Abstract
The microenvironment of cancerous cells includes endoplasmic reticulum (ER) stress the resistance to which is required for the survival and growth of tumors. Acute ER stress triggers the induction of a family of ER stress proteins that promotes survival and/or growth of the cancer cells, and also confers resistance to radiation and chemotherapy. Prolonged or severe ER stress, however, may ultimately overwhelm the cellular protective mechanisms, triggering cell death through specific programmed cell death (pcd) pathways. Thus, downregulation of the protective stress proteins may offer a new therapeutic approach to cancer treatment. In this regard, recent reports have demonstrated the roles of the phytochemical curcumin in the inhibition of proteasomal activity and triggering the accumulation of cytosolic Ca2+ by inhibiting the Ca2+-ATPase pump, both of which enhance ER stress. Using a mouse melanoma cell line, we investigated the possibility that curcumin may trigger ER stress leading to programmed cell death. Our studies demonstrate that curcumin triggers ER stress and the activation of specific cell death pathways that feature caspase cleavage and activation, p23 cleavage, and downregulation of the anti-apoptotic Mcl-1 protein.
Keywords: Endoplasmic reticulum, Curcumin, ER stress, Caspase, Apoptosis, Programmed cell death
Introduction
The efficient functioning of the endoplasmic reticulum (ER) is essential for proper cellular activities and survival. Any condition that interferes with ER functions triggers the accumulation and aggregation of misfolded or unfolded proteins and induction of an ER stress response to restore normal ER function [1–3]. The induction of a family of ER signaling proteins is required to block ER stress signals, maintain ER function and integrity, ensure protein folding, and protect cells from misfolded protein toxicity [1–6]. Prolonged ER stress impairs the protective mechanisms designed to promote correct folding and degrade faulty proteins, ultimately leading to organelle dysfunction and programmed cell death (pcd) [7–9].
The microenvironment of cancerous cells and solid tumors is characterized by an inherent physiological ER stress response that provides an overall protective role in tumor development. The levels of a family of ER proteins are elevated correlating with tumor progression, metastasis and drug resistance. It is believed that this family of ER stress proteins is anti-apoptotic and confers protection to cancer cells against the natural immune defense mechanisms of the host and anti-cancer drugs [10, 11]. This suggests that down-regulation of the protective stress proteins, or the provocation of an ER stress-mediated cell death process by small molecules, may retard tumor development, growth, and invasion, and potentially improve treatment outcome [10–12].
Curcumin, the main biologically active phytochemical isolated from turmeric root, is a powerful antioxidant and anti-inflammatory compound, and is a potent inhibitor of the proliferation of cancer cells [12–15]. Numerous studies have demonstrated its role in suppressing the expression of cyclin D1 and other transcription factors that are implicated in carcinogenesis, blocking the activation of nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1), inducing apoptosis in tumor cells by activating caspases and down-regulating anti-apoptotic Bcl-2 family proteins [13, 16]. Recent reports have also demonstrated that curcumin (a) inhibits proteasomal activity [17], (b) triggers the accumulation of cytosolic Ca2+ by inhibiting the Ca2+-ATPase pump [18, 19], and (c) disrupts protein disulfide bond formation [12], all of which trigger an ER stress response. Therefore, we evaluated the possibility that curcumin may trigger ER stress-induced apoptosis in mouse melanoma cells. Our studies demonstrate that curcumin triggers ER stress and activates the intrinsic apoptotic pathway, affecting both Bcl-2 family proteins and caspases.
Material and methods
Cells, culture conditions and cell extracts
Murine melanoma B16–F10 cells, human embryonic kidney HEK293 and NIH3T3 mouse fibroblasts were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin. Curcumin (Sigma Chemical Co, St. Louis MO, USA) was dissolved in absolute alcohol at a stock concentration of 10 mM and was diluted to the required concentration immediately before use with cell culture media. A 10 mM stock solution of BAPTA/AM (Sigma Chemical Co, St. Louis MO, USA) was prepared in DMSO and was diluted in cell culture media just before addition to the cells. Salubrinal (Sal; EMD Biosciences) was dissolved in DMSO and diluted in cell culture media before addition to cells. Q-VD-OPH (MP Biomedicals, Aurora, Ohio, USA), a cell permeable irreversible caspase inhibitor, was dissolved in DMSO according to manufacturer’s data sheet and further diluted with cell culture media.
Total cell extracts were prepared as described [7, 9, 20, 21]. Briefly, cells from untreated or curcumin-treated cells were resuspended in RIPA buffer (50 mM Tris, pH 7.5, 0.5% deoxycholate, 1% Triton X-100, 0.1% SDS, 150 mM NaCl) containing protease inhibitors (complete Mini; Roche, Penzberg, Germany). 100–200 µg protein from total extracts was used for Western blotting.
Western blotting
SDS-PAGE and Western blot analyses were performed as described earlier [7–9, 22]. Membranes were probed with 1:500 dilutions of mouse-specific anti-caspase-9, anti-caspase-12, anti-caspase-7, anti-caspase-3 and anti-Bax antibodies (all from Cell Signaling Laboratories), a 1:1,000 dilution of anti-p23 monoclonal antibody (BD Biosciences), a 1:500 dilution of anti-KDEL monoclonal antibody (Stressgen), a 1:500 dilution of anti-Mcl-1 antibody (Santa Cruz), and a 1:50,000 dilution of anti-GAPDH rabbit polyclonal antibody (Research Diagnostics, Inc).
Flow cytometry
Flow cytometry analysis was performed as described [21]. Media and cells from untreated and treated samples were collected by trypsinization, stained with 3 µg/ml GFP-Annexin-V and 2.5 µg/ml propidium iodide in Annexin-V binding buffer and incubated at room temperature for 15 min, and read on a BD LSR flow cytometer (BD Biosciences, San Jose, CA, USA). Data were processed with CellQuest Pro (BD Biosciences).
Immunoprecipitation
Cell lysis and immunoprecipitation were performed as previously described [8, 9, 22]. Cell extracts from untreated and curcumin-treated cells were prepared as described [7, 22]. A total of 200 µg protein from the total extract was subjected to immunoprecipitation. Following an overnight incubation at 4°C with the antibodies, protein A/G-Sepharose was added to the samples and incubated at 4°C for an additional 6 h. Samples were spun briefly to pellet the protein A/G-Sepharose conjugate. The supernatant was subjected to SDS-PAGE and Western blotting.
