Abstract
The phosphorylated inositol diphosphates, including the diphosphoinositol pentakisphosphate regioisomers, play critical roles in signal-transduction and cellular regulation. In particular, the IP7 isomer 5-PP-Ins(1,2,3,4,6)P5 is implicated in a non-enzymatic phosphate transfer converting a protein serine phosphate residue to a serine diphosphate. A scalable, practical new synthesis of 5-PP-Ins(1,2,3,4,6)P5 is described that also allows access to a variety of IP7 and IP8 regioisomers. The identity of the synthetic 5-PP-Ins(1,2,3,4,6)P5 was validated using IP6K1 to catalyze the conversion of IP7 + ADP to ATP + IP6.
Inositides, particulary inositol phosphates and phosphatidylinositol lipids are crucially important cell signaling molecules that are linked to a series of signaling events.1–3 These signaling events include ion-channel function,4,5 vesicle trafficking,6 apoptosis,7,8, transcriptional regulation,9 motility,10 cell proliferation and transformation.11 Inositol 1,4,5-trisphosphate (IP3) is a ubiquitous second messenger, which couples agonist stimulation of a wide variety of receptors to the mobilization of intracellular calcium.12 The more highly phosphorylated inositides, particularly the “omnipotent” inositol hexakisphosphate13 and phosphorylated inositol diphosphates (commonly called pyrophosphates in the biological literature), play critical roles in signal-transduction and cellular regulation.2,6
In 1993, two inositol diphosphates, diphosphoinositol pentakisphosphate (5-PP-Ins(1,2,3,4,6)P5, or IP7) and bisdiphosphoinositol tetrakisphosphate (PP2-InsP4, or IP8), were purified from Dictyostelium.6,14 The diphosphate bond of the 5-PP-Ins(1,2,3,4,6)P5 has a calculated phosphorylation potential that equals or exceeds that of ATP,6,15 suggesting that it could serve a similar function. 5-PP-Ins(1,2,3,4,6)P5 is biosynthesized from inositol hexakisphosphate (IP6) by a family of three inositol hexakisphosphate kinases (InsP6Ks).16–18 Inositol hexakisphosphate kinase-2 (InsP6K2), one of the InsP6Ks, was found to be an important physiologic mediator of cell death. More recent studies revealed that IP7 plays an important role in regulation of insulin secretion.19–22 Recently, fluorometric detection of IP7 in the presence of IP6 and lower IPn congeners was achieved by the Matile lab using a synthetic multifunctional pore.23 A different regioisomer of IP7 is produced by yeast Vip1 and is necessary for