Abstract
Despite decades of progress in cardiovascular biology, heart disease remains the leading cause of death in the developed world. Recently, cell-based therapy has emerged as a promising avenue for future therapeutics. However, the molecular signals that regulate cardiac progenitor cells are not well-understood. Wnt/β-catenin signaling is essential for expansion and differentiation of cardiac progenitors in mouse embryos and in the embryonic stem cell system. Studies from our laboratory and others highlight the pivotal roles of Wnt/β-catenin signaling in the multiple steps of cardiogenesis and provide insights into understanding the complex regulation of cardiac progenitors. Here we discuss the required roles of Wnt/β-catenin signaling at the different stages of heart development.
Keywords: Wnt/β-catenin signaling, cardiogenesis, cardiac progenitors, embryonic stem cells, cardiomyocytes
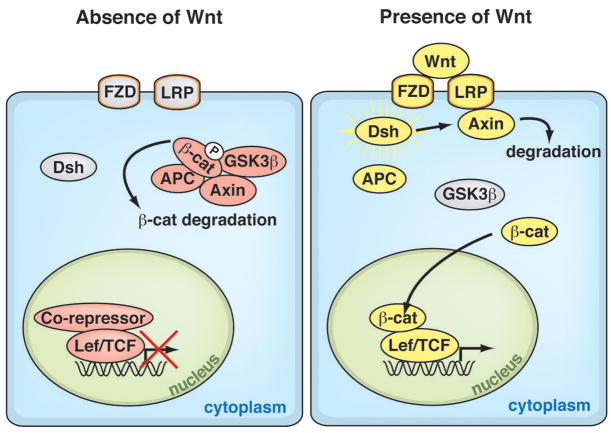
The Wnt/β-catenin (canonical Wnt) signaling pathway is an evolutionarily conserved cascade that regulates many developmental and stem-cell processes, such as cell-fate, proliferation, differentiation and survival1–3. The central component of canonical Wnt signaling is β-catenin, the obligatory transcriptional mediator. In the absence of Wnt, the destruction complex phosphorylates β-catenin for proteolytic degradation. Wnt/β-catenin signal transduction is initiated when Wnts bind to the transmembrane proteins, Frizzled and Lrp, which stabilize β-catenin by inhibiting the kinase activity of the destruction complex of adenomatous polyposis coli, axin, and glycogen synthase kinase-3β. Stabilized β-catenin translocates into the nucleus, where it interacts with the Lef/TCF transcription factors to activate Wnt target genes (Figure 1)2–4.
Figure 1. The Wnt/β-Catenin Signaling Pathway.
Canonical Wnt signaling is initiated when Wnts bind to their cognate receptor complex of the transmembrane proteins Frizzled and Lrp. This interaction activates the cytoplasmic protein Dishevelled, which stabilizes β-catenin by inhibiting the kinase activity of the destruction complex of adenomatous polyposis coli (APC), Axin, and glycogen synthase kinase-3β (GSK3β). Unphosphorylated β-catenin on its N-terminus translocates into the nucleus, where it interacts with the TCF/lymphoid enhancer factor (LEF) transcription factors to activate Wnt target genes. In the absence of Wnt, the destruction complex phosphorylates β-catenin, targeting it for degradation.
Contrasting roles for Wnt/β-catenin signaling were initially reported in fly and vertebrate cardiogenesis. In flies, Wnt signals were necessary for development of cardiac progenitors5, 6, but in vertebrates, inhibition of Wnt signals was required for differentiation of cardiac mesoderm7–9. However, in the vertebrate experiments, canonical Wnt signaling was inactivated globally and without temporal regulation, leaving the question of a spatio-temporal and mesoderm-specific function of Wnt/β-catenin signaling unclear. We addressed this question in an in vivo mouse model and in the embryonic stem (ES) cell system and found that Wnt/β-catenin signaling in cardiac mesoderm is required for cardiogenesis at discrete developmental stages. In this report, we summarize our recent findings on the role of Wnt/β-catenin signaling in early mammalian cardiogenesis and examine important questions for future studies.
