, sex, BMI z-score, total calories, smoking, alcohol, dietary fiber intake, milk, and diet beverage consumption. The final model for systolic blood pressure z-scores simultaneously adjusted for age, race/ethnicity, sex, BMI z-score, height z-score, total calories, smoking, alcohol, caffeine, sodium, and milk consumption. The final model for diastolic blood pressure z-scores simultaneously adjusted for age, race/ethnicity, sex, BMI z-score, total calories, smoking, alcohol, and sodium intake.
To evaluate for potential interactions by sex and obesity (BMI ≥95th percentile for sex and age) on the association of beverage consumption with serum uric acid and blood pressure, we added interaction terms for sex and obesity to the unadjusted and fully adjusted models. Interaction terms with P < 0.20 were designated à priori as significant.
We performed sensitivity analysis by excluding adolescents who reported taking antihypertensive medications, as some antihypertensive medications can affect serum uric acid levels. We performed sensitivity analysis by including unsweetened fruit juices in the category of sugar sweetened beverages, as fruit juices include naturally occurring fructose. We also performed a sensitivity analysis by excluding sweetened coffee or tea in the category of sugar sweetened beverages, as some sweetened coffee or tea may include drinks sweetened with sucrose.
RESULTS
Demographic and clinical characteristics of the study population are shown in Table I. 82.5% of adolescents report drinking any amount of sugar sweetened beverages in a day. Adolescents who drank more sugar sweetened beverages tended to be older and male. Sugar sweetened beverage consumption did not differ according to race/ethnicity, BMI z-score, height z-score, or dietary fiber intake. Sugar sweetened beverage consumption was associated with higher intake of total calories, sodium, and caffeine. Sugar sweetened beverage consumption was associated with less milk and diet beverage consumption. Adolescents in the highest category of sugar sweetened beverage consumption were more likely to smoke and to drink alcohol than adolescents in the lowest categories of sugar sweetened beverage consumption.
Table 1.
Demographic and Clinical Characteristics by Categories of Sugar Sweetened Beverage Consumption in Adolescents 12–18 Years in the NHANES 1999–2004.
| Categories of Sugar Sweetened Beverage Intake (oz/day) | Pa | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | 0 | 1–12 | 13–24 | 25–36 | >36 | |||
| Nb | 4867 | 774 | 569 | 1,410 | 916 | 1,198 | ||
| %c | 17.4% | 9.6% | 27.6% | 18.1% | 27.3% | |||
| Age, median, yrs. | 15.5 | 15.4 | 14.6 | 15.0 | 15.3 | 16.4 | <0.0001 | |
| Boys, % | 52.1% | 43.3% | 36.9% | 50.5% | 54.3% | 63.3% | <0.0001 | |
| Race/Ethnicity, % | White | 63.5% | 67.5% | 53.6% | 60.9% | 63.3% | 67.1% | 0.42 |
| Black | 15.1% | 12.3% | 21.3% | 16.8% | 15.6% | 12.7% | ||
| Hispanic | 16.6% | 14.8% | 20.5% | 18.0% | 16.7% | 15.0% | ||
| Other | 4.8% | 5.4% | 4.6% | 4.4% | 4.5% | 5.2% | ||
| BMI Z-score, median | 0.48 | 0.42 | 0.51 | 0.50 | 0.38 | 0.51 | 0.55 | |
| Height Z-score, median | 0.13 | 0.12 | −0.11 | 0.22 | 0.07 | 0.20 | 0.08 | |
| Total Calories, median, kcal/d | 2172 | 1915.7 | 1741.0 | 2049.5 | 2249.0 | 2673.0 | <0.0001 | |
| Dietary Fiber, median. gm/d | 11.9 | 12.6 | 10.5 | 11.5 | 11.6 | 12.6 | 0.15 | |
| Sodium, median, mg/d | 3150 | 2946 | 2842 | 2886 | 3080 | 3739 | <0.0001 | |
| Caffeine, median, mg/d | 37 | 2.5 | 8.0 | 34.9 | 56.2 | 105.0 | <0.0001 | |
| Milk, oz/d | <1 | 46.8% | 42.8% | 45.2% | 41.7% | 45.7% | 55.6% | 0.0002 |
| 1–8 | 10.8% | 11.0% | 13.2% | 11.4% | 10.0% | 9.7% | ||
| 9–16 | 22.2% | 19.1% | 25.5% | 24.9% | 25.9% | 18.0% | ||
| >16 | 20.2% | 27.1% | 16.1% | 22.0% | 18.4% | 16.7% | ||
| Diet Beverages, oz/d | <1 | 83.6% | 67.8% | 83.4% | 81.5% | 91.1% | 90.8% | <0.0001 |
| 1–12 | 5.8% | 9.2% | 7.7% | 6.9% | 3.5% | 3.3% | ||
| >12 | 10.6% | 23.0% | 8.9% | 11.6% | 5.4% | 5.9% | ||
| Smoking, % | 15.1% | 10.9% | 9.6% | 11.1% | 16.4% | 23.1% | <0.0001 | |
| Alcohol, % | 1.3% | 1.0% | 0.1% | 1.1% | 0.9% | 2.6% | 0.003 | |
P for trend.
