Abstract
The taccalonolides are a class of structurally and mechanistically distinct microtubule-stabilizing agents isolated from Tacca chantrieri. A crucial feature of the taxane family of microtubule stabilizers is their susceptibility to cellular resistance mechanisms including overexpression of P-glycoprotein, MRP7 and the βIII isotype of tubulin. The ability of four taccalonolides, A, E, B and N, to circumvent these multidrug resistance mechanisms was studied. Taccalonolides A, E, B and N were effective in vitro against cell lines that overexpress P-glycoprotein and MRP7. In addition, taccalonolides A and E were highly active in vivo against a doxorubicin- and paclitaxel- resistant Pgp-expressing tumor, Mam17/ADR. An isogenic HeLa-derived cell line that expresses the βIII isotype of tubulin was generated to evaluate the effect of βIII-tubulin on drug sensitivity. When compared with parental HeLa cells, the βIII-tubulin overexpressing cell line was less sensitive to paclitaxel, docetaxel, epothilone B and vinblastine. In striking contrast, the βIII-tubulin overexpressing cell line showed greater sensitivity to all four taccalonolides. These data cumulatively suggest that the taccalonolides have advantages over the taxanes in their ability to circumvent multiple drug resistance mechanisms. The ability of the taccalonolides to overcome clinically relevant mechanisms of drug resistance in vitro and in vivo confirms that the taccalonolides represent a valuable addition to the family of microtubule-stabilizing compounds with clinical potential.
Keywords: microtubule stabilizers, drug resistance, P-glycoprotein, multidrug-resistant protein 7, βIII-tubulin
Introduction
Some of the most clinically effective antitumor drugs developed over the past 25 years are those that target cellular microtubules [1]. Microtubule-targeting drugs are often classified as microtubule stabilizers, which include the taxanes and epothilones, or destabilizers, represented by the vinca alkaloids and combretastatin A4. These designations, stabilizer or destabilizer, refer to the ability of these drugs to cause striking changes in the microtubule structures of interphase cells. The extensive work of Jordan and Wilson showed that at lower, clinically relevant concentrations, microtubule stabilizers and microtubule destabilizers have the same general mechanism of action: they inhibit microtubule dynamics leading to apoptosis [2, 3].
Paclitaxel and docetaxel are taxanes that effectively treat breast, ovarian, prostate and non-small-cell lung cancer [1, 4, 5]. Although the taxanes are arguably some of the most valuable chemotherapeutic agents available today, intrinsic and acquired drug resistance limit their anticancer actions. The identification of new microtubule stabilizers that can overcome taxane resistance mechanisms could provide significant breakthroughs in the treatment of cancer [6]. The search for new classes of microtubule-targeting agents has been successful, and the epothilones and discodermolide have been evaluated in the clinic. In October 2007, ixabepilone (Ixempra™) was the first epothilone approved for the treatment of multidrug-resistant breast cancer [7]. Discodermolide was evaluated in a Phase I trial but failed to advance due to lung toxicity [8]. Other chemically diverse classes of microtubule stabilizers, including the laulimalides, peloruside A and the taccalonolides, have potential to be useful in cancer therapy.
We previously discovered the taccalonolides, a unique class of microtubule stabilizers that are highly acetylated steroids isolated from the tropical plant Tacca chantrieri [9]. In cells, the effects of the taccalonolides (taccas) are almost identical to the effects of the taxanes. The most abundant taccalonolides isolated, A and E (taccas A and E), cause an increase in the density of interphase microtubules and shift cellular tubulin to the polymerized form. Consistent with the effects of other microtubule stabilizers, the taccas induce the formation of abnormal mitotic spindles leading to mitotic arrest, Bcl-2 phosphorylation and initiation of apoptosis [9]. However, certain findings indicate that the taccas have a different mechanism of action as compared with other microtubule stabilizers [10]. Unlike other compounds that are able to exert taxane-like effects in cells, the taccas do not robustly stimulate the polymerization of purified bovine brain tubulin or microtubule protein [10]. Further studies indicate that the taccas do not bind to the taxane-binding site of tubulin [10]. Studies are ongoing to identify the cellular binding site of the taccas and the mechanism of their antimitotic activity.
A significant proportion of cancer cell lines and tumors are multidrug-resistant due to expression of ABC (ATP-binding cassette) transporters. These transporters have multiple physiological roles, including the capacity to extrude xenobiotics from cells in an ATP-dependent manner. The MDR1 gene product P-glycoprotein (Pgp, ABCB1) functions in the cellular export of a wide spectrum of compounds, including complex natural products including the taxanes [11]. Expression of Pgp in tissues such as the intestinal tract, liver and kidney contributes to the intrinsic taxane resistance of epithelial tumors derived from these tissues. In cells selected in vitro and in vivo for resistance to agents including the taxanes, upregulation of Pgp expression often leads to diminished intracellular drug accumulation and attenuated cytotoxic effects [11, 12]. Tumors in genetic murine models of breast cancer (Brca 1−/−, p53−/−) that are initially sensitive to doxorubicin and docetaxel develop resistance following exposure to these drugs, which is attributable to expression of Pgp [13, 14]. Clinically, expression of Pgp in both hematological and solid tumors has been reported to be associated with poor treatment response and subsequent treatment failure [12, 15–17]. The overwhelming lack of success of Pgp inhibitors in the clinic indicates that the identification of agents that are not susceptible to Pgp-mediated resistance will be a crucial feature of new microtubule stabilizers [6, 12]. Hence, the anticancer efficacy of ixabepilone in multidrug-resistant breast cancer might relate to its ability to circumvent export by Pgp. Our previous work using the multidrug-resistant NCI/ADR cell line suggested that the taccas A and E are not transported by Pgp [9]. In this study, we further explore the efficacy of the taccas in cells transduced with a Pgp-expression vector compared with an isogenic parental cell line.
In addition to Pgp, other resistance factors may affect the efficacy of microtubule-targeting agents. Expression of the ABC transporter MRP7 (multidrug-resistance protein 7; ABCC10) has recently been identified as one such factor. MRP7 is an ABC transporter with little structural homology to other MRP family members, although it shares their capacity to transport amphipathic anions [18, 19]. Noted substrates for MRP7 include 17β-estradiol-(17-β-D-glucuronide), docetaxel, paclitaxel, vinblastine and vincristine [19, 20]. MRP7 is normally expressed in stomach, colon, kidney, brain, pancreas, liver, lung, ovary and lymph nodes and has been detected in cancer cell lines and tumor samples [19, 21, 22]. In cancer cell lines, the overexpression of MRP7 leads to 10-fold resistance to docetaxel and lower but significant resistance to paclitaxel and the vinca alkaloids [20]. Additionally, MRP7 expression was found to be induced in non-small-cell lung cancer cell lines upon paclitaxel treatment. MRP7 expression levels correlated with both paclitaxel accumulation and sensitivity [23]. These findings suggest that the ability of novel chemotherapeutic agents to circumvent MRP7-mediated efflux may provide a significant advantage for the treatment of cancer.