WNT/β-CATENIN SIGNALING IN UNDIFFERENTIATED ES CELLS AND MESODERM FORMATION
ES cells, derived from the inner cell mass of the blastocyst, can self-renew indefinitely and can differentiate into the three germ layers (endoderm, mesoderm, ectoderm) and their derivatives. Canonical Wnt signaling promotes the self-renewal of undifferentiated and pluripotent human and mouse ES cells10. However, maintaining long-term pluripotency of ES cells appears to require other factors as well11.
The heart is an ancient organ whose early morphogenesis takes place in a highly conserved fashion from insects to vertebrates. After formation of the three germ layers, precardiac mesoderm arises bilaterally from the mesoderm. Cardiac progenitors in the precardiac mesoderm migrate into the midline and differentiate to give rise to the contractile heart12.
In mice, Wnt3 or β-catenin loss-of-function mutants fail to develop the primitive streak and mesoderm and to express their respective marker genes13, 14. In like manner, inhibiting canonical Wnt signaling in ES cells blocks expression of primitive streak and mesodermal genes15. To confirm the role of Wnt/β-catenin signaling in early stages of mouse development, we inactivated β-catenin with Mox2-Cre16, which is activated in the epiblast from embryonic day 5 (E5.0) but not in the primitive endoderm. We observed embryonic defects similar to those described above (C. K. and D. S., unpublished data), suggesting an essential role of Wnt/β-catenin signaling in mesoderm formation. Therefore, Wnt/β-catenin signaling is required for mesoderm formation, which is a pre-requisite for pre-cardiac mesoderm commitment.
WNT/β-CATENIN SIGNALING IN INDUCTION AND EXPANSION OF PRECARDIAC MESODERM
Induction of precardiac mesoderm has been extensively studied in various model systems5–9, 17. In flies, Wg (the founding member of the canonical Wnt family) is required in a temporally restricted manner for the induction and proliferation of cardiac precursors, as shown by studies with temperature-sensitive alleles of Wg5, 6. However, canonical Wnt signaling negatively affects cardiogenesis in vertebrates. In the chick, ectopic overexpression of the canonical Wnt ligands Wnt8c or Wnt3a blocked induction of early cardiac genes and cardiogenesis9. Similarly, overexpression of Wnt3A or Wnt8 inhibited cardiogenesis in Xenopus explants8. In zebrafish, a global decrease in the transcriptional activity of the β-catenin/TCF complex caused hyperproliferation of cardiomyocytes, suggesting that β-catenin negatively regulates cardiac differentiation or expansion7. Contrary to these, Wnt3a and Wnt8 were upregulated in pluripotent P19 cells treated with DMSO, which induces cardiomyogenesis, and Wnt3a treatment consistently enhanced cardiac gene transcription in P19 cells17. Since early cardiogenesis is a well-conserved process across metazoans, these discrepancies may be due to precise spatio-temporal requirements of Wnt modulation in a cell-specific manner. Therefore, we focused on evaluating roles of Wnt/β-catenin signaling in a spatio-temporally restricted manner in mouse embryos and the ES cell system.
In mammals and birds, the bilateral pre-cardiac mesoderm cells migrate and merge at the anterior midline to generate the cardiac crescent (CC)12, 18. Cardiomyocyte differentiation begins at the onset of CC formation12. The CC, characterized as the first heart field (FHF), migrates to the midline and fuses to form a linear heart tube. The resulting tubular heart, composed of outer myocardial and inner endocardial layers, undergoes rightward looping to give rise to the future left ventricle (LV) and future atria. Second heart field (SHF) cells also appear at the CC stage, located more medially in splanchinic mesoderm and immediately dorsal to the CC. The SHF cells migrate into the anterior and posterior poles of the heart tube to give rise to the outflow tract (OT), right ventricle (RV), and a subset of atrial cells19–22. Unlike FHF cells, SHF cells remain undifferentiated until they migrate into the looping heart tube.
To investigate the cell-autonomous role of Wnt/β-catenin signaling in pre-cardiac mesoderm, we conditionally inactivated or stimulated Wnt/β-catenin signaling with Islet1-Cre in the SHF, where cells are fated to form the OT and RV19. Expression of Cre recombinase in the model is initiated just after the commitment of mesoderm to the pre-cardiac mesoderm fate. Tissue-specific deletion of β-catenin in the SHF resulted in nearly complete loss of the RV23. This observation suggests that β-catenin is required for RV formation. However, the deletion did not affect the induction of the SHF cells, as evidenced by indistinguishable expression domains of Islet1 in wildtype and mutant embryos23.