Raw number of adolescents in study population not adjusted for sample survey design.
Except where otherwise noted, data shown is adjusted for the complex sample survey design of NHANES 1999–2004.
The association of sugar sweetened beverage consumption with serum uric acid levels is shown in Table II. In the unadjusted model, serum uric acid levels increased by 0.50 mg/dL in adolescents in the highest category of sugar sweetened beverage consumption compared with adolescents in the lowest category of sugar sweetened beverage consumption. In the adjusted model (Table II, Models 1) increasing sugar sweetened beverage consumption was associated with higher serum uric acid levels. However in the model additionally adjusted for alcohol, smoking, dietary fiber, milk, and diet beverage consumption (Table II, Model 2), serum uric acid levels increased by only 0.18 mg/dL in adolescents in the highest category of sugar sweetened beverage consumption compared with adolescents in the lowest category of sugar sweetened beverage consumption (p for trend = 0.01) (Figure). We found that the correlation of sugar sweetened beverage consumption with serum uric acid levels was not modified by sex or obesity (P for interaction > 0.2 each).
Table 2.
Difference in serum uric acid according to categories of sugar sweetened beverage (SSB) consumption.
| Uric acid (mg/dL) | ||||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | |||||
| SSB (oz/d) | Unadjusted difference | 95%CI | Adjusted difference | 95%CI | Adjusted difference | 95%CI |
| 0 | Reference | Reference | Reference | |||
| 1–12 | −0.18 | −0.38, 0.03 | 0.02 | −0.20, 0.23 | 0.03 | −0.19, 0.24 |
| 13–24 | 0.10 | −0.06, 0.26 | 0.05 | −0.09, 0.20 | 0.05 | −0.10, 0.20 |
| 25–36 | 0.25 | 0.08, 0.41 | 0.16 | 0.03, 0.30 | 0.16 | 0.01, 0.30 |
| ≥36 | 0.50 | 0.35, 0.65 | 0.22 | 0.08, 0.36 | 0.18 | 0.02, 0.33 |
| P for trend | <0.00001 | 0.002 | 0.01 | |||
Model adjusted for age, race/ethnicity, sex, total calories, and BMI z-score
Model adjusted for age, race/ethnicity, sex, total calories, BMI z-score, alcohol, smoking, dietary fiber intake, milk and diet beverage consumption.
Figure 1.