Overexpression of the βIII isotype of tubulin [reviewed in 24] has been established as another clinically relevant resistance factor for the taxanes. Microtubules are formed of polymers of α/β heterodimers. Seven α-tubulin isotypes and nine β-tubulin isotypes have been described and tubulin isotype distribution is highly tissue specific. Under normal circumstances βIII-tubulin is found predominantly in neuronal tissues. However, in cancer cell lines and human tumors, the expression of βIII-tubulin has been implicated in drug resistance to microtubule-targeting drugs including the taxanes [24]. Additionally, expression of βIII is often observed in cell lines made resistant to the taxanes by serial drug exposure [24]. Incorporation of βIII into microtubules decreases microtubule dynamics [25] and the removal of βIII from bovine brain tubulin results in microtubules that are more dynamic and more sensitive to paclitaxel [26]. Inducible expression of βIII-tubulin has also been shown to diminish the ability of paclitaxel to inhibit microtubule dynamics in cells [27]. Depletion of βIII by siRNA-mediated knockdown increases the sensitivity of cell lines to microtubule binding agents, further demonstrating the link between βIII-tubulin expression and drug resistance [28].
Clinically, a number of studies indicate that the expression of the βIII isotype is associated with a lack of response to taxanes and poor clinical prognosis [24]. In four clinical trials of non-small-cell lung cancer, the expression of βIII-tubulin was associated with resistance to a therapeutic regimen that included a taxane or vinorelbine, but not gemcitabine/cisplatin-based therapy [24]. The correlation between high βIII-tubulin levels and resistance to taxane-based therapies has also been shown in ovarian, breast and gastric cancers, suggesting that the expression of this tubulin isotype might be a predictive marker for the success or failure of tubulin-targeting therapies.
Multidrug resistance is clearly multifactorial and often limits the success of chemotherapy. It is clear that alterations in the β-tubulin isotype composition and expression of specific ABC transporters, including MRP7 and Pgp, can lead to resistance to the microtubule-stabilizing drugs currently in clinical use. The successes and failures of the taxanes have prompted intense drug discovery efforts to find new classes of microtubule stabilizers. Here, we characterize the activity of the taccas in a variety of taxane-resistant cell lines and one murine tumor. We demonstrate that that taccas are not susceptible to multidrug resistance associated with overexpression of Pgp, MRP7 or the βIII-tubulin isotype. These findings indicate that taccas may have advantages over other microtubule-targeting agents and support their future preclinical evaluation.
Materials and Methods
Isolation and Chemical Modification of the Taccalonolides
The taccas A and E were isolated from the roots and rhizomes of Tacca chantrieri. The plant material was washed free of dirt, frozen and lyophilized. The freeze-dried material was ground to a fine powder and extracted with CO2 and methanol using a supercritical fluid extractor. Non-polar lipids were removed by hexane extraction. The taccas were further enriched by extraction with dichloromethane and water and the resultant fraction dried by evaporation. The crude tacca extract was fractionated by flash chromatography on a silica column with hexanes and isopropanol. High performance liquid chromatography (HPLC) was used to separate the taccas A and E. A preparative Waters Resolve™ silica column was used initially and was followed by HPLC using a semi-preparative column. Isopropanol and isooctane solvent mixtures were used for HPLC. Taccas B and N were generated by treating taccas A and E, respectively, with mild base hydrolysis as described previously [29]. The chemical identification of the individual taccalonolides was confirmed by proton nuclear magnetic resonance.
Cell Culture
HeLa, SK-OV-3, MDA-MB-435 and NCI/ADR cell lines were obtained and grown as previously described [9]. The SK-OV-3-MDR-1 cell line, which was generated from SK-OV-3 cells by adenoviral-mediated expression of MDR1, was obtained from Dr. Susan Kane [30]. The SK-OV-3-MDR-1 cells were subcloned by limiting dilution techniques to isolate single-cell clones. The clone used in these studies was designated SK-OV-3-MDR-1-6/6. HEK293 cells and isogenic MPR7-expressing clones were isolated and maintained as previously described [19]. A single-cell clone from transfection of HeLa cells with βIII-tubulin, designated wildtype βIII, was constructed and maintained as described [31].
Indirect Immunofluorescence
HeLa and wildtype βIII cells were grown on glass coverslips for 2 days after which cellular microtubules and mitotic spindles were evaluated by indirect immunofluorescence techniques as previously described [9]. Images were acquired by digital camera and colorized and compiled using MetaMorph software.
Inhibition of Cell Proliferation
The sulforhodamine B assay was used to evaluate the antiproliferative effects of microtubule disrupting drugs in the SKOV-3 and HeLa cell line pairs [32, 33]. The concentration that causes 50% inhibition of proliferation (IC50) after 48 hours of treatment was calculated as previously described [9] in three independent experiments, each using triplicate points, with at least 7 drug concentrations that span the entire range of growth inhibition. The drug sensitivity of the MRP7-transfected HEK293 cells was assessed using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt/phenazine methosulfate (MTS/PMS) microtiter plate assay (CellTiter 96 Cell Proliferation Assay, Promega, Madison, WI). The cell lines were seeded in triplicate at 3000 cells per well in 96-well plates in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum. The next day the cells were treated with various drug concentrations. Colorimetric analyses were performed after 72 hours of growth in the presence of drug. Relative resistance values (Rr) were calculated by dividing the IC50 of the transfected or transduced cell line by the IC50 obtained in the parental cell line.
Immunoblot Analysis
HeLa and wildtype βIII cells in log phase growth were harvested and cellular protein was isolated using Cell Extraction Buffer (Invitrogen) in the presence of protease inhibitors. Protein concentrations were measured and equal amounts of total cellular protein were separated by SDS-PAGE and transferred to nitrocellulose membranes that were probed with monoclonal β-tubulin isotype specific antibodies for βI-tubulin (SAP.4G5), βII-tubulin (JDR.3B8), βIII-tubulin (SDL.3D10) and βIV-tubulin (ONS.1A6) [34] as previously reported [31].