Caspase activity assay
The fluorogenic substrate benzyloxycarbonyl-Asp-Glu-Val-Asp-7-amino-4 trifluoromethylcoumarin was purchased from Enzyme Systems Products and dissolved in dimethyl formamide as a 10 mM stock solution. Cell extracts (50–100 µg of protein) from untreated or curcumin-treated cells were incubated with 100 µM peptide substrate. Caspase activity was determined by measuring the release of amino-4-trifluoromethylcoumarin from the synthetic substrate using continuous recording instruments as described earlier [7]. Caspase activity was analyzed using a SpectraMAX 340 plate reader (Molecular Devices) at excitation and emission wavelengths of 444 and 538 nm, respectively, and expressed as DEVDase activity/mg protein.
Evaluation of cell death
Assessment of cell death was carried out by pelleting floating and adherent cells (after trypsinization) as previously described [7, 9, 20]. The cell pellet was resuspended in 1 × PBS/0.4% Trypan blue and cells were counted using a hemocytometer. Cell death was determined as the percentage of dead cells over the total number of cells. Statistical significance was determined by two-way analysis of variance (ANOVA).
Alternatively, assessment of cell death was carried out by the MTT assay as previously described [23]. Briefly, cells were seeded in a 24-well plate at a density of 50,000 per well. Following treatment with curcumin, viable cells were measured using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). In brief, 50 µl of 1 mg/ml MTT was added to the cells (500 µl) and incubated at 37°C for 2 h. The medium was discarded, the dark blue formazan crystalline product was dissolved in dimethyl sulfoxide and the absorbance was analyzed in a Spectramax plate reader (Molecular Devices) at 570 nm. Cell death was determined as the percentage of live cells over the total number of cells.
Results
Curcumin triggers ER stress-induced cell death in melanoma cells
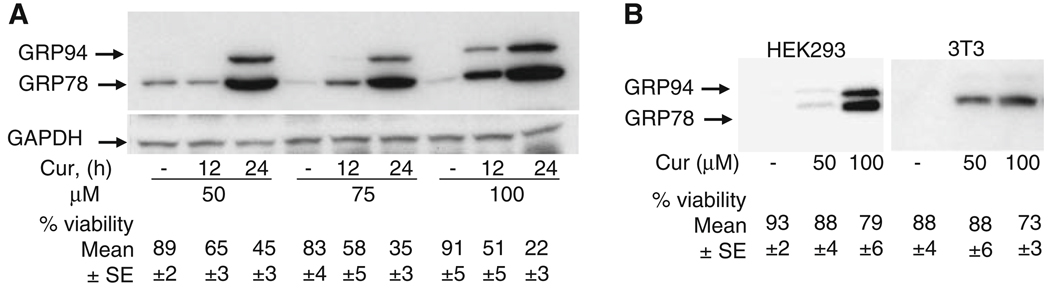
Several studies have reported the use of curcumin at various concentrations varying from 1 to 100 µM [12, 13, 17, 24]. In order to test whether melanoma cells are susceptible to ER stress and cell death, B16 mouse melanoma cells were treated with different concentrations of curcumin. As shown in Fig. 1a, exposure of cells to curcumin led to a decrease in cell viability in a time and dose-dependent manner. This is similar to results reported earlier [13] demonstrating induction of apoptosis by curcumin in human melanoma cells. In contrast, NIH3T3 mouse fibroblasts and HEK-293 cells were resistant to curcumin treatment and displayed minimal cell death at 100 µM (Fig. 1b) suggesting curcumin’s specificity and its ability to distinguish cancer cells from transformed cells (HEK293) or spontaneously immortalized cells (3T3).
Fig. 1.
Curcumin triggers ER stress induced cell death in melanoma cells. GRP78 and GRP94 expression in (a) mouse melanoma cells treated with different concentrations of curcumin for different time periods and (b) in HEK293 cells and NIH3T3 mouse fibroblasts treated with different concentrations of curcumin for 24 h. Cell extracts (150 µg protein) were analyzed by Western blot analysis for GRP78, GRP94 and GAPDH (loading control) protein expression. Surviving versus dead cells were quantified as described in Section ‘Methods’. Data (mean ± SE) are from at least three independent experiments
A high level of glucose regulated protein (GRP) expression is indicative of ER stress [7–9, 25], and curcumin treatment of melanoma cells resulted in the induction of GRP78 and GRP94 expression (Fig. 1a). Interestingly, despite showing minimal cell death toxicity, curcumin treatment of both NIH3T3 mouse fibroblasts and HEK293 cells resulted in the induction of GRP expression (Fig. 1b) suggesting that in contrast to melanoma cells, upregulation of GRP family of proteins in NIH3T3 and HEK293 cells may confer survival advantage through their anti-apoptotic properties [11, 26].
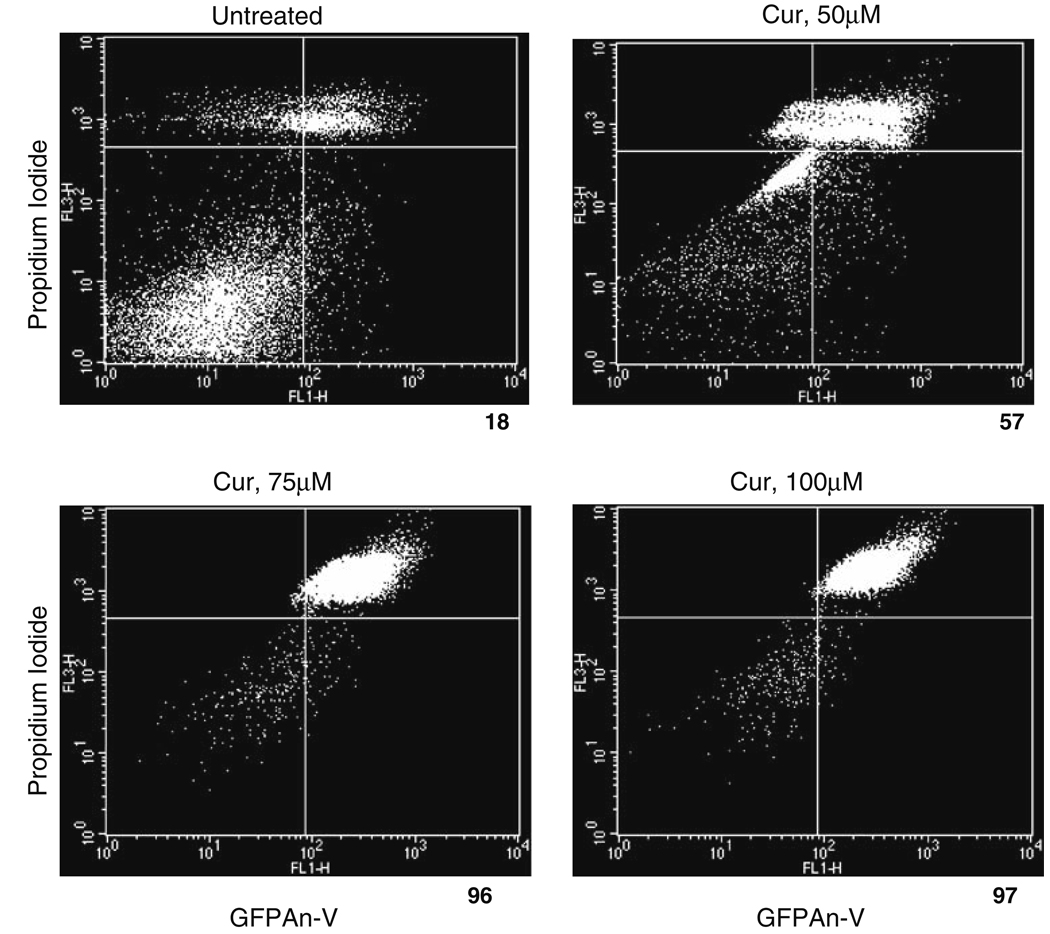
Cells exposing phosphatidylserine, which serves as a marker of apoptosis, can be labeled with a His-GFP-Annexin-V fusion protein [21]. Loss of plasma membrane integrity can be followed by uptake of propidium iodide [21]. After 24 h of ER stress, fluorescence-activated cell-sorting analysis revealed that 60% or more of the cells were GFPAn-V-positive with curcumin treatment compared to 18% in untreated cells (Fig. 2). This method seems more sensitive and reliable for measuring dying cells as opposed to the trypan blue method (Fig. 1) that involves manual counting of dead cells and may explain the discrepancy in the viability of melanoma cells at 24 h of curcumin treatment as measured by the above two methods (Fig. 1 and Fig. 2).