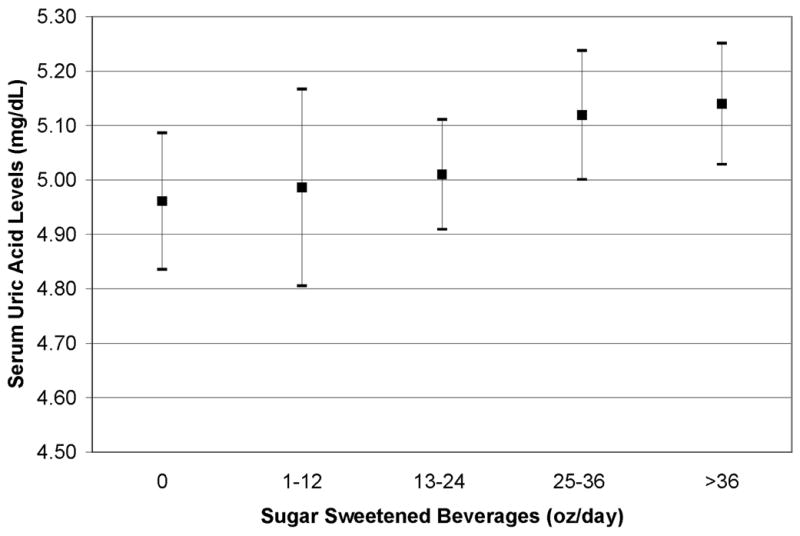
Sample mean of serum uric acid with 95% confidence intervals by categories of sugar sweetened beverage consumption adjusted for age, race/ethnicity, sex, total calories, BMI z-score, alcohol, smoking, dietary fiber intake, diet beverage consumption, and milk consumption. P for trend = 0.01
The association of sugar sweetened beverage consumption with systolic blood pressure z-score is shown in Table III. In the unadjusted model, higher sugar sweetened beverage consumption was not significantly associated with higher systolic blood pressure z-score. However, when adjusted for age, race/ethnicity, sex, total calories, and BMI z-score, sugar sweetened beverage consumption was associated with higher systolic blood pressure, a z-score difference of 0.18 (Table III, Model 1). Both age and total calories was positively associated with sugar sweetened beverage consumption, but negatively associated with systolic blood pressure z-score (data not shown), and therefore masked the association in the unadjusted model. In the model additionally adjusted for height z-score, sodium, caffeine, smoking, alcohol, and milk consumption (Table III, Model 2), sugar sweetened beverage consumption was associated with higher systolic blood pressure, a z-score difference of 0.17 (P for trend = 0.03). There was no association between sugar sweetened beverage consumption with diastolic blood pressure z-score in the unadjusted or adjusted models (Table III). We found that the correlation of sugar sweetened beverage consumption with blood pressure was not modified by sex or obesity (P for interaction > 0.2 each).
Table 3.
Difference in blood pressure z-scores according to categories of sugar sweetened beverage (SSB) consumption.
| Systolic blood pressure z-score | ||||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | |||||
| SSB (oz/d) | Unadjusted difference | 95%CI | Adjusted difference | 95%CI | Adjusted difference | 95%CI |
| 0 | Reference | Reference | Reference | |||
| 1–12 | 0.11 | −0.02,0.23 | 0.06 | −0.07, 0.19 | 0.02 | −0.11, 0.15 |
| 13–24 | 0.17 | 0.03, 0.31 | 0.15 | 0.01, 0.29 | 0.15 | 0.00, 0.29 |
| 25–36 | 0.10 | 0.00, 0.20 | 0.11 | 0.00, 0.21 | 0.09 | −0.03, 0.21 |
| > 36 | 0.14 | 0.00, 0.27 | 0.18 | 0.02, 0.34 | 0.17 | 0.02, 0.32 |
| P for trend | 0.08 | 0.03 | 0.03 | |||
| Diastolic blood pressure z-score | ||||||
| Model 1a | Model 2c | |||||
| SSB (oz/d) | Unadjusted difference | 95%CI | Adjusted difference | 95%CI | Adjusted difference | 95%CI |
|
| ||||||
| 0 | Reference | Reference | Reference | |||
| 1–12 | −0.03 | −0.17, 0.11 | −0.05 | −0.19, 0.09 | −0.05 | −0.18, 0.09 |
| 13–24 | −0.04 | −0.16, 0.09 | 0.00 | −0.13, 0.12 | −0.01 | −0.13, 0.12 |
| 25–36 | 0.04 | −0.08, 0.17 | 0.10 | −0.03, 0.22 | 0.10 | −0.02, 0.23 |
| > 36 | −0.07 | −0.18, 0.04 | 0.05 | −0.19, 0.09 | 0.04 | −0.07, 0.16 |
| P for trend | 0.64 | 0.09 | 0.09 | |||
Model adjusted for age, race/ethnicity, sex, total calories, and BMI z-score.
Model adjusted for age, race/ethnicity, sex, total calories, BMI z-score, height z-score, sodium intake, caffeine intake, smoking, alcohol, and milk consumption.
Model adjusted for age, race/ethnicity, sex, total calories, BMI z-score, sodium intake, smoking, and alcohol.
There were 3458 (73.4%), 330 (5.4%), 622 (11.9%) and 457 (9.3%) adolescents who reported drinking 0 oz/day, 1–8 oz/day, 9–16 oz/day and >16 oz/day of fruit juice, respectively. Fruit juice consumption was not independently associated with serum uric acid or blood pressure. When fruit juices were included with sugar sweetened beverages, we observed a similar associations between sugar sweetened beverage consumption with serum uric acid and diastolic blood pressure. When fruit juices were included with sugar sweetened beverages, we observed a similar effect size between sugar sweetened beverage consumption and systolic blood pressure however it did not reach conventional levels of statistical significance.