In Vivo Studies
The antitumor activity of taccas A and E were evaluated in the doxorubicin- and paclitaxel-resistant syngeneic Mammary 17/ADR model as previously described [35]. All mice were obtained from the NCI-Frederick Animal Production Program (Frederick, MD), maintained in an AALAC-approved facility (Wayne State University, Detroit, MI) and given food and water ad libitum. On day 0, tumor fragments (30–50 mg) were bilaterally implanted subcutaneously in 8-week-old female C3H mice. Mice were then randomly placed into various treatment and control groups (n = 5 mice/group). Mice had a mean 25 g body weight at the start of treatment. All drugs were administered by IV bolus injections. Taccalonolides were injected in a volume of 0.2 mL/injection as a solution diluted with cyclodextrin from 18 mg/mL liquid stocks (65/35 v/v DMSO/Cremophor EL, pH 7.0). Doxorubicin (Adriamycin; Adria) and paclitaxel (Taxol; Mead-Johnson) were diluted appropriately with USP grade water from clinical grade stocks. Mice were observed and weighed daily prior to treatment and tumors were measured 2 to 3 times weekly by caliper. Tumor mass was calculated using the formula: mass (mg) = length (mm) × width (mm) 2 /2. Efficacy was assessed using multiple endpoints as follows: 1) T/C, defined as median tumor mass of treated group divided by the median tumor mass of the non-treated control and expressed as percent; growth inhibition (GI) is the inverse of T/C. 2) Tumor growth delay (TGD or T-C), where T is defined as the median time in days required for treatment group tumors to reach 1000 mg and C is the median time for the control group tumors to reach the same size. 3) Tumor cell kill, defined as: (T-C)/[(Td)(3.32)]. Td is the tumor volume doubling time in days of the control tumors, which was estimated from the best fit straight line from a log-linear growth plot of the control group tumors in exponential growth (100–800 mg range) [36].
Results
Isolation and Chemical Modification of the Taccalonolides
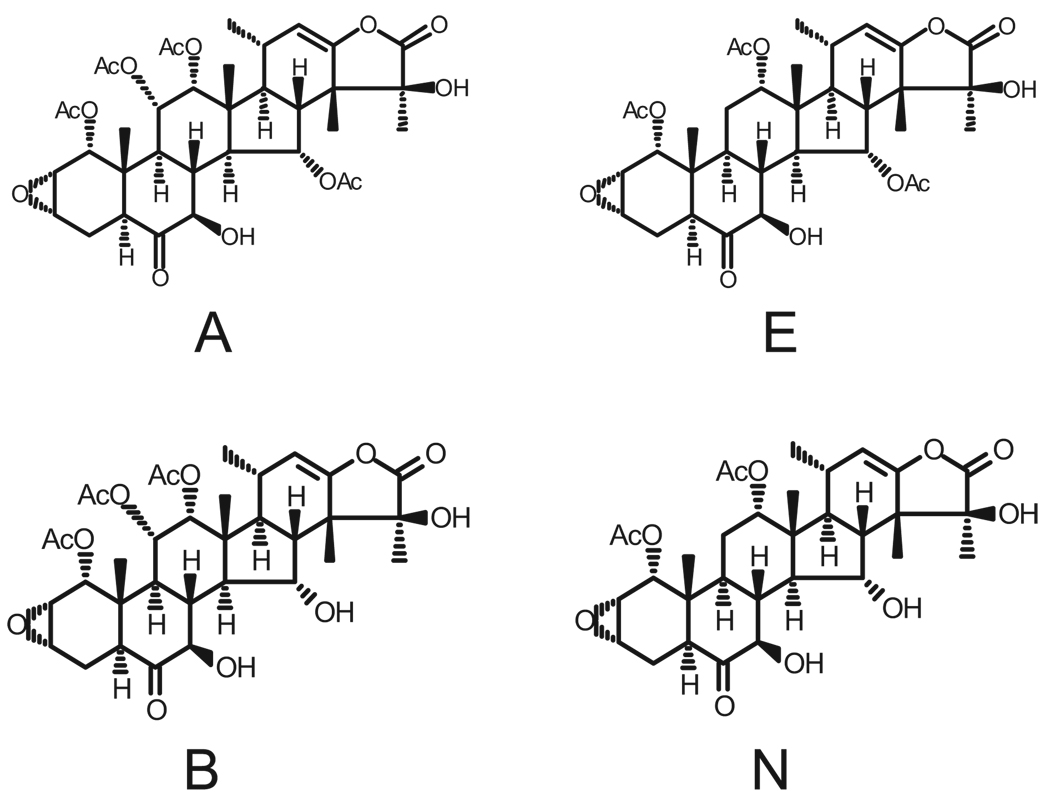
A number of different approaches have been used to extract and purify the taccalonolides from the roots and rhizomes of Tacca chantrieri. In previous studies, we performed large-scale solvent extractions followed by HPLC purification and preparative thin-layer chromatography to separate taccas A and E [9]. In the present study, we utilized a supercritical fluid extractor with a solvent modifier followed by flash chromatography separations and HPLC purifications to isolate taccas A and E (Figure 1). The taccas A and E were each modified at the C15 position by mild base hydrolysis as initially described by Chen and colleagues [29] to generate taccas B and N, respectively (Figure 1).
Figure 1.
Chemical structures of the taccalonolides used in this study.
The Taccas Are Not Susceptible to Pgp-mediated Multidrug Resistance In Vitro
The susceptibility of taccas A, E, B and N to Pgp-mediated multidrug resistance in vitro was analyzed using SK-OV-3/MDR-1-6/6, an ovarian cancer cell line transduced with an MDR1 expression vector [30]. The activity of Pgp in this cell line was confirmed using paclitaxel, an established substrate of the transporter, as well as 2-methoxyestradiol (2ME2) and epothilone B, drugs that are not Pgp substrates [6, 37]. As expected, SK-OV-3/MDR-1-6/6 exhibited robust resistance to paclitaxel (860-fold), whereas only very modest levels of resistance were observed for 2ME2 and epothilone B (2.6 and 6.8-fold respectively; Table 1). These data indicate that the major mechanism of resistance in the SK-OV-3/MDR-1-6/6 cell line is Pgp, but that a low level of resistance might be observed even with drugs that are not substrates of Pgp. By comparison with paclitaxel, SK-OV-3/MDR-1-6/6 exhibited modest resistance towards the taccas. Relative resistance values of 4.1, 12, 5.1 and 6.1, respectively were observed for tacca A, B, E and N (Table 1). Our results suggest that all 4 taccalonolides are significantly better than paclitaxel at circumventing Pgp-mediated drug resistance.
Table 1.
Drug sensitivity of parental and Pgp-transduced SK-OV-3 cells
| IC50 ± SD (nM)a | |||
|---|---|---|---|
| Drug | SK-OV-3 | SK-OV-3/MDR-1-6/6 | Relative Resistanceb |
| Paclitaxel | 3.01 ± 0.06 | 2576 ± 265 | 860 |
| 2ME2 | 867 ± 36 | 2268 ± 235 | 2.6 |
| Epothilone B | 0.90 ± 0.13 | 6.09 ± 0.43 | 6.8 |
| Tacca A | 622 ± 39 | 2523 ± 317 | 4.1 |
| Tacca B | 208 ± 14 | 2547 ± 282 | 12.2 |
| Tacca E | 708 ± 32 | 3608 ± 429 | 5.1 |
| Tacca N | 201 ± 7 | 1220 ± 142 | 6.1 |
IC50 values were determined in each of the cell lines from a graph of at least seven drug concentrations that span the entire curve of growth inhibition as means of 3–4 independent experiments ± SD.