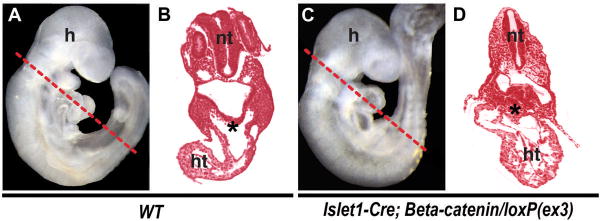
In contrast, stabilization of β-catenin in the SHF led to a greatly enlarged and hypercellular RV23. Strikingly, the undifferentiated SHF progenitor pool dorsal to the developing heart tube, identified by Islet1 expression (C. K. and D. S., unpublished data), was greatly expanded (Figure 2). This pool contained an increased percentage of phosphohistone H3 (PH3)-positive cells23, indicating proliferation of SHF progenitors. In agreement with this, agonizing Wnt/β-catenin signaling in ES cells after mesoderm commitment promoted the expansion of cardiac progenitors23. These findings suggest that Wnt/β-catenin signaling positively regulates proliferation of cardiac progenitors. Similar results were reported by other groups24, 25. Together, Wnt/β-catenin signaling is required for expansion of precardiac cells during mammalian cardiogenesis.
Figure 2. β-Catenin Promotes Expansion of Cardiac Progenitors.
(A, B) Wild-type (WT) embryos. (C–D) Mutant embryos with stabilized β-catenin (Islet1-cre, β-catenin/loxP(ex3)). Lateral views of E9.5 embryos are shown in A and C. The dotted lines indicate the planes of each section shown in B, D. The mutant embryos show hyperplasia of cardiac progenitors (asterisks, B, D). A–D were adapted from Kwon et al., 2007. h, head; ht heart; nt, neural tube.
WNT/β-CATENIN SIGNALING IN CARDIOMYOCYTE DIFFERENTIATION AND PROLIFERATION
To investigate the role of Wnt/β-catenin signaling in the differentiation and proliferation of committed cardiomyocytes, we deleted β-catenin with the Nkx2.5-Cre line, in which Cre expression is under the control of a ventricular-specific enhancer of the cardiac regulatory gene, Nkx2.526. Unlike the endogenous Nkx2.5 gene, which is also expressed in the pre-cardiac mesoderm, this Nkx2.5 enhancer activates Cre only in differentiating and proliferating ventricular cells after CC formation26. Loss of β-catenin in the Nkx2.5-Cre domain caused embryonic lethality around E12.5 with greatly reduced ventricular chamber size23. Sections of the heart revealed poorly developing myocardial cells with a significantly reduced proliferation rate in both ventricles23. Conversely, the β-catenin-stabilized heart showed enlarged ventricles with an increased proliferation rate and cell number until E13.523. This proliferation appears to be mediated in part by CyclinD2, which is a direct target of Wnt/β-catenin signaling and promotes cell cycling27, 28, as its levels are downregulated or upregulated in β-catenin-deleted or stabilized ventricles, respectively23, 29. Levels of CyclinD1, another known target of Wnt/β-catenin signaling, were unchanged (not shown), implying a context-dependence of CyclinD1.
These in vivo data suggest that β-catenin is required for proliferation of committed cardiomyocytes, and may be involved in cardiomyocyte differentiation. However, it is difficult to determine if β-catenin regulates the commitment of undifferentiated cardiac progenitors to the cardiomyocyte lineage in vivo, since differentiating cells also proliferate. Because of this inherent difficulty, we turned to the ES cell system. We found that inhibition of canonical Wnt signaling after formation of mesoderm progenitor cells completely abolished beating embryonic bodies (EBs), despite the early presence of Nkx2.5+ cardiac progenitors23. This defect was further confirmed by decreased expression of sarcomeric genes23. Similarly, the deletion of Wnt2, a canonical Wnt gene, impaired terminal cardiomyocyte differentiation in ES cells30, suggesting a requirement of canonical Wnt signaling in the differentiation process. In contrast to these studies, Qyang et al. showed that differentiation of ES cell-derived Isl1+ cardiac progenitors was suppressed upon the addition of Wnt3a conditioned medium24. Although the role of Wnt/β-catenin signaling in the commitment of pre-cardiac mesoderm to cardiomyocytes remains to be determined in greater detail, these findings suggest that Wnt/β-catenin signaling can have biphasic effects on the differentiation event.