When sweetened coffee or tea was excluded from the sugar sweetened beverage category, there were 879 (19.7%), 587 (10.1%), 1437 (28.8%), 886 (17.5%), and 1078 (23.9%) adolescents who reported drinking 0 oz/day, 1–12 oz/day, 13–24 oz/day, 25–36 oz/day, or >36 oz/day of sugar sweetened beverages. After exclusion of sweetened coffee or teas, we observed a similar association between sugar sweetened beverage consumption with serum uric acid, systolic blood pressure, and diastolic blood pressure.
There were 17 adolescents in our sample who report taking antihypertensive medications. When they were excluded from our analysis, we observed similar associations between sugar sweetened beverage consumption with serum uric acid and blood pressure.
We did not exclude adolescents with chronic kidney disease as only 61% of adolescents had a serum creatinine level measured. However, in the subset of adolescents who had a serum creatinine measured, there were 11 adolescent with an estimated GFR < 90 ml/min per 1.73m2 and none less than 60ml/min per 1.73m2.23 We did not exclude adolescents who took medications (allopurinol and probenecid) which affect uric acid levels, because prescription medications were not recorded for adolescents 16 years old or younger. However in the subset of adolescents between 16–18 years old who had prescription medications recorded, there were no adolescents who took medications that would affect the serum uric acid level.
DISCUSSION
Adult studies have shown serum uric acid levels to be predictive of major outcomes even when values are within the normal range.8 It should be noted that the adjusted difference in serum uric acid between the lowest and highest category of sugar sweetened beverage consumption was relatively small. In adult studies, the risk associated with serum uric acid varies with the outcome. The observed serum uric acid difference between extreme categories, 0.18mg/dL, in the NHANES III, was associated with an 50% increased risk of incident gout in a large prospective adult cohort study.11, 24 On the other hand, for every 1mg/dL increase in serum uric acid, there was a 7% increase in the development of incident kidney disease or death after adjusting for multiple variables such as age, sex, race, systolic blood pressure, alcohol, smoking, HDL-cholesterol, and baseline kidney function.25
The mean systolic blood pressure z-score increased by 0.17 for the highest sugar sweetened beverage category. This represents a difference of 2mmHg (95% CI 1–2mmHg). In a general normotensive adult population, a 2mmHg reduction of systolic blood pressure would lower stroke mortality by 10% and ischemic heart disease by 7%.26
Sugar sweetened beverages are primarily sweetened with high fructose corn syrup, a mixture of fructose and glucose. Unlike glucose, fructose is solely metabolized in the liver where phosphorylation of fructose by fructokinase depletes intracellular phosphate. Activation of the enzyme AMP deaminase-1 increases adenine nucleotide catabolism, producing uric acid.28 Serum uric acid levels, even within the normal range, are associated with other cardiovascular risk factors and predict cardiovascular events in adults.8 Serum uric acid levels have been associated with cardiovascular risk factors like hypertension and the metabolic syndrome in adolescents.14–17
We found that higher sugar sweetened beverage consumption was associated with higher systolic blood pressure in adolescents. Our results are consistent with studies that suggest that fructose consumption may contribute to the pathogenesis of hypertension through its action on serum uric acid.2 Serum uric acid may raise systemic blood pressure by increasing renal inflammation, activating the renin-angiotensin system, and decreasing nitric oxide production.29 Secondly, fructose feeding can acutely decrease urinary sodium excretion, thereby potentially raising systemic blood pressure.30 Lastly, sugar sweetened beverage consumption is associated with increased salt intake which may contribute to higher blood pressure.31 In a referral based population, Feig et al found that children with primary hypertension had higher serum uric acid than healthy controls.15 Using NHANES data, Goldstein et al found that adolescents with higher serum uric acid had higher blood pressure.17 In the Bogalusa Heart study, serum uric acid levels correlated with childhood systolic and diastolic blood pressure, but results were unadjusted for age, sex, race, or BMI.14 Using the NHANES data, Ford et al found that adolescents in the highest tertile of serum uric acid levels were more likely to have high blood pressure and other components of the metabolic syndrome.16 In a cohort of healthy adolescents, Savoca et al showed that those who had more caffeinated soda consumption had higher systolic blood pressure;32 however, these observations by were not adjusted for age, sex, BMI, caffeine intake, or smoking, thereby confounding their results.