Fig. 2.
Curcumin triggers ER stress-induced cell death in melanoma cells. Representative flow cytometry dot plots from curcumin-treated melanoma cells stained with GFPAn-V/Propidium iodide after 24 h of treatment. The average cell death counts are listed under each dot-plot and are expressed as a percentage of total dead cells
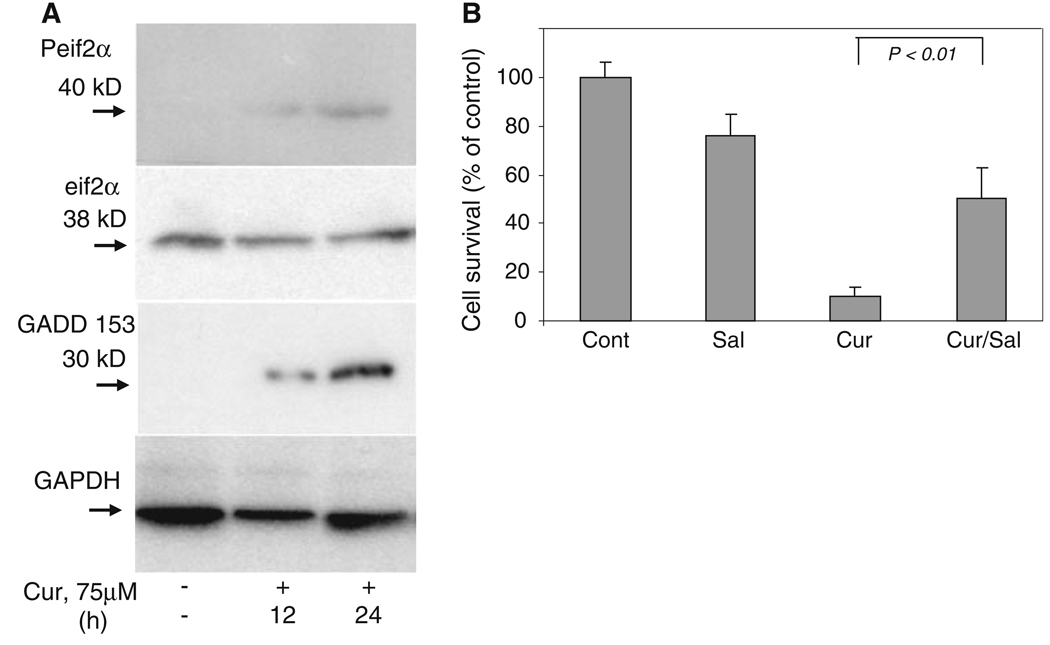
GADD 153/CHOP is a 30 kDa protein that triggers growth arrest and DNA damage. While GADD 153 is ubiquitously expressed at very low levels in basal conditions, it is robustly expressed by perturbations that induce ER stress in a wide variety of cells [4, 5, 27]. Similarly, phosphorylation of the alpha subunit of the eukaryotic initiation factor-2 (eIF2α) is a well documented mechanism of down-regulating protein synthesis triggered by agents that induce ER stress and protein misfolding [4, 5]. As shown in Fig. 3a, curcumin treatment resulted in increased phosphorylation of eIF2α and increased protein expression of GADD 153.
Fig. 3.
GADD 153 and Phospho-eIF2α protein expression during ER stress. (a) Cell extracts (150 µg protein) from untreated or curcumin-treated (75 µM) melanoma cells were analyzed by Western blot analysis with Phospho-eIF2α antibody, eIF2α antibody or GADD 153 antibody. Membranes were also probed with anti-GAPDH antibody to serve as a loading control. (b) Melanoma cells were either left untreated or exposed to curcumin (75 µM) in the presence or absence of salubrinal (Sal, 50 µM), a specific inhibitor of phospho-eIF2α phosphatase. Surviving versus apoptotic cells were analyzed by MTT assay as described in Section ‘Methods’
Salubrinal (Sal) is an inhibitor of serine/threonine phosphatase PP1 and inhibits eIF2α dephosphorylation that in turn blocks ER stress-induced cell death [28, 29]. To study the effect of Sal on curcumin-treated melanoma cells, we added Sal in combination with curcumin. As shown in Fig 3b, addition of Sal to cells significantly decreased the number of dying cells and increased survival by more than 50%.