Pho81 cyclic-dependent kinase (CDK) inhibition of the the cyclin-CDK complex Pho80-Pho85, thereby regulating an important metabolic network.24,25 Although first identified24 as 4(6)-PP-InsP5, this was recently revised26 to 1(3)-PP-InsP5, a result that has been independently validated and extended by synthesis and assay of the enantiopure 1-PP and 3-PP-InsP5 isomers.27
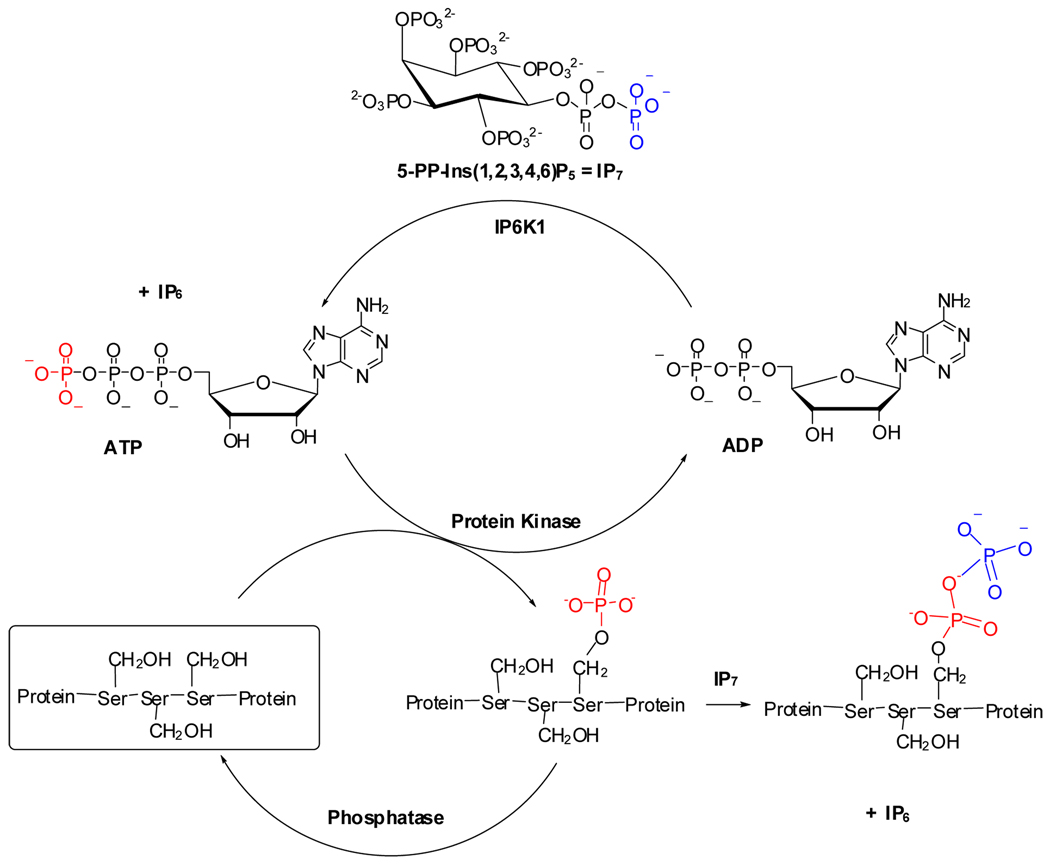
The reversible phosphorylation of proteins regulates nearly every aspect of cell physiology.28 Phosphorylation and dephosphorylation, catalyzed by protein kinases29 and protein phosphatases, is depicted in Figure 1. Some 30% of the proteins encoded by the human genome are phosphorylated, and abnormal phosphorylation is now recognized as a cause or a consequence of many human pathologies. As a result, protein kinases are already the second largest group of drug targets after G-protein-coupled receptors, and they account for 20–30% of the drug discovery programs of many companies.30
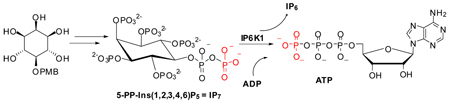
Figure 1.
Reversible protein phosphorylation and pyrophosphorylation mechanism.
Unlike ATP, however, the inositol diphosphate IP7 appears to phosphorylate serine phosphate residues non-enzymatically (Figure 1).31 5-PP-Ins(1,2,3,4,6)P5 has been demonstrated to phosphorylate a variety of Ser-rich protein targets in yeast and mammals.15 The resulting 5-PP-Ins(1,2,3,4,6)P5-phosphorylated peptides are more acid-labile and more resistant to phosphatases, suggesting that a protein diphosphate bond had been formed (Figure 1).31 Moreover, only the Ser-rich regions of target proteins that had been previously phosphorylated by a protein kinase were substrates, strongly implicated Ser-PP, a serine diphosphate (pyrophosphate), as the product of the non-enzymatic diphosphorylation. This diphosphorylation may represent a novel mode of signaling.31 This diphosphorylation may represent a new fundamental signaling mechanism, but its study in many cases is limited by the availability of 5-PP-Ins(1,2,3,4,6)P5 and a family of chemical and biological tools to probe the structure and function of diphosphorylated proteins. To address this unmet need, we describe herein a scalable and efficient new method for the synthesis of 5-PP-Ins(1,2,3,4,6)P5 based on modifications of previous inositide syntheses by the laboratories of Prestwich31 and Falck.32,33
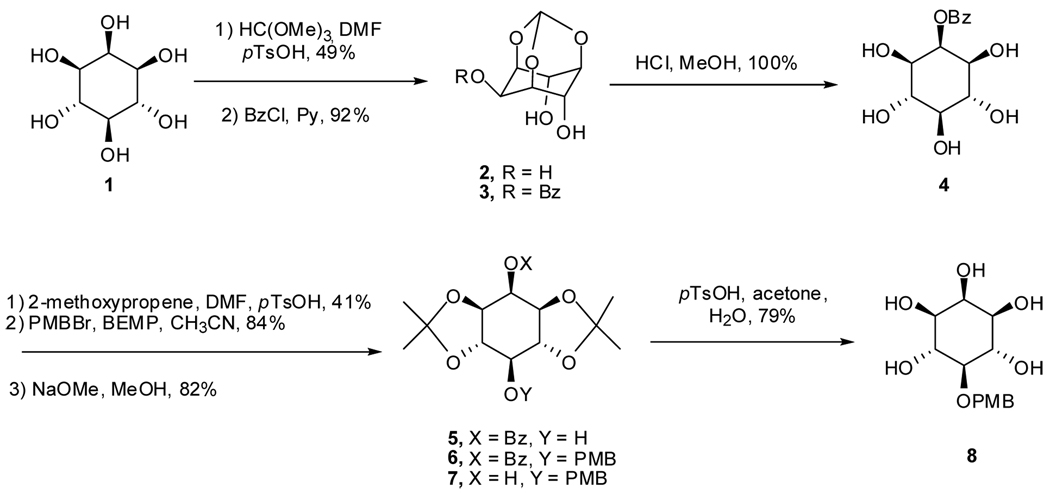