The strength of this study is that it includes a nationally representative population of adolescents, which increases generalizability. The study population was also large and rigorously characterized, which allowed us to adjust for important potential confounding factors, for which previous studies in children could not account, such as BMI, smoking, alcohol, caffeine intake, and other dietary factors.
However, there are also limitations. 24-hour dietary recalls, although providing reliable population means for intake,33 is not the best tool to estimate the usual diet as it only assesses intake for one day. Food frequency questionnaires are a better measure of long-term patterns of intake.34 We were unable to use the food frequency questionnaires for this study because the food frequency questionnaires in NHANES 1999–2002 did not include sugar sweetened beverage consumption.35 Random measurement errors from 24-hour dietary recall would likely create a non-differential form of misclassification bias, which will bias against finding an association between sugar sweetened beverage consumption and uric acid. This misclassification bias may also diminish the observed effect size of the sugar sweetened beverage consumption and systolic blood pressure association. At the same time, the p-value for trend in the sugar sweetened beverages and systolic blood pressure association is very close to the conventional threshold for statistical significance so it is possible that this is a false positive association due to chance. Data on family history of gout or hypertension was not collected in the NHANES surveys for participants less than 20 years old. The effect of family history of gout and hypertension could affect serum uric acid and systolic blood pressure through several potential pathways. Offspring of hypertensive parents have higher serum uric acid and lower fractional excretion of uric acid than offspring of normotensive parents.36, 37 Secondly, offspring of hypertensive parents have higher blood pressure.38, 39 As families with gout or hypertension may have shared environmental factors like dietary patterns, family history may be a residual confounding variable we were unable to account for in our analysis. Because of the cross-sectional study design, we can not demonstrate that it was the consumption of sugar sweetened beverage which raised serum uric acid. However, it seems reasonable to assume that higher serum uric acid or systolic blood pressure did not cause adolescents to drink more sugar sweetened beverages.
The current study provides new information on how sugar sweetened beverage consumption among adolescents may affect cardiovascular risk factors, namely serum uric acid and blood pressure. Because this association does not appear to be modified by the presence or absence of obesity, our data suggests that reducing the sugar sweetened beverage consumption will have beneficial cardiovascular effects in adolescents, regardless of weight status.
Acknowledgments
The authors would like to thank the following individuals for help with preparation and review of the manuscript: Eric Vittinghoff (NIH, Amgen, Wyeth, and Berlex Labs), Michele Mietus-Snyder (American Heart Association), Paul Brakeman (NIH), and Anthony Portale (UCSF).
S.N. was supported by the American Heart Association (0725258Y) and the Department of Pediatrics, University of California San Francisco. C-y.H. was supported by NIH DK70939 and DK67126.
ABBREVIATIONS
- NHANES
National Health and Nutrition Examination Survey
- BMI
body mass index
Footnotes
The authors disclose no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr. 2004 April 1;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007 October 1;86:899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 3.Rutledge AC, Adeli K. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr Rev. 2007 Jun;65:S13–23. doi: 10.1111/j.1753-4887.2007.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 4.Emmerson BT. Effect of oral fructose on urate production. Ann Rheum Dis. 1974 May 1;33:276–280. doi: 10.1136/ard.33.3.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox IH, Kelley WN. Studies on the mechanism of fructose-induced hyperuricemia in man. Metabolism. 1972 Aug;21:713–721. doi: 10.1016/0026-0495(72)90120-5. [DOI] [PubMed] [Google Scholar]
- 6.Perheentupa J, Raivio K. Fructose-induced Hyperuricaemia. The Lancet. 1967;290:528. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- 7.Reiser S, Powell AS, Scholfield DJ, Panda P, Ellwood KC, Canary JJ. Blood lipids, lipoproteins, apoproteins, and uric acid in men fed diets containing fructose or high-amylose cornstarch. Am J Clin Nutr. 1989 May;49:832–839. doi: 10.1093/ajcn/49.5.832. [DOI] [PubMed] [Google Scholar]
- 8.Gagliardi ACM, Miname MH, Santos RD. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.05.022. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 9.Johnson RJ, Feig DI, Herrera-Acosta J, Kang DH. Resurrection of uric acid as a causal risk factor in essential hypertension. Hypertension. 2005 Jan;45:18–20. doi: 10.1161/01.HYP.0000150785.39055.e8. [DOI] [PubMed] [Google Scholar]