Processing of caspases in ER stress-induced cell death triggered by curcumin
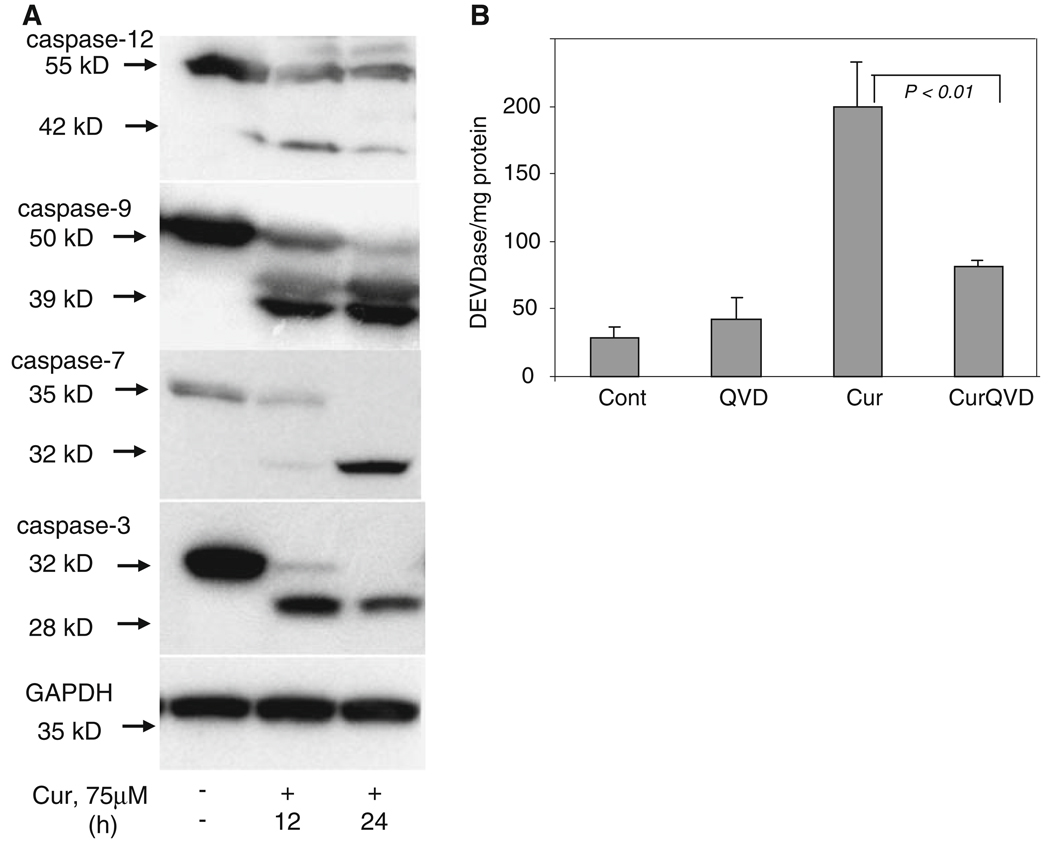
Caspase-dependent apoptotic cell death features caspase activation that, in the case of executioner caspases (caspases-3 and -7) requires cleavage into a large and small subunit. Cleavage and activation of downstream caspases is promoted by proximal initiator caspases (caspases-12, -9 and -8) which themselves undergo dimerization and cleavage [30]. In order to determine whether caspases are cleaved during ER stress-induced apoptosis in melanoma cells, cell-free extracts from curcumin-treated cells were analyzed by Western blotting. As shown in the time course in Fig. 4a, curcumin treatment resulted in the processing and cleavage of caspases-12, -9, -7 and -3. Processing of caspase-12 was seen initially at 12 h of curcumin treatment. The antibody we employed for caspase-9 recognized full length (50 kDa) and an amino-terminally cleaved product (39 kDa, prodomain + large subunit) of caspase-9 [7]. Cleavage of caspase-7 was very similar to that of caspase-9, with more of the cleaved form present at 24 h of treatment. Processing of the pro-form of caspase-3 (32 kDa) to the p28 form was also observed at 24 h of curcumin treatment.
Fig. 4.
Curcumin triggered ER stress-induced cell death involves cleavage of caspases. (a) Melanoma cells were treated with 75 µM curcumin for the indicated times. Cell-free cytosolic extracts (150 µg of protein) from untreated and curcumin-treated cells were prepared as described under Section “Experimental Procedures” and analyzed by Western blot analysis. Membranes were probed with anti-caspase-12, anti-caspase-9, anti-caspase-7 or anti-caspase-3 antibodies. All caspase antibodies were capable of detecting the pro and active forms. Cell extracts were also probed with GAPDH as a loading control. Each Western blot is representative of three independent experiments. (b) Melanoma cells were either left untreated or exposed to curcumin (75 µM) in the presence or absence of the cell permeable caspase specific inhibitor Q-VD-OPH (25 µM). Surviving versus apoptotic cells were analyzed by MTT assay as described in Section ‘Methods’. Data are from three independent experiments
Caspase activity measurements on cell extracts were performed using Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin as substrate. As shown in Fig. 4b, melanoma cells treated with curcumin activated caspases as demonstrated by increased DEVDase activity. Q-VD-OPH (QVD), a broad spectrum cell-permeable caspase inhibitor that irreversibly binds to active caspases, blocks cell death with minimal toxicity. As shown in Fig. 4b, the DEVDase activity triggered by curcumin was significantly suppressed by QVD. QVD not only inhibited DEVDase activity but also enhanced melanoma cell viability (data not shown).