Relative resistance values were calculated by dividing the IC50 obtained in the SK-OV-3/MDR-1-6/6 cell line by the IC50 measured in the parental SK-OV-3 cell line.
The Taccas A and E Circumvent Pgp-mediated Drug Resistance In Vivo
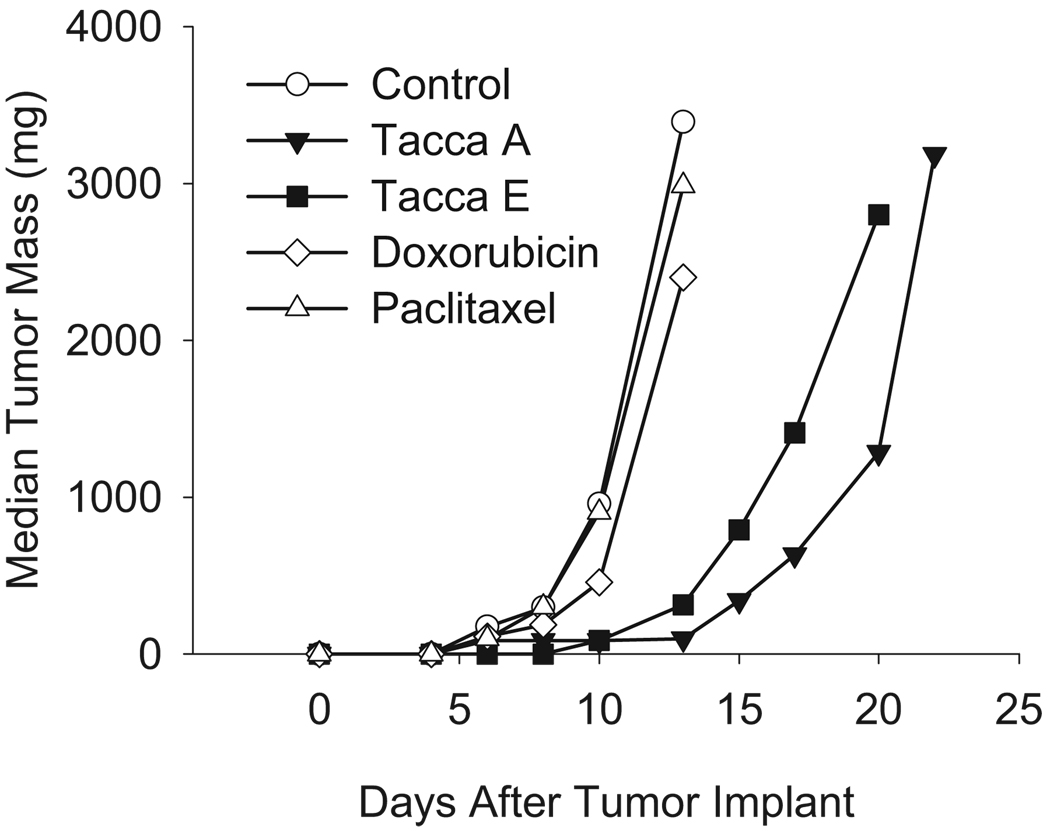
The efficacy of taccas in Pgp-expressing cancer cell lines led us to test their antitumor activity in a Pgp-expressing syngeneic murine mammary adenocarcinoma model. The Mam17/ADR tumor is a rapidly growing, metastatic tumor that is resistant to both paclitaxel and doxorubicin, providing a rigorous test for antitumor efficacy. As expected, paclitaxel, administered at a total dose of 75 mg/kg (historic highest non-toxic total dose (HNTTD) range: 60–90mg/kg for C3H mice) was inactive, resulting in only 6% growth inhibition (94% T/C, 0.1 log kill and 0.5 day tumor growth delay (TGD)). Mice sustained a moderate 9.4% body weight loss, indicative of appropriate dosing. Similarly, doxorubicin given at a total dose of 14.4 mg/kg (historical HNTTD range: 12–16mg/kg) produced 52% growth inhibition (48% T/C, 0.3 log kill and 1.0 day TGD) with a moderate 8.1% body weight loss.
Preliminary studies with a tacca A- and E-enriched crude preparation showed that the taccas had in vivo potency in the same dose range as paclitaxel, much lower than was anticipated from their in vitro potency (data not shown). Taccas A and E were used for the efficacy trials described here. Tacca A had excellent antitumor activity when given at a total dose of 38 mg/kg, resulting in a 91% growth inhibition (9%T/C, 2.3 log kill and 9 day TGD; Figure 2). Agents that show growth inhibition greater than 90% are considered to be highly active by NCI standards and this level of antitumor activity is rarely achieved against the aggressive Mam17/ADR model. Although no drug deaths occurred with this dosing schedule, a mean weight loss nadir of 25.8% occurred, a significant toxicity, most likely due to high doses given once every 2 days. It is encouraging to note that despite the large weight loss, these mice recovered 91.5% of their starting weights within 6 days of nadir, indicating good host recovery potential. Another tacca A treatment group with a 33.2 mg/kg total dose (lower individual doses given more frequently) resulted in less than 2% weight loss but provided minimal antitumor action (33% growth inhibition, 67% T/C, 0.3 log kill and 1 day TGD). These results demonstrate that tacca A has excellent dose and timing-dependent antitumor potential against a highly drug resistant model.
Figure 2.
The in vivo antitumor activity of taccas A and E against a Pgp-expressing, multidrug-resistant mouse mammary adenocarcinoma, Mam17/ADR. Paclitaxel was injected at 12.5 mg/kg on days 1, 3, 5, 7, 9 and 10 for a total dose of 75 mg/kg. Doxorubicin was injected at 4.8 mg/kg on days 1, 6 and 10 for a total dose of 14.4 mg/kg. Tacca A was injected at 8 mg/kg on day 1 and 15 mg/kg on days 3 and 5 for a total dose of 38 mg/kg. Tacca E was injected at 8 mg/kg on day 1, 15 mg/kg on day 3 and 21 mg/kg on days 4, 5 and 8 for a total dose of 86 mg/kg.