CONCLUSION AND OPEN QUESTIONS
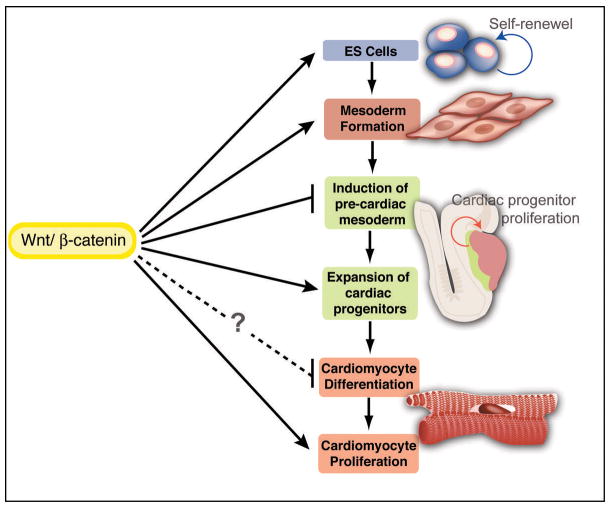
Heart malformation is the leading cause of human birth defects, and heart disease remains the number one killer of adults in the developed world. Recent advances have provided insights into the potential of cardiac stem/progenitor cell–based therapies. For example, cardiac progenitors found at early stages of mouse or ES cell development can be manipulated in culture to differentiate into various types of cardiac cells31–33. However, the molecular signals that regulate cardiac progenitors are less clear. Our findings suggest that canonical Wnt signaling is an essential positive regulator of cardiac progenitors at multiple steps of cardiogenesis in vivo and in vitro (Figure 3). These data provide important clues for understanding the factors that control early cardiac commitment, expansion, and differentiation, a prerequisite for future cell-mediated therapeutics.
Figure 3. Positive Inputs of Canonical Wnt Signaling at Multiple Stages of Mammalian Cardiogenesis.
The diagram summarizes involvement of Wnt/β-catenin signaling during the course of mammalian heart development. Wnt/β-catenin signaling promotes the self-renewal of ES cells and is required for mesoderm formation (in vivo and in vitro). After mesoderm forms, Wnt/β-catenin signaling promotes the proliferation of cardiac progenitors (in vivo and in vitro) and is required for cardiomyocyte proliferation (in vivo). The antagonistic role of Wnt/β-catenin signaling in the induction of cardiac mesoderm in a cell-autonomous fashion has not been addressed in vivo, but inhibition of Wnt function in a discrete developmental window is likely required for induction of cardiac mesoderm. The precise role of Wnt/β-catenin signaling in cardiomyocyte differentiation remains to be determined.
Although our in vivo studies of canonical Wnt signaling in a spatio-temporally restricted manner provide compelling evidence that Wnt/β-catenin signaling is required in a cell-autonomous fashion for the expansion and development of pre-cardiac mesoderm and cardiac mesoderm, narrow developmental windows apparently exist during which canonical Wnt signaling sequentially inhibits then promotes cardiac development7–9, 24, 34. Thus, the precise temporal control of Wnt signaling in endoderm and mesoderm is essential for the proper emergence and subsequent differentiation of cardiac progenitors. This may be similar to the function of Bmp signaling during cardiogenesis35.
Despite numerous loss-of-function studies of individual canonical Wnts, cardiac defects have not been reported2, 36. This could be due to the redundancy of Wnt proteins, or a key Wnt may not yet have been identified. In any case, it will be important to identify the essential endogenous canonical Wnts and their sources at different phases of mammalian cardiogenesis. Equally important, the molecular mechanisms by which Wnt/β-catenin signaling controls cardiac progenitors need to be elucidated. A thorough understanding of the Wnt/β-catenin signaling-mediated regulation of cardiogenesis may enable manipulation of cardiac progenitors/ES cells for therapeutic applications.