The three key problems in the synthesis of any IP7 stereoisomer are (i) the method of stereoselective introduction of the protected 5-diphosphate, (ii) the stability of the protected diphosphorylated intermediate(s), and (iii) the complete removal of protecting groups under mild conditions with minimal degradation of the desired IP7. We selected the synthetic route as shown in Scheme 1 to prepare the key intermediate 8. It is important to note that the starting materials, intermediates, and final products are all formally meso compounds, since the 5-diphosphate will be positioned on the C-2-C-5 plane of symmetry. Following known procedures, myo-inositol 1,3,5-orthoformate 2, available in one step from myo-inositol,34 was selectively benzoylated at 2-OH with benzoyl chloride.35 Hydrolysis of orthoformate 2 in 4 M HCl/MeOH afforded benzoate 4,35 which was converted to the acetonide 5 as a mixture with other isomers.36 Compound 5 was easily separated from other isomers by flash chromatography. Introduction of the 4-methoxybenzyl ether was unexpectedly problematic. Under standard benzylation conditions (NaH or nBuLi, 4-MeOBnCl or 4-MeOBnBr, THF or DMF) or phase-transfer catalysis conditions (4-MeOBnCl, Bu4NHSO4, aqueous NaOH), starting material was converted to complex product mixtures. Nonetheless, reaction of 5 with 4-methoxybenzyl bromide in the presence of the hindered organic base, 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine (BEMP)37 in CH3CN afforded the fully protected inositol 6 in 84% yield. Treatment benzoate 6 with NaOMe gave 2-OH inositol 7 in 82% yield, and hydrolysis of the acetonides with pTsOH in acetone/H2O, gave the 5-PMB protected inositol 8 in 79% yield. Since 8 was poorly soluble in EtOAc and CH2Cl2, purification was accomplished simply by trituration of the white solid with these two solvents.
Scheme 1.
Synthesis of advanced intermediate 8
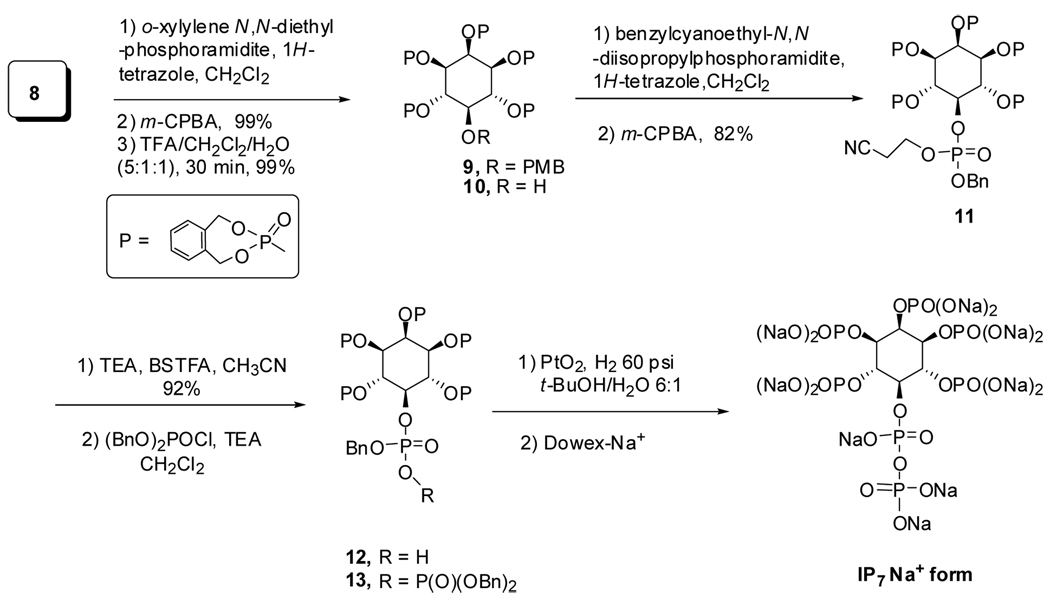
With the advanced intermediate 5-PMB inositol in hand, different phosphoramidites coupling with compound 8 would generate different phosphates. Considering the stability of the IP7, dimethyl phosphate and diethyl phosphate could not be used as protecting groups because the use of TMSBr to remove these protecting groups could cause decomposition of diphosphate moiety. The dibenzyl phosphate32,33 and o-xylylene phosphate are the best candidates because they are readily removed by hydrogenolysis. In addition, since the