Tacca E, with a total dose of 86 mg/kg, also provided excellent antitumor activity with 91% growth inhibition (9% T/C, 1.5 log kill and 6 day TGD; Figure 2). A maximal weight loss of 12.6% occurred with treatment, with 95% weight recovery within 7 days of nadir. The antitumor efficacy of tacca E also appeared to be dose-dependent because equivalent total dosing on a less intense schedule resulted in diminished antitumor effects (data not shown). These initial studies demonstrate that taccas A and E have excellent antitumor effects in this multidrug-resistant Pgp murine tumor model and that tacca E might be better tolerated than tacca A. Further refinement of dose and schedule of administration will be important factors to optimize antitumor effects and reduce toxicities for both tacca A and E.
The Taccas Overcome Resistance Due to MRP7 Expression
We next analyzed the ability of the taccas to circumvent resistance due to overexpression of the ABC transporter MRP7. Two HEK293 cell lines in which MRP7 was ectopically expressed (HEK-MRP7-C17 and HEK-MRP7-C18) and control cells transfected with parental vector (HEK-pcDNA3) were used for this analysis. In accord with a previous report [20] HEK-MRP7-C17 and HEK-MRP7-C18 exhibited robust resistance to docetaxel (8.7- and 7.2-fold resistance; Table 2). In contrast, minimal resistance was observed for tacca A (1.4- and 1.3-fold resistance; Table 2). In the case of taccas B, E and N, the MRP7-transfected cells were actually more sensitive compared to the parental control cells. Although there were some minor differences, the 4 taccalonolides had similar potency in HEK293 cells whether or not MRP7 was overexpressed. These findings provide evidence that the taccalonolides A, E, B and N are able to circumvent MRP7-mediated drug resistance, suggesting a potential advantage over the taxanes in the treatment of tumors expressing MRP7.
Table 2.
Drug sensitivity of HEK293 cells transfected with MRP7 or vector
| IC50 ± SEM (µM)a | Relative Resistanceb | ||||
|---|---|---|---|---|---|
| Drug | HEK-pcDNA3 | HEK-MRP7-C | HEK-MRP7-C18 | HEK-MRP7-C17 | HEK-MRP7-C18 |
| Tacca A | 1.76 ± 0.45 | 2.40 ± 0.31 | 2.20 ± 0.56 | 1.4 | 1.3 |
| Tacca B | 1.66 ± 0.52 | 1.30 ± 0.37 | 2.07 ± 0.33 | 0.78 | 1.3 |
| Tacca E | 2.78 ± 0.81 | 1.97 ± 0.62 | 2.47 ± 0.95 | 0.71 | 0.89 |
| Tacca N | 2.19 ± 0.56 | 1.76 ± 0.36 | 1.93 ± 0.18 | 0.8 | 0.88 |
| Docetaxel (nM) | 17.3 ± 5.5 | 151 ± 52 | 125 ± 25 | 8.7 | 7.2 |
IC50 values were determined in each of the cell lines as means of 4 independent experiments ± SE.
Relative resistance values were calculated by dividing the IC50 of the MRP7 transfectants (HEK-MRP7-C17 and HEK-MRP7-C18) by the IC50 of the parental vector-transfected cell line (HEK-pcDNA3).
The Taccalonolides Overcome βIII-Tubulin-Mediated Drug Resistance
To test the effect of βIII-tubulin expression on the activity of the taccas, we generated a HeLa-derived cell line that ectopically expresses the human βIII-tubulin gene, referred to as wildtype βIII [34]. The HeLa and wildtype βIII cell line pair provides the ability to test isogenic cell lines for the effect of βIII-tubulin expression on drug sensitivity. Others have reported that the expression of β-tubulin isotypes in CHO cells can reduce cell viability [38]. However, we found that the growth rate of the wildtype βIII cell line was essentially identical to the parental HeLa cell line (data not shown).
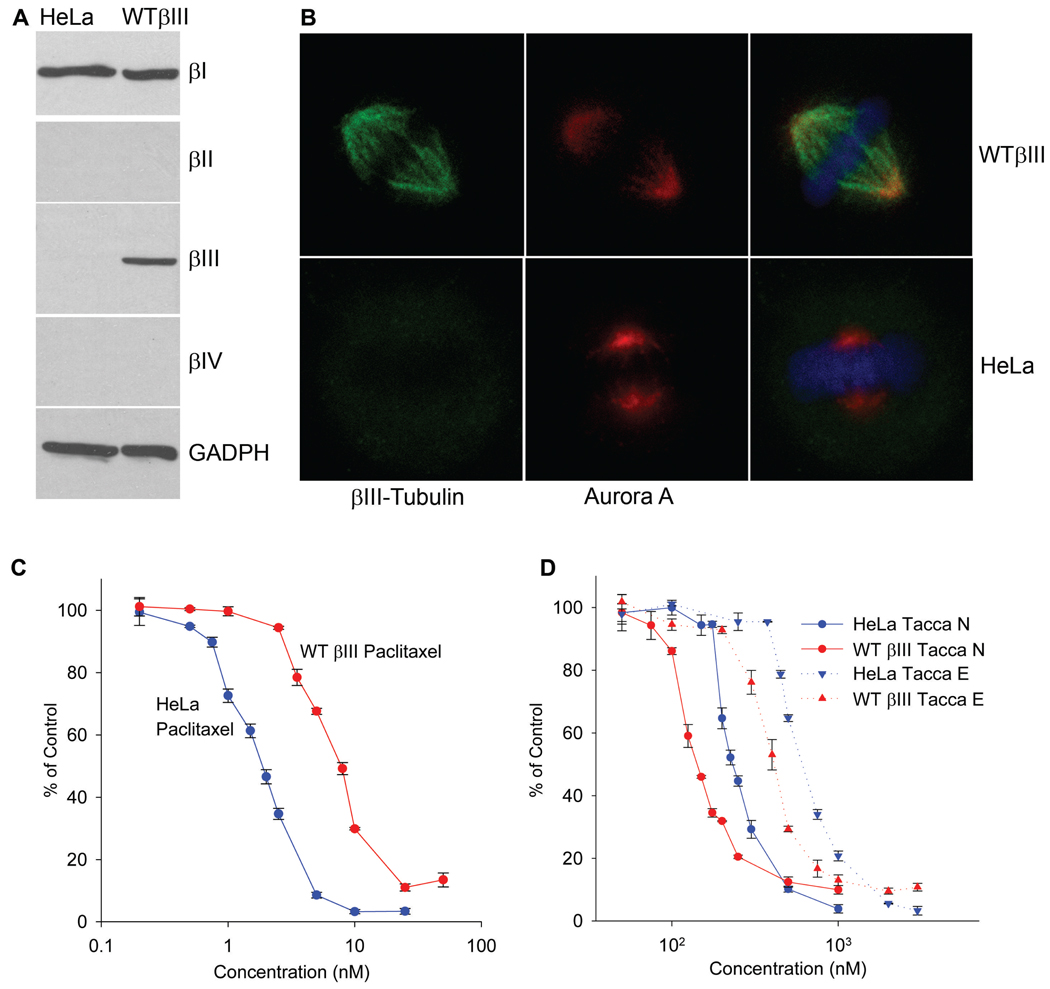
As shown in Figure 3A, expression of βIII-tubulin was readily detected in wildtype βIII cells, but was not detected to any extent in the parental HeLa cell line. Expression of βIII-tubulin did not result in alterations in the expression levels of other tubulin isotopes in that βI-, βII- and βIV-tubulins were expressed at equivalent levels in HeLa and wildtype βIII cells (Figure 3A). Although βII- and βIV-tubulins were not detected in either cell line, they were found in PC-tubulin preparations at relative levels that were consistent with previously published findings [39, 40] (Supplemental Figure 1). The expression and localization of the βIII-tubulin isotype in HeLa and wildtype βIII cells was examined by immunofluorescence. Consistent with the immunoblot data, βIII-tubulin was not detected in either interphase microtubules (Supplemental Figure 2) or in mitotic spindles (Figure 3B) of HeLa cells. However, in wildtype βIII cells, βIII-tubulin was observed in cytoplasmic interphase microtubules (Supplemental Figure 2) and in the mitotic spindle of dividing cells (Figure 3B). Aurora A, a kinase localized to centrosomes and the mitotic spindle, was used to visualize mitotic spindle structures independently of βIII-tubulin expression. The extensive overlap of βIII and Aurora A in wildtype βIII cells substantiated the conclusion that βIII-tubulin was correctly localized to the mitotic spindle. In addition, chromosomes were appropriately oriented in the metaphase plate in both HeLa and wildtype βIII cells (Figure 3B). In combination, these experiments indicate that βIII-tubulin was properly localized to microtubules in both interphase and dividing wildtype βIII cells and that this expression of βIII-tubulin did not affect either cell growth or the apparent function of the mitotic spindle. The incorporation of βIII into the mitotic spindle structures is of paramount importance because it is the ability of microtubule-disrupting drugs to interfere with mitotic events that underlies their antiproliferative and cytotoxic effects.