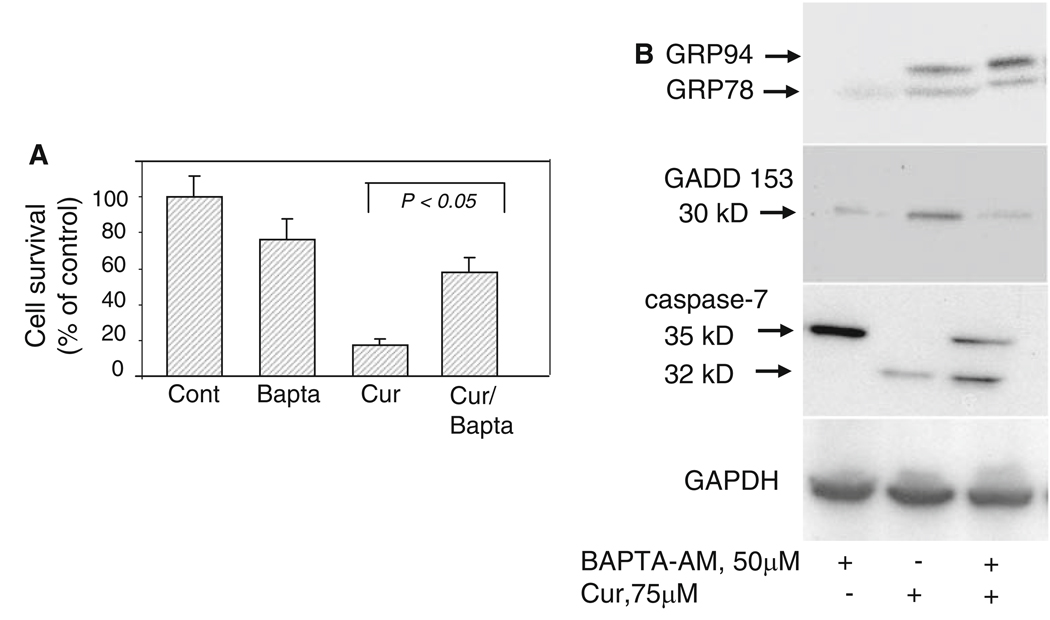
BAPTA-AM blocks GADD 153 induction and caspase cleavage
Recent reports have demonstrated the role of curcumin in triggering the accumulation of cytosolic Ca2+ by several mechanisms including inhibiting Ca2+-ATPase pump [18, 19, 31, 32] that in turn could trigger an ER stress response leading to cell death. The calcium chelator BAPTA-AM blocks Ca2+ release from the ER, prevents mitochondrial ROS accumulation and blocks apoptosis [29, 33–35]. This meant that blocking Ca2+ release by the calcium chelator BAPTA-AM could potentially inhibit ER stress-induced cell death triggered by curcumin. We therefore analyzed the effects of BAPTA-AM on cell death, GADD153 induction and caspase cleavage after exposing mouse melanoma cells to curcumin. As shown in Fig. 5, preincubation of melanoma cells with 50 µM BAPTA-AM followed by curcumin treatment not only inhibited cell death (Fig. 5a) but also blocked induction of GADD 153 expression and caspase-7 cleavage (Fig. 5b), suggesting that a combination of ER stress and cytosolic Ca2+ influx facilitates and accelerates curcumin-induced apoptosis in melanoma cells. Interestingly, BAPTA-AM did not block the induction of expression of GRP family proteins triggered by curcumin (Fig. 5b) suggesting that Ca2+ release may be downstream of GRP accumulation. BAPTA-AM alone did not have any effect on GADD153 expression or caspase processing.
Fig. 5.
Effects of BAPTA-AM on curcumin-treated melanoma cells. (a) Melanoma cells were either left untreated or preincubated with BAPTA-AM (50 µM) for 3 h followed by treatment with 75 µM curcumin for 24 h. Surviving versus apoptotic cells were analyzed by MTT assay as described in Section ‘Methods’. Data are from three independent experiments. (b) Melanoma cells were preincubated with BAPTA-AM (50 µM) for 3 h followed by treatment with 75 µM curcumin for 24 h. Cell extracts (150 µg of protein) were prepared as described under Section “Experimental Procedures” and analyzed by Western blot analysis. Membranes were probed with anti KDEL antibody, anti-GADD 153 antibody or anti-caspase-7 antibody
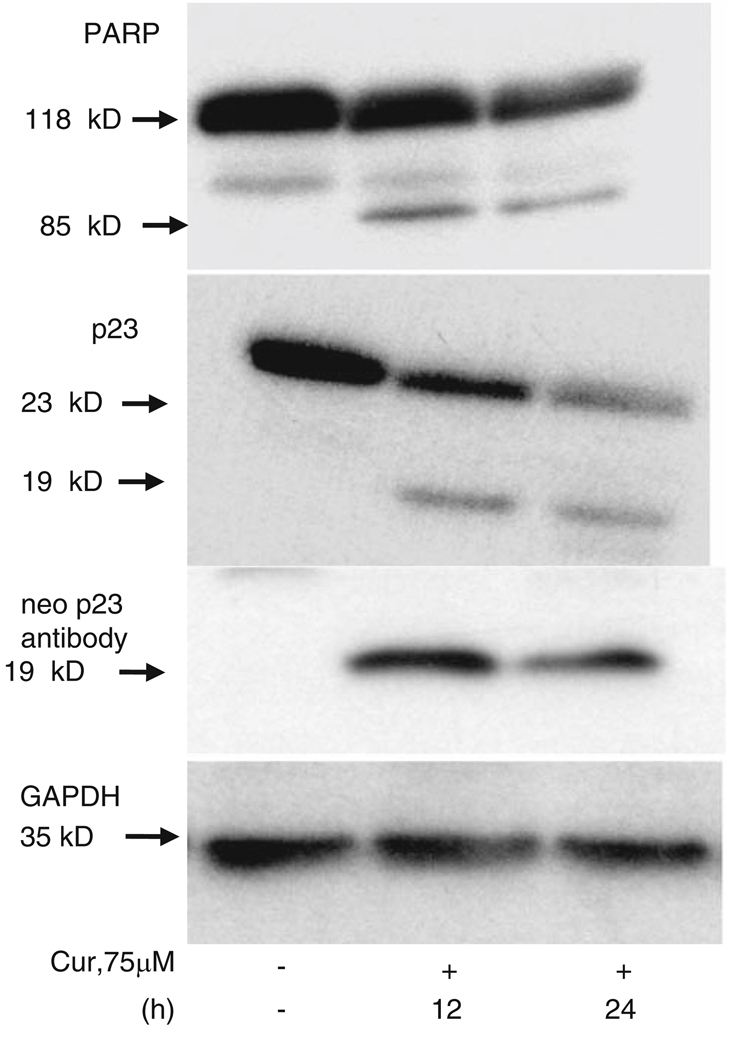
Expression of PARP and p23 in curcumin-treated melanoma cells
PARP, a 116 kDa nuclear poly (ADP-ribose) polymerase that is involved in DNA repair predominantly in response to environmental stress, is cleaved by caspases in vitro and is one of the main cleavage targets of caspase-3 in vivo [36]. As shown in Fig. 6 (top panel), ER stress-induced cell death triggered by curcumin resulted in cleavage of PARP to yield an 85 kDa cleaved fragment, as described earlier [7]. In an earlier study, we found that p23, an HSP90 co-chaperone protein, plays a role in mediating ER stress-induced cell death [37]. p23 is cleaved to a 19 kDa product during ER stress-induced cell death, irrespective of triggering agent. As shown in Fig. 6, curcumin treatment of melanoma cells also resulted in the processing of p23 and formation of the 19 kDa cleaved fragment [37]. The antibody we employed recognized full length p23 and the 19 kDa cleaved product (Fig. 6, middle panel). In addition, we also generated a neo-epitope antibody that recognized only the cleaved fragment (19 kDa) in curcumin-treated samples and not the parent p23; this confirmed p23 cleavage (Fig. 6, bottom panel).
Fig. 6.