o-xylylene phosphate is sterically smaller than dibenzyl phosphate, we expected that the o-xylylene phosphate intermediate 13 would be more stable in this highly sterically congested intermediate. Thus, the pentakis(o-xylylene phosphate) 9 was prepared in 99% yield by global phosphorylation of pentaol 8 with o-xylylene N,N-diethylphosphoramidite and 1H-tetrazole in CH2Cl2. The 4-methoxybenzyl functional group was removed with TFA/CH2Cl2/H2O (5:1:1, v/v) to furnish alcohol 10.
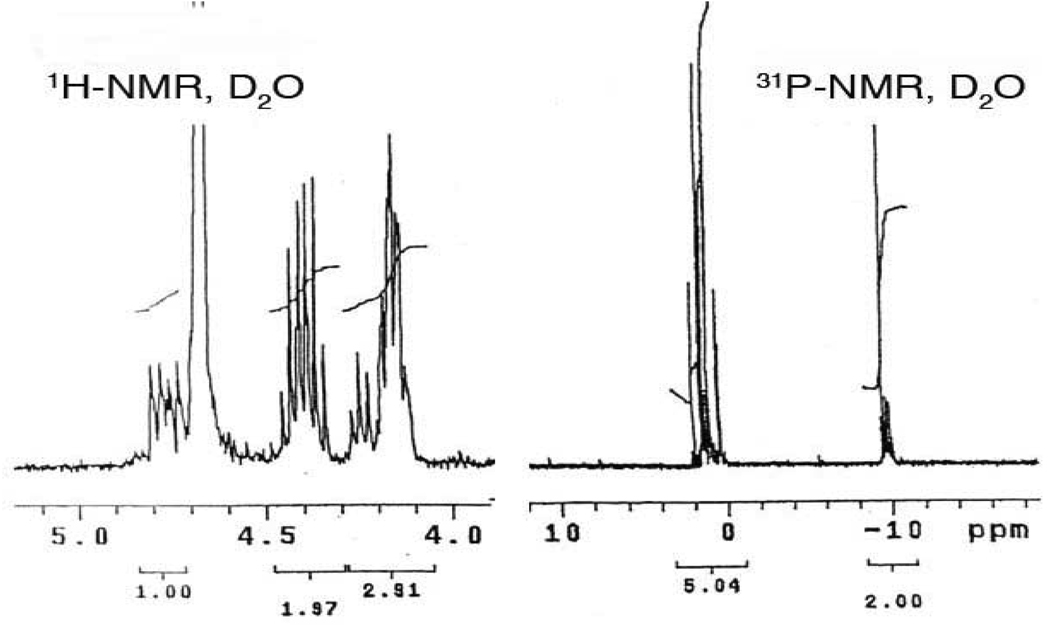
Here again, our route diverges from previous methods. The unsymmetrical phosphoramidite, benzyl-2-cyanoethyl N,N-diisopropylphosphoramidite, was prepared in 86% yield from commercial 2-cyanoethyl N,N-diisopropyl-chlorophosphoramidite and benzyl alcohol in the presence of Hunig’s base. Reaction of 10 with this phosphoramidite in the presence of 1H-tetrazole, followed by m-CPBA oxidation generated the differentially protected hexakisphosphate 11. Selective removal of the cyanoethyl group under mild conditions (TEA/BSTFA/CH3CN)38–40 and purification by Dowex-H+ chromatography afforded pure monophosphoric acid 12 in 92% yield (Scheme 2). After treatment of 12 (1 equiv) with triethylamine (2 equiv) in anhydrous CH2Cl2 at 0 °C followed by dibenzylphosphoryl chloride (2 equiv) for 2 h at rt, solvents were removed to stop the reaction and give a crude product. The crude residue 13 was then deprotected by stirring an aqueous t-butanol solution under H2 (60 psi) with PtO2 for 4 h. After removal of the catalyst and concentration, the residue was dissolved in water and washed with EtOAc and CH2Cl2. The aqueous solution was concentrated and purified through ion exchange chromatography (Dowex 50W × 8 − 200 Na+ exchange resin) by elution with water to afford the 5-PP-Ins(1,2,3,4,6)P5 sodium form in 81% yield as white solid. The 31P NMR spectrum of 5-PP-Ins(1,2,3,4,6)P5 in D2O displayed a characteristic pyrophosphate peak, at −9.40 to −10.20 ppm, integrating at a 2.0 : 5.04 ratio relative to the monophosphate resonances between 0.2 and 1.2 ppm (Figure 2). The complexity of the signals reflects a distribution of sodium salt and protonated species each with characteristic chemical shifts. Similarly, the key feature of the complex proton spectrum is the 1.0 : 4.88 ratio of peaks for H-5 : H1–4, H6. Both 1H NMR and 31P NMR were consistent with the 5-PP-Ins(1,2,3,4,6)P5 isolated from Polysphondylium.41
Scheme 2.