Figure 3.
βIII-tubulin expression confers resistance to paclitaxel and sensitivity to the taccalonolides. (A) Lysates were prepared from HeLa and isogenic wildtype βIII-tubulin expressing cells. Tubulin isotype expression was analyzed by immunoblotting with isotype-specific antibodies. Immunodetection of GAPDH was used as a control for equivalent loading and transfer of total protein between the two cell lines. (B) Mitotic spindles containing βIII-tubulin (green) or Aurora A (red) were visualized alone or as merged images with DAPI (blue) by indirect immunofluorescence in HeLa and wildtype βIII cells. The SRB assay was used to define the dose-response curves of paclitaxel (C) or taccalonolides E and N (D) in HeLa and wildtype βIII cells. The data represent the means of three independent experiments for each drug concentration ± SE.
The HeLa and wildtype βIII pair of isogenic cell lines was used to analyze the impact of βIII-tubulin expression on the activity of a variety of microtubule-stabilizing and destabilizing drugs. These included drugs that bind to the taxane binding site: paclitaxel, docetaxel and epothilone B; colchicine site binding agents: colchicine, combretastatin A4 phosphate and 2ME2; and the vinca domain binding drug vinblastine. The wildtype βIII cells were 4.7-, 6.9- and 2.3-fold resistant to paclitaxel, docetaxel and epothilone B respectively, as compared to the HeLa cell line (Table 3, Supplemental Figure 3A). The βIII expressing cells were also 3-fold more resistant to vinblastine (Table 3). Interestingly, the two cell lines were equally sensitive to drugs that bind within the colchicines-binding site on tubulin (Table 3).
Table 3.
The effect of βIII-tubulin on drug sensitivity
| IC50 ± SD (nM)a | |||
|---|---|---|---|
| Drug | HeLa | Wildtype βIII | Relative Resistanceb |
| Paclitaxel | 1.63 ± 0.15 | 7.73 ± 0.23 | 4.7 |
| Docetaxel | 0.58 ± 0.03 | 3.97 ± 0.10 | 6.8 |
| Epothilone B | 0.53 ± 0.08 | 1.24 ± 0.05 | 2.3 |
| Vinblastine | 6.85 ± 0.02 | 18.3 ± 3.1 | 2.7 |
| Colchicine | 12.2 ± 1.0 | 11.5 ± 0.5 | 0.9 |
| Combretastatin A4P | 4.69 ± 0.17 | 5.73 ± 0.42 | 1.2 |
| 2ME2 | 608 ± 55 | 586 ± 37 | 1.0 |
| Tacca A | 594 ± 43 | 541 ± 24 | 0.9 |
| Tacca B | 190 ± 3 | 120 ± 8 | 0.6 |
| Tacca E | 644 ± 10 | 381 ± 28 | 0.6 |
| Tacca N | 247 ± 16 | 138 ± 8 | 0.6 |
IC50 values were determined in each of the cell lines from a graph of at least seven drug concentrations that span the entire curve of growth inhibition as means of 3 independent experiments ± SD.
Relative resistance values were calculated by dividing the IC50 of the βIII transfected cell line (wildtype βIII) by the IC50 measured in the parental HeLa cell line.
The sensitivity of the two cells lines to the taccas was also evaluated. The βIII-expressing cell line reproducibly showed sensitivity to the taccas as compared to the parental HeLa cell line, suggesting that incorporation of the βIII isotype of tubulin into mitotic spindles does not confer resistance to the taccas (Table 3). Dose-response curves suggest that βIII-expressing wildtype βIII cells are less sensitive to the antiproliferative effects of paclitaxel and more sensitive to taccas E, N and B (Figure 3C, 3D, Supplemental Figure 3). These results suggest that the taccalonolides may have a unique niche as microtubule stabilizers that circumvent βIII-tubulin-mediated drug resistance.
Discussion
Drug resistance is clearly multifactorial and the identification of new drugs that can circumvent multiple resistance mechanisms would be beneficial. In the present study, we analyze the susceptibility of the taccas to several established resistance factors to gain insights into their potential clinical utility. Our finding that the taccas are effective in vitro and in vivo against Pgp-expressing tumor cells and in vitro against MRP7 and βIII-tubulin-expressing cell lines indicates that the taccas may offer advantages over well established microtubule-targeting agents. Additionally, tacca A and E do not appear to bind to tubulin [10], providing an additional advantage in that mutations in the taxane binding site do not cause resistance to the taccas [9]. The unique mechanism of action of the taccalonolides may prove valuable not only in overcoming resistance, but in acting in concert with tubulin-binding microtubule stabilizers to initiate mitotic arrest leading to apoptosis. This hypothesis will be tested in future studies.