- 10.Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008 Jun;121:e1604–1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- 11.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008 Jan 15;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 12.Gao X, Qi L, Qiao N, et al. Intake of Added Sugar and Sugar-Sweetened Drink and Serum Uric Acid Concentration in US Men and Women. Hypertension. 2007 Aug;50:306–312. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 13.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006 Aug;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alper AB, Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005 Jan;45:34–38. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 15.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003 Sep;42:247–252. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007 May 15;115:2526–2532. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein HS, Manowitz P. Relation between serum uric acid and blood pressure in adolescents. Ann Hum Biol. 1993 Sep–Oct;20:423–431. doi: 10.1080/03014469300002832. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1999–2004. [Accessed Sept. 2006]. National Center for Health Statistics (NCHS) http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm. [Google Scholar]
- 19.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993 November 1;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 20.Weitzman M, Cook S, Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005 Aug 9;112:862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. NHANES III 1988–94 Reference Manuals and Reports (on CD-ROM) Hyattsville, Md: National Center for Health Statistics; 1996. [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Survey Analytic and Reporting Guidelines. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Sept. 2006]. National Center for Health Statistics (NCHS) http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf. [Google Scholar]
- 23.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002 Feb;39:S1–266. [PubMed] [Google Scholar]
- 24.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008 Feb 9;336:309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008 Jun;19:1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002 Dec 14;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 27.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002 Nov;76:911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005 Dec;1:80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 29.Feig DI, Mazzali M, Kang D-H, et al. Serum Uric Acid: A Risk Factor and a Target for Treatment? J Am Soc Nephrol. 2006 April 1;17:S69–73. doi: 10.1681/ASN.2005121331. [DOI] [PubMed] [Google Scholar]
- 30.Rebello T, Hodges RE, Smith JL. Short-term effects of various sugars on antinatriuresis and blood pressure changes in normotensive young men. Am J Clin Nutr. 1983 Jul;38:84–94. doi: 10.1093/ajcn/38.1.84. [DOI] [PubMed] [Google Scholar]
- 31.He FJ, Marrero NM, MacGregor GA. Salt intake is related to soft drink consumption in children and adolescents: a link to obesity? Hypertension. 2008 Mar;51:629–634. doi: 10.1161/HYPERTENSIONAHA.107.100990. [DOI] [PubMed] [Google Scholar]
- 32.Savoca MR, Evans CD, Wilson ME, Harshfield GA, Ludwig DA. The association of caffeinated beverages with blood pressure in adolescents. Arch Pediatr Adolesc Med. 2004 May;158:473–477. doi: 10.1001/archpedi.158.5.473. [DOI] [PubMed] [Google Scholar]
- 33.Rockett HR, Colditz GA. Assessing diets of children and adolescents. Am J Clin Nutr. 1997 Apr;65:1116S–1122S. doi: 10.1093/ajcn/65.4.1116S. [DOI] [PubMed] [Google Scholar]
- 34.Willett W. Nutritional Epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) Food Frequency Questionnaire - Raw Questionnaire Responses. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Feb, 2008. National Health and Nutrition Examination Survey 2003–2004. [Google Scholar]
- 36.Simsolo RB, Romo MM, Rabinovich L, Bonanno M, Grunfeld B. Family history of essential hypertension versus obesity as risk factors for hypertension in adolescents. Am J Hypertens. 1999 Mar;12:260–263. doi: 10.1016/s0895-7061(98)00253-2. [DOI] [PubMed] [Google Scholar]
- 37.Ueno M, Tomita Y, Tsuchihashi T, Takishita S, Fujishima M. Clinical alterations in normotensive offspring with parental hypertension. J Hypertens Suppl. 1988 Dec;6:S50–51. doi: 10.1097/00004872-198812040-00011. [DOI] [PubMed] [Google Scholar]
- 38.Fuentes RM, Notkola IL, Shemeikka S, Tuomilehto J, Nissinen A. Familial aggregation of blood pressure: a population-based family study in eastern Finland. J Hum Hypertens. 2000 Jul;14:441–445. doi: 10.1038/sj.jhh.1001049. [DOI] [PubMed] [Google Scholar]
- 39.Cook BB, Treiber FA, Mensah G, Jindal M, Davis HC, Kapuku GK. Family history of hypertension and left ventricular mass in youth: possible mediating parameters. Am J Hypertens. 2001 Apr;14:351–356. doi: 10.1016/s0895-7061(00)01275-9. [DOI] [PubMed] [Google Scholar]