Curcumin triggers cleavage of caspase substrates. Melanoma cells were treated with 75 µM curcumin for the indicated times. Cell extracts (150 µg of protein) from untreated and curcumin-treated cells were prepared as described under Section “Experimental Procedures” and analyzed by Western blot analysis. Membranes were probed with anti-PARP, anti-p23, or neo specific anti-p19 antibody. Cell extracts were also probed with GAPDH as a loading control. Each Western blot is representative of three independent experiments
Effect of curcumin on the expression of pro and anti-apoptotic proteins of the Bcl-2 family
Recent studies have disclosed the role of several ER stress-induced cell death effectors, including pro and anti-apoptotic members of the Bcl-2 family [1–3, 38–41]. Mcl-1 is an anti-apoptotic member of the Bcl-2 family that localizes to the mitochondria and inhibits apoptosis [42–44]. Recent studies have shown that expression of Mcl-1 protein is downregulated in cells undergoing pcd [42, 43, 45, 46]. Similarly, proapoptotic Bax undergoes a conformational change and accumulates on the mitochondrial surface triggering the release of cytochrome c to activate the final steps of pcd [47–49].
To further understand the role of curcumin in ER stress-induced apoptosis in mouse melanoma cells, we asked whether curcumin has any effect on the expression of these two proteins. Exposure of melanoma cells to curcumin led to a time-dependent decrease in Mcl-1 expression (Fig. 7a). The antibody that we employed failed to detect the expression of Mcl-1S (short chain, a pro-apoptotic protein). For detecting Bax expression before and after curcumin treatment, immunoprecipitation was performed with an anti-Bax antibody that recognizes conformationally-altered Bax, and the resulting immunoprecipitate was analyzed by immunoblotting using non-conformation-dependent antisera specific for Bax [27, 37, 50]. As shown in Fig. 7b, while Bax expression in total cell lysates decreased with time of curcumin treatment, immunoprecipitation with the conformation-specific antibody revealed increased levels of conformationally altered Bax only in the curcumin-treated cell lysates, suggesting that a population of pro-apoptotic Bax is present as an active conformer (i.e., with the N-terminal region exposed) in curcumin-treated cells.
Fig. 7.
Effect of curcumin treatment on Bcl-2 family of proteins. Melanoma cells were treated with 75 µM curcumin for the indicated times. Cell extracts (150 µg of protein) from untreated and curcumin-treated cells were prepared as described under Section “Experimental Procedures” and analyzed by Western blot analysis. (a) Membranes were probed with anti-Mcl-1 antibody for detection of the anti apoptotic Mcl-1 protein. (b) Immunoprecipitation was performed with anti-Bax6A7 antibody that recognizes conformationally changed Bax, and the resulting immunoprecipitate was analyzed by immunoblotting using antisera specific for Bax protein
Discussion
The endoplasmic reticulum (ER) is extremely sensitive to changes that affect its structure, integrity and function. Changes in calcium homeostasis, inhibitors of protein disulfide bond formation, oxidative stress, inhibition of proteasomal activity can all disrupt protein synthesis and folding, resulting in unfolded or misfolded proteins [4, 11, 51, 52]. The cell responds by initiating a cascade of quality control signaling mechanisms (termed “ER stress response”) that restore normal ER function [51]. Several of these signaling molecules, which include the GRP family of chaperone proteins, also play critical roles in cytoprotection by triggering survival signals [4]. Cancerous cells adapt to local cellular environments by triggering protective ER stress responses [10]. Therefore, approaches to down-regulate the protective ER stress response in cancerous cells may significantly improve treatment outcome [11]. Prolonged or severe ER stress overwhelms cellular protective mechanisms, ultimately triggering cell death. ER stress-induced cell death is coupled to specific independent death pathways, as well as demonstrating cross-talk with the classic intrinsic (Apaf-dependent) and extrinsic apoptotic pathways [7–9, 53–55]. This implies that the use of small molecules to provoke a chronic ER stress-mediated cell death process may also retard tumor development, growth, and invasion, thus providing novel targets for therapeutic intervention.
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), the major yellow pigment isolated from turmeric root (curcuma longa) and commonly used as a flavoring agent in food, has drawn much attention because of its effect on several biochemical pathways. It has been demonstrated to have anti-inflammatory, antioxidant, and anti-proliferative properties [15]. Its in vivo actions include suppression of carcinogenesis of the skin, stomach, colon, breast, and liver in mice [15], and in vitro, it has been shown to inhibit the growth of a wide variety of tumor cells [14, 56]. Curcumin’s anti-cancer effects appear to be due to its ability to block the transformation, proliferation, and invasion of tumor cells by suppressing the activation of certain transcription factors and promoting the activation of cell death proteases [12, 14, 16, 17]. Similarly, topical application of curcumin normally used to accelerate wound healing [57], markedly inhibits tumor progression by blocking DNA and RNA synthesis [58–60]. Several of these effects were demonstrated using 5,000–12,000 mg of curcumin in clinical trials and up to 100 µM curcumin for in vitro studies with no obvious toxicity [61]. The advantage of using curcumin over other compounds stems from the fact that it is relatively non-toxic even at high concentrations, and can be taken orally or applied topically without causing any significant undesirable side effects [15].
Recent evidence also suggest that curcumin inhibits cellular proteasome activity thus triggering the accumulation of proteins destined for degradation [17], disrupts protein disulfide bond formation [12, 19], and also inhibits the Ca2+-ATPase activity, resulting in cytosolic Ca2+ accumulation [12, 19]. All of these events disrupt the balance between ER protein synthesis and degradation, resulting in ER stress and pcd [12]. Our data confirm that melanoma cells are sensitive to curcumin treatment at concentrations ranging from 50 to 100 µM. Elevation in the expression of the GRP family of proteins, phosphorylation of eIF2α, inhibition of cell death by salubrinal, induction of GADD 153 protein expression, and cleavage of downstream caspases including ER specific caspase-12 and caspase-7, all indicate the involvement of ER stress in triggering cell death. Blockage of GADD 153 protein expression and caspase-7 cleavage by the calcium chelator BAPTA-AM suggests that a combination of ER stress and accumulation of cytosolic Ca2+ accelerates curcumin-induced apoptosis in melanoma cells. Failure to block the induction of expression of GRP family proteins by BAPTA-AM suggests the involvement of multiple ER associated signaling pathways triggered by curcumin that finally converge on the caspase/Bcl-2 pathway to trigger melanoma cell death. The mechanism by which curcumin exhibits specific toxicity towards cancerous cells is still not clear. Our studies on a class of transformed cells (HEK293 and 3T3) indicate that these cells are resistant to curcumin treatment. The plausible explanation is that cancer cells including melanomas may be selectively vulnerable due to their inability to cope with prolonged ER stress due to the accumulation of misfolded proteins and Ca2+ leakage [10, 11]. While prolonged ER stress eliminates the protective properties of ER stress and GRP family of proteins and drives the melanoma cells to death pathways, upregulation of anti-apoptotic GRP family of proteins in 293 and 3T3 cells may actually trigger survival signaling pathways [10, 11]. The present study warrants further investigation into the anti-apoptotic roles of the GRP family of proteins and the survival pathways that they regulate.