Although the taccas clearly circumvent many drug-resistance mechanisms, they are significantly less potent than other microtubule stabilizers in drug-sensitive cell lines. In this context, it is important to remember that anticancer drugs are often used at their maximally tolerated dose. Therefore, even slight dose increases of very potent drugs in resistant tumors are prohibitive. Our in vitro results demonstrate that the taccalonolides are effective against taxane-resistant cell lines at concentrations comparable to (or even less than) those used in sensitive lines, suggesting the possibility that the taccas may be effective against resistant tumors in vivo at tolerated doses. Indeed, we found that a concentration of tacca E essentially equal to the maximum tolerated dose of paclitaxel was effective against an aggressive multidrug-resistant tumor. Therefore, while the taccas A, B, E and N are less potent than the taxanes in many cell lines, their enhanced efficacy in clinically relevant, multidrug-resistant tumors makes them attractive drug candidates.
Having four distinct taccalonolides allows us to gain insight on the importance of molecular structure as it affects function. For instance, the removal of the C15 hydroxyl significantly increases potency as evidenced by at least a 3-fold decrease in the IC50 value of taccas B and N compared with A and E in nearly every cell line tested. Identification of additional naturally occurring taccalonolides, along with further chemical modification of those already isolated, should provide further insight into what chemical moieties are important for optimal antimitotic actions. The presence of the C11 acetate group makes little difference in tacca potency in vitro as shown by the similar IC50 values of tacca A and E in several cell types. However, this difference does seem to have a significant impact in vivo against an aggressive Pgp-expressing tumor. Additionally, our data indicate that, in vivo, tacca A and E are substantially more potent than we would expect from their in vitro potency. Pharmacokinetic studies are planned.
Although the most well established mechanism of resistance to the taxanes is overexpression of Pgp, there are multiple lines of evidence to suggest that up-regulation of βIII-tubulin is another clinically relevant taxane resistance mechanism [24]. However, much of the cell line and clinical data are only correlative. The wildtype βIII cell line provides an opportunity for a systematic determination of resistance conferred solely by βIII-tubulin overexpression. In accordance with siRNA knockdown experiments in established cell lines with elevated βIII-tubulin expression, we found that βIII expression confers resistance to the microtubule stabilizers paclitaxel, docetaxel and epothilone B, as well as the microtubule depolymerizer vinblastine, which bind within the taxane and vinca domain respectively [28]. The additional finding that βIII-tubulin overexpression has no effect on the sensitivity of cells to three colchicine-site binding destabilizers further demonstrates that the mechanism of resistance conferred by βIII-tubulin overexpression is not as simple as net microtubule stabilization or destabilization, but may relate specifically to the tubulin/microtubule binding site. The isogenic wildtype βIII and HeLa cell lines will be valuable in the future to further probe these relationships. The fact that βIII expression does not cause resistance to the taccas is not totally unexpected considering that they do not appear to bind directly to tubulin [10]. The finding that a cell line expressing βIII is more sensitive to all 4 taccas than the parental line further suggests that these agents do indeed stabilize microtubules through a novel mechanism of action and supports additional study of their use in taxane-resistant tumors.
There is an increasing body of evidence correlating aggressive, non-taxane responsive tumors with βIII-tubulin expression [24]. βIII-tubulin is not normally found in epithelial tissues and there is a question as to why it is often expressed in tumors of epithelial origin. A mechanistic rationale for this observation has been missing. In contrast to most other β-tubulin isotypes, βIII-tubulin lacks Cys239. This cysteine is very reactive and oxidation of Cys239 has been shown to inhibit microtubule assembly [41]. βIII-tubulin has a serine at position 239 and, consistent with the lower reactivity of Ser239, tubulin polymers formed of αβIII are less sensitive to oxidizing agents including reactive oxygen species (ROS) [41]. Therefore, expression of βIII could provide a mechanism to protect microtubules from oxidative damage.
The hypoxic conditions of the tumor environment enhance the generation of ROS [42]. A recent report by Raspaglio and colleagues demonstrated that βIII-tubulin is upregulated in cells grown in hypoxic conditions and that this expression occurred through activation of HIF-1α [43]. Mechanistically, this suggests that cells that express βIII-tubulin as a result of hypoxic conditions might be better equipped to survive the high oxidative stress environment of a tumor. These findings give a rational molecular basis for the finding that βIII-tubulin is often up-regulated in solid tumors that are refractory to current treatments and supports efforts to identify drugs that are effective against these notably aggressive tumors.
Supplementary Material
Acknowledgements
Grant Support: National Cancer Institute CA121138 (SLM), CA073728 (GDK), CA114574 (GDK), United States Army Breast Cancer Research Program W81XWH-05-1-0238 (RFL) and the NCI P30 CA054174 (SLM and RFL). This work was funded in part by the Zachry Endowed Chair.
We thank Susan Kane for the SK-OV-3-MDR-1 cell line and April Hopstetter for her help in preparation of the manuscript.