Acknowledgments
The authors thank G. Howard and S. Ordway for editorial assistance and members of the Srivastava lab for helpful discussions. C.K. was supported by California Institute for Regenerative Medicine; D.S. is an Established Investigator of the American Heart Association and was supported by grants from NHLBI/NIH and the March of Dimes Birth Defects Foundation.
References
- 1.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 2.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 3.Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120:11–4. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Tolwinski NS, Wieschaus E. Rethinking WNT signaling. Trends Genet. 2004;20:177–81. doi: 10.1016/j.tig.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Park M, Wu X, Golden K, Axelrod JD, Bodmer R. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol. 1996;177:104–16. doi: 10.1006/dbio.1996.0149. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Golden K, Bodmer R. Heart development in Drosophila requires the segment polarity gene wingless. Dev Biol. 1995;169:619–28. doi: 10.1006/dbio.1995.1174. [DOI] [PubMed] [Google Scholar]
- 7.Rottbauer W, Saurin AJ, Lickert H, Shen X, Burns CG, Wo ZG, Kemler R, Kingston R, Wu C, Fishman M. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell. 2002;111:661–72. doi: 10.1016/s0092-8674(02)01112-1. [DOI] [PubMed] [Google Scholar]
- 8.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–15. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–27. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature medicine. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 11.Dravid G, Ye Z, Hammond H, Chen G, Pyle A, Donovan P, Yu X, Cheng L. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem cells (Dayton, Ohio) 2005;23:1489–501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 12.Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91:457–69. doi: 10.1161/01.res.0000034152.74523.a8. [DOI] [PubMed] [Google Scholar]
- 13.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–5. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 14.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–37. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 15.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–96. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 16.Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function Genesis. 2000;26:113–5. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura T, Sano M, Songyang Z, Schneider MD. A Wnt- and beta -catenin-dependent pathway for mammalian cardiac myogenesis. Proc Natl Acad Sci U S A. 2003;100:5834–9. doi: 10.1073/pnas.0935626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery MO, Litvin J, Gonzalez-Sanchez A, Bader D. Staging of commitment and differentiation of avian cardiac myocytes. Dev Biol. 1994;164:63–71. doi: 10.1006/dbio.1994.1180. [DOI] [PubMed] [Google Scholar]
- 19.Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–89. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–88. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- 21.Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- 22.Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–40. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- 23.Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qyang Y, Martin-Puig S, Chiravuri M, Chen S, Xu H, Bu L, Jiang X, Lin L, Granger A, Moretti A, Caron L, Wu X, Clarke J, Taketo MM, Laugwitz KL, Moon RT, Gruber P, Evans SM, Ding S, Chien KR. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by a Wnt/beta-catenin pathway. Cell stem cell. 2007;1:165–79. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. The Journal of clinical investigation. 2007;117:1794–804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- 27.Huang W, Chang HY, Fei T, Wu H, Chen YG. GSK3beta mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene. 2006 doi: 10.1038/sj.onc.1210033. [DOI] [PubMed] [Google Scholar]
- 28.Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell. 2002;111:673–85. doi: 10.1016/s0092-8674(02)01084-x. [DOI] [PubMed] [Google Scholar]
- 29.Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–24. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H, Gilner JB, Bautch VL, Wang DZ, Wainwright BJ, Kirby SL, Patterson C. Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. The Journal of biological chemistry. 2007;282:782–91. doi: 10.1074/jbc.M606610200. [DOI] [PubMed] [Google Scholar]
- 31.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuasa S, Itabashi Y, Koshimizu U, Tanaka T, Sugimura K, Kinoshita M, Hattori F, Fukami S, Shimazaki T, Ogawa S, Okano H, Fukuda K. Transient inhibition of BMP signaling by Noggin induces cardiomyocyte differentiation of mouse embryonic stem cells. Nat Biotechnol. 2005;23:607–11. doi: 10.1038/nbt1093. [DOI] [PubMed] [Google Scholar]
- 36.Garriock RJ, Warkman AS, Meadows SM, D’Agostino S, Krieg PA. Census of vertebrate Wnt genes: isolation and developmental expression of Xenopus Wnt2, Wnt3, Wnt9a, Wnt9b, Wnt10a, and Wnt16. Dev Dyn. 2007;236:1249–58. doi: 10.1002/dvdy.21156. [DOI] [PubMed] [Google Scholar]