Our earlier studies demonstrated the role of p23, a small chaperone protein, in ER stress-induced cell death [37]. Our present results suggest that in addition to PARP, p23 is also susceptible to cleavage following curcumin treatment of melanoma cells. A specific neo-epitope antibody recognizing only the cleaved fragment (19 kDa) confirmed this finding. While the significance of p23 cleavage is still not clear, it is possible that ER stress-induced cleavage of p23 resulting in the 19 kDa product abolishes the ability of p23 to act as an anti-apoptotic protein, thus rendering the melanoma cells more susceptible to pcd [37]. It is also possible that the 19 kDa cleaved product may display proapoptotic activity analogous to proteins such as Bcl-2 and Bid [62, 63].
Our results also indicate that downregulation of the anti-apoptotic Mcl-1 protein expression and increased expression of pro-apoptotic Bax facilitates curcumin-mediated cell death in mouse melanoma cells. A short splice variant of Mcl-1 mRNA encoding a protein termed Mcl-1 short was recently identified [64–66]. Furthermore, it was suggested that Mcl-1S dimerizes with Mcl-1, downregulates its expression, blocks its anti-apoptotic properties and triggers pcd [42, 44]. While we were unable to detect the expression of Mcl-1S, exposure of melanoma cells to curcumin triggered a time-dependent decrease in Mcl-1 expression that could lead to its inactivation. ER stress inducers including cellular stress inducers have also been shown to induce a change in Bax conformation resulting in its accumulation on the mitochondria and inducing the release of cytochrome c to activate the final steps of pcd [27, 50, 67]. Thus the balance between anti-apoptotic Bcl-2 family proteins (in this case Mcl-1) and pro-apoptotic Bcl-2 proteins (in this case, Bax) modulates ER—mitochondrial-dependent melanoma cell survival versus death.
In summary, the present work suggests that curcumin triggers ER stress and induces apoptosis in melanoma cells featuring the up-regulation of GRP family proteins, activation of caspases, cleavage of p23, and disruption of the balance between anti- and pro-apoptotic Bcl-2 in favor of pro-apoptotic Bax activity. Aspects of the present study that are appealing are the facts that curcumin is a commonly used food spice, it can be classified as a small molecule, it can be used for topical application, and it does not trigger serious side effects, thus making it an attractive candidate for possible treatment and prevention of melanoma.
Acknowledgements
We thank members of the Bredesen laboratory for helpful comments and discussions and Molly Susag for administrative assistance. This work was supported by grants from the National Institutes of Health (NS33376 to D.E.B. & R.V.R, AG12282 and NS45093 to D.E.B) and Elisabeth R. Levy and Family Foundation award.
Abbreviations
- ER
Endoplasmic reticulum
- pcd
Programmed cell death
- eIF2α
Eukaryotic initiation factor-2 alpha
- GRP
Glucose regulated protein
Contributor Information
Jason Bakhshi, Terra Linda High School, 320 Nova Albion Way, San Rafael, CA 94903, USA.
Lee Weinstein, Undergraduate Program, University of California, 2200 University Ave, Berkeley, CA 94720, USA.
Karen S. Poksay, The Buck Institute for Age Research, 8001 Redwood Blvd, Novato, CA 94945, USA
Brian Nishinaga, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Bronx, NY 10461, USA.
Dale E. Bredesen, The Buck Institute for Age Research, 8001 Redwood Blvd, Novato, CA 94945, USA University of California, San Francisco, CA 94143, USA.
Rammohan V. Rao, The Buck Institute for Age Research, 8001 Redwood Blvd, Novato, CA 94945, USA, e-mail: rrao@buckinstitute.org
References
- 1.Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 2.Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16:653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802. doi: 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 5.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 7.Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, Del Rio G, Bredesen DE, Ellerby HM AN Apaf-1-INDEPENDENT INTRINSIC PATHWAY. Coupling endoplasmic reticulum stress to the cell death program. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- 8.Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao RV, Hermel E, Castro-Obregon S, del Rio G, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- 10.Lee AS, Hendershot LM. ER stress and cancer. Cancer Biol Ther. 2006;5:721–722. doi: 10.4161/cbt.5.7.3120. [DOI] [PubMed] [Google Scholar]
- 11.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 12.Pae HO, Jeong SO, Jeong GS, Kim KM, Kim HS, Kim SA, Kim YC, Kang SD, Kim BN, Chung HT. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem Biophys Res Commun. 2007;353:1040–1045. doi: 10.1016/j.bbrc.2006.12.133. [DOI] [PubMed] [Google Scholar]
- 13.Bush JA, Cheung KJ, Jr, Li G. Curcumin induces apoptosis in human melanoma cells through a Fas receptor/caspase-8 pathway independent of p53. Exp Cell Res. 2001;271:305–314. doi: 10.1006/excr.2001.5381. [DOI] [PubMed] [Google Scholar]
- 14.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149–172. doi: 10.1007/978-0-387-46401-5_5. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 16.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Curr Probl Cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Jana NR, Dikshit P, Goswami A, Nukina N. Inhibition of proteasomal function by curcumin induces apoptosis through mitochondrial pathway. J Biol Chem. 2004;279:11680–11685. doi: 10.1074/jbc.M310369200. [DOI] [PubMed] [Google Scholar]
- 18.Logan-Smith MJ, East JM, Lee AG. Evidence for a global inhibitor-induced conformation change on the Ca(2+)-ATPase of sarcoplasmic reticulum from paired inhibitor studies. Biochemistry. 2002;41:2869–2875. doi: 10.1021/bi011938n. [DOI] [PubMed] [Google Scholar]
- 19.Logan-Smith MJ, Lockyer PJ, East JM, Lee AG. Curcumin, a molecule that inhibits the Ca2+-ATPase of sarcoplasmic reticulum but increases the rate of accumulation of Ca2+ J Biol Chem. 2001;276:46905–46911. doi: 10.1074/jbc.M108778200. [DOI] [PubMed] [Google Scholar]
- 20.Ellerby HM, Martin SJ, Ellerby LM, Naiem SS, Rabizadeh S, Salvesen GS, Casiano CA, Cashman NR, Green DR, Bredesen DE. Establishment of a cell-free system of neuronal apoptosis: comparison of premitochondrial, mitochondrial, and postmitochondrial phases. J Neurosci. 1997;17:6165–6178. [PMC free article] [PubMed] [Google Scholar]