References
- 1.Rowinsky EK, Tolcher AW. Antimicrotubule agents. In: DeVita VTJ, Hellman S, Rosenberg SA, editors. Cancer principles and practice of oncology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. pp. 431–447. [Google Scholar]
- 2.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 3.Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7:730–742. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- 4.Chu Q, Vincent M, Logan D, Mackay J, Evans W. Taxanes as first-line therapy for advanced non-small cell lung cancer: a systematic review and practice guideline. Lung Cancer. 2005;50:355–374. doi: 10.1016/j.lungcan.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Montero A, Fossella F, Hortobagyi G, Valero V. Docetaxel for treatment of solid tumours: a systematic review of clinical data. Lancet Oncol. 2005;6:229–239. doi: 10.1016/S1470-2045(05)70094-2. [DOI] [PubMed] [Google Scholar]
- 6.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18 Suppl 5:3–8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 7.Conlin A, Fornier M, Hudis C, Kar S, Kirkpatrick P. Ixabepilone. Nat Rev Drug Discov. 2007;6:953–954. [Google Scholar]
- 8.Mita A, Lockhart AC, Chen T-L, et al. A phase I pharmacokinetic (PK) trial of XAA296A (discodermolide) administered every 3 wks to adult patients with advance solid malignancies. J Clin Oncol. 2004;22(14S):2025. [Google Scholar]
- 9.Tinley TT, Randall-Hlubek DA, Leal RM, et al. Taccalonolides E and A: plant-derived steroids with microtubule-stabilizing activity. Cancer Res. 2003;63:3211–3220. [PubMed] [Google Scholar]
- 10.Buey RM, Barsoain I, Jackson E, et al. Microtubule interactions with chemically diverse stabilizing agents: thermodynamics of binding to the Taxol site predicts cytotoxicity. Chem Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537–592. doi: 10.1146/annurev.biochem.71.102301.093055. [DOI] [PubMed] [Google Scholar]
- 12.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 13.Rottenberg S, Nygren AOH, Pajic M, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci USA. 2007;104:12117–12122. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borst P, Jonkers J, Rottenberg S. What makes tumors multidrug resistant? Cell Cycle. 2007;6:2782–2787. doi: 10.4161/cc.6.22.4936. [DOI] [PubMed] [Google Scholar]
- 15.Yeh JJ, Hsu WH, Wang JJ, Ho ST, Kao A. Predicting chemotherapy response to Taxol-based therapy in advanced non-small-cell lung cancer with P-glycoprotein expression. Respiration. 2003;70:32–35. doi: 10.1159/000068411. [DOI] [PubMed] [Google Scholar]
- 16.Chiou J-F, Liang J-A, Hsu W-H, Wang J-J, Ho S-T, Kao A. Comparing the relationship of Taxol-based chemotherapy response with P-glycoprotein and lung resistance-related protein expression in non-small cell lung cancer. Lung. 2003;181:267–273. doi: 10.1007/s00408-003-1029-7. [DOI] [PubMed] [Google Scholar]
- 17.Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89:917–931. doi: 10.1093/jnci/89.13.917. [DOI] [PubMed] [Google Scholar]
- 18.Hopper E, Belinsky MG, Zeng H, Tosolini A, Testa JR, Kruh GD. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett. 2001;162:181–191. doi: 10.1016/s0304-3835(00)00646-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZS, Hopper-Borge E, Belinsky MG, Shchaveleva I, Kotova E, Kruh GD. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10) Mol Pharmacol. 2003;63:351–358. doi: 10.1124/mol.63.2.351. [DOI] [PubMed] [Google Scholar]
- 20.Hopper-Borge E, Chen ZS, Shchaveleva I, Belinsky MG, Kruh GD. Analysis of the drug resistance profile of multidrug resistance protein 7 (ABCC10): resistance to docetaxel. Cancer Res. 2004;64:4927–4930. doi: 10.1158/0008-5472.CAN-03-3111. [DOI] [PubMed] [Google Scholar]
- 21.Dabrowska M, Sirotnak FM. Regulation of transcription of the human MRP7 gene. Characteristics of the basal promoter and identification of tumor-derived transcripts encoding additional 5' end heterogeneity. Gene. 2004;341:129–139. doi: 10.1016/j.gene.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi S, Kataoka T, Ohara O, Oishi M, Kuo MT, Ishikawa T. Human ATP-binding cassette transporter ABCC10: expression profile and p53-dependent upregulation. J Exp Ther Oncol. 2004;4:239–246. [PubMed] [Google Scholar]
- 23.Oguri T, Ozasa H, Uemura T, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Mol Cancer Ther. 2008;7 doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
- 24.Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–175. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 25.Panda D, Miller HP, Banerjee A, Ludueña RF, Wilson L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc Natl Acad Sci USA. 1994;91:11358–11362. doi: 10.1073/pnas.91.24.11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Q, Ludueña RF. Removal of beta III isotype enhances taxol induced microtubule assembly. Cell Struct Funct. 1993;18:173–182. doi: 10.1247/csf.18.173. [DOI] [PubMed] [Google Scholar]
- 27.Kamath K, Wilson L, Cabral F, Jordan MA. βIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J Biol Chem. 2005;280:12902–12907. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 28.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67:9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z-L, Shen J-H, Gao Y-S. Some chemical reactions of taccalonolide A - a bitter substance from Tacca plantaginea. Heterocycles. 1989;29:2103–2108. [Google Scholar]
- 30.Sadava D, Coleman A, Kane SE. Liposomal daunorubicin overcomes drug resistance in human breast, ovarian and lung carcinoma cells. J Liposome Res. 2002;12:301–309. doi: 10.1081/lpr-120016196. [DOI] [PubMed] [Google Scholar]
- 31.Joe PA, Banerjee A, Ludueña RF. The roles of Cys124 and Ser239 in the functional properties of human βIII tubulin. Cell Motil Cytoskeleton. 2008 April 24; doi: 10.1002/cm.20274. epub. [DOI] [PubMed] [Google Scholar]
- 32.Boyd MR, Paull KD. Some practical considerations and applications of the National Cancer Institute in vitro anticancer discovery screen. Drug Devel Res. 1995;34:91–109. [Google Scholar]
- 33.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee A, Roach MC, Trcka P, Ludueña RF. Preparation of a monoclonal antibody specific for the class IV isotype of β-tubulin. Purification and assembly of αβII, αβIII, and αβIV tubulin dimers from bovine brain. J Biol Chem. 1992;267:5625–5630. [PubMed] [Google Scholar]
- 35.Weiderhold KN, Randall-Hlubek DA, Polin LA, Hamel E, Mooberry SL. CB694, a novel antimitotic with antitumor activities. Int J Cancer. 2006;118:1032–1040. doi: 10.1002/ijc.21424. [DOI] [PubMed] [Google Scholar]
- 36.Corbett T, Polin L, Roberts BJ, et al. Transplantable Syngeneic Rodent Tumors: Solid Tumors in Mice. In: Teicher BA, editor. Tumor Models in Cancer Research. Totowa, NJ: Humana Press; 2002. [Google Scholar]
- 37.Schumacher G, Hoffmann J, Cramer T, et al. Antineoplastic activity of 2-methoxyestradiol in human pancreatic and gastric cancer cells with different multidrug-resistant phenotypes. J Gastro Hepatology. 2007;22:1469–1473. doi: 10.1111/j.1440-1746.2007.05033.x. [DOI] [PubMed] [Google Scholar]
- 38.Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III beta-tubulin reduces microtubule assembly and confers resistance to Taxol. Cell Motil Cytoskeleton. 2003;56:45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- 39.Fellous A, et al. Microtubule Assembly in vitro. Eur J Biochem. 1977;78:167–174. doi: 10.1111/j.1432-1033.1977.tb11726.x. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee A, et al. A monoclonal antibody against the type II isotype of beta-tubulin. Preparation of isotypically altered tubulin. J Biol Chem. 1988;263(6):3029–3034. [PubMed] [Google Scholar]
- 41.Ludueña R, Banerjee A. The isotypes of tubulin: distribution and functional significance. In: Fojo T, editor. Microtubules. Totowa, NJ: Humana Press; 2005. [Google Scholar]
- 42.Furuta E, Pai SK, Zhan R, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 43.Raspaglio G, Filippetti F, Prislei S, et al. Hypoxia induces class III β-tubulin gene expression by HIF-1α binding to its 3' flanking region. Gene. 2008;409:100–108. doi: 10.1016/j.gene.2007.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.