- 21.Egger L, Madden DT, Rheme C, Rao RV, Bredesen DE. Endoplasmic reticulum stress-induced cell death mediated by the proteasome. Cell Death Differ. 2007;14:1172–1180. doi: 10.1038/sj.cdd.4402125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao RV, Poksay KS, Castro-Obregon S, Schilling B, Row RH, Del Rio G, Gibson BW, Ellerby HM, Bredesen DE. Molecular components of a cell death pathway activated by endoplasmic reticulum stress. J Biol Chem. 2004;279:177–187. doi: 10.1074/jbc.M304490200. [DOI] [PubMed] [Google Scholar]
- 23.Chinta SJ, Andersen JK. Reversible inhibition of mitochondrial complex I activity following chronic dopaminergic glutathione depletion in vitro: implications for Parkinson’s disease. Free Radic Biol Med. 2006;41:1442–1448. doi: 10.1016/j.freeradbiomed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 25.Liu H, Bowes RC, III, van de Water B, Sillence C, Nagelkerke JF, Stevens JL. Endoplasmic reticulum chaperones GRP78 and calreticulin prevent oxidative stress, Ca2+ disturbances, and cell death in renal epithelial cells. J Biol Chem. 1997;272:21751–21759. doi: 10.1074/jbc.272.35.21751. [DOI] [PubMed] [Google Scholar]
- 26.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 28.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 29.Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27:901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Nat Rev Mol Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 31.Tan TW, Tsai HR, Lu HF, Lin HL, Tsou MF, Lin YT, Tsai HY, Chen YF, Chung JG. Curcumin-induced cell cycle arrest and apoptosis in human acute promyelocytic leukemia HL-60 cells via MMP changes and caspase-3 activation. Anticancer Res. 2006;26:4361–4371. [PubMed] [Google Scholar]
- 32.Bae JH, Park JW, Kwon TK. Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem Biophys Res Commun. 2003;303:1073–1079. doi: 10.1016/s0006-291x(03)00479-0. [DOI] [PubMed] [Google Scholar]
- 33.Nakano T, Watanabe H, Ozeki M, Asai M, Katoh H, Satoh H, Hayashi H. Endoplasmic reticulum Ca2+ depletion induces endothelial cell apoptosis independently of caspase-12. Cardiovasc Res. 2006;69:908–915. doi: 10.1016/j.cardiores.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 34.Bartlett JD, Luethy JD, Carlson SG, Sollott SJ, Holbrook NJ. Calcium ionophore A23187 induces expression of the growth arrest and DNA damage inducible CCAAT/enhancer-binding protein (C/EBP)-related gene, gadd153. Ca2+ increases transcriptional activity and mRNA stability. J Biol Chem. 1992;267:20465–20470. [PubMed] [Google Scholar]
- 35.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 36.Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 37.Rao RV, Niazi K, Mollahan P, Mao X, Crippen D, Poksay KS, Chen S, Bredesen DE. Coupling endoplasmic reticulum stress to the cell-death program: a novel HSP90-independent role for the small chaperone protein p23. Cell Death Differ. 2006;13:415–425. doi: 10.1038/sj.cdd.4401761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Germain M, Shore GC. Cellular distribution of Bcl-2 family proteins. Sci STKE. 2003;2003:pe10. doi: 10.1126/stke.2003.173.pe10. [DOI] [PubMed] [Google Scholar]
- 39.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 40.Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 42.Yeh TC, Chiang PC, Li TK, Hsu JL, Lin CJ, Wang SW, Peng CY, Guh JH. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol. 2007;73:782–792. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Koshikawa N, Maejima C, Miyazaki K, Nakagawara A, Takenaga K. Hypoxia selects for high-metastatic Lewis lung carcinoma cells overexpressing Mcl-1 and exhibiting reduced apoptotic potential in solid tumors. Oncogene. 2006;25:917–928. doi: 10.1038/sj.onc.1209128. [DOI] [PubMed] [Google Scholar]
- 44.Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian T, Vijayalingam S, Lomonosova E, Zhao LJ, Chinnadurai G. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J Virol. 2007;81:10486–10495. doi: 10.1128/JVI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiang PC, Kung FL, Huang DM, Li TK, Fan JR, Pan SL, Shen YC, Guh JH. Induction of Fas clustering and apoptosis by coral prostanoid in human hormone-resistant prostate cancer cells. Eur J Pharmacol. 2006;542:22–30. doi: 10.1016/j.ejphar.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–688. doi: 10.1042/0300-5127:0290684. [DOI] [PubMed] [Google Scholar]
- 48.Liu FT, Newland AC, Jia L. Bax conformational change is a crucial step for PUMA-mediated apoptosis in human leukemia. Biochem Biophys Res Commun. 2003;310:956–962. doi: 10.1016/j.bbrc.2003.09.109. [DOI] [PubMed] [Google Scholar]
- 49.Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 52.Feldman DE, Chauhan V, Koong AC. The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 53.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 55.Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and A{beta}-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–480. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 57.Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006;290:87–96. doi: 10.1007/s11010-006-9170-2. [DOI] [PubMed] [Google Scholar]
- 58.Huang MT, Ma W, Yen P, Xie JG, Han J, Frenkel K, Grunberger D, Conney AH. Inhibitory effects of topical application of low doses of curcumin on 12-O-tetradecanoylphorbol-13-acetateinduced tumor promotion and oxidized DNA bases in mouse epidermis. Carcinogenesis. 1997;18:83–88. doi: 10.1093/carcin/18.1.83. [DOI] [PubMed] [Google Scholar]
- 59.Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extra-cellular signal-regulated kinase activity and NF-kappaB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- 60.Huang MT, Newmark HL, Frenkel K. Inhibitory effects of curcumin on tumorigenesis in mice. J Cell Biochem Suppl. 1997;27:26–34. [PubMed] [Google Scholar]
- 61.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, Ko JY, Lin JT, Lin BR, Ming-Shiang W, Yu HS, Jee SH, Chen GS, Chen TM, Chen CA, Lai MK, Pu YS, Pan MH, Wang YJ, Tsai CC, Hsieh CY. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 62.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 63.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 64.Le Gouill S, Podar K, Harousseau JL, Anderson KC. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle. 2004;3:1259–1262. doi: 10.4161/cc.3.10.1196. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J, D’Ercole AJ. Expression of Mcl-1 in cerebellar granule neurons is regulated by IGF-I in a developmentally specific fashion. Brain Res Dev Brain Res. 2004;152:255–263. doi: 10.1016/j.devbrainres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 66.Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 67.Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, Banares S, Cui J, Digicaylioglu M, Ke N, Kitada S, Monosov E, Thomas M, Kress CL, Babendure JR, Tsien RY, Lipton SA, Reed JC. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]