Global phosphorylation and final conversion to 5-PP-Ins(1,2,3,4,6)P5
Figure 2.
1H NMR and 31P NMR of 5-PP-InsP5.
In this new synthetic route, intermediate 5 could be readily prepared in 10 g amounts in four steps, including removal of stereoisomers, in 18% yield from inexpensive commercially available materials. With this known material, the final 5-PP-InsP5 was prepared in 33% overall yield for seven steps. Importantly, the o-xylylene phosphate 13 was more stable than the globally benzyl-protected 5-PP-InsP5 intermediate, which only gave a 25% yield for the final three steps described by us previously.31
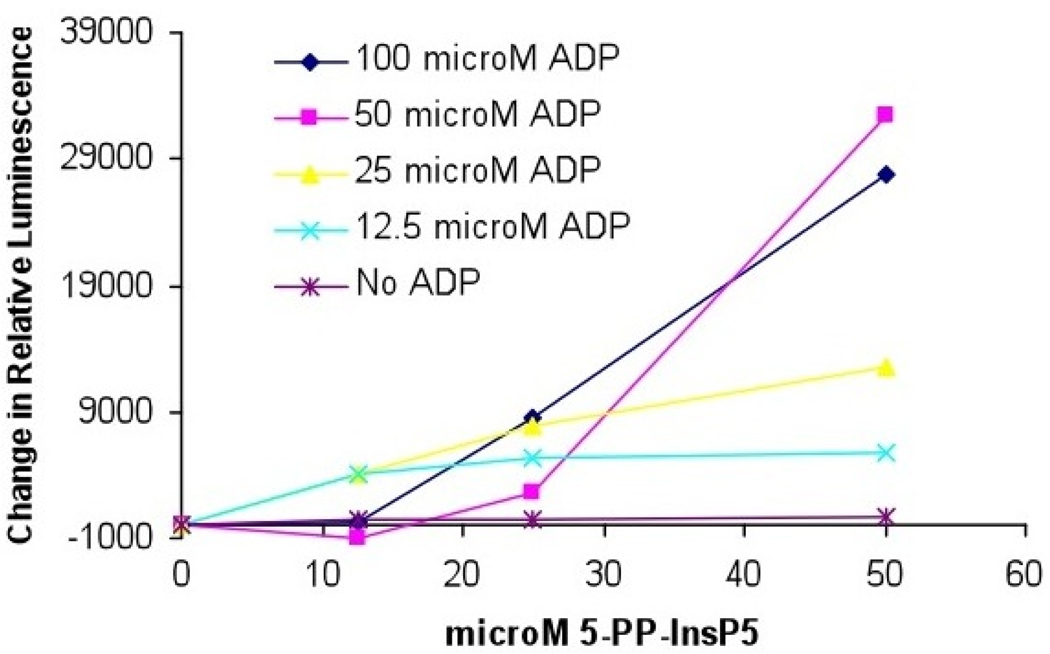
To validate the biological activity of the synthetic 5-PP-Ins(1,2,3,4,6)P5, we used it as a substrate to phosphorylate ADP. Inositol hexakisphosphate kinase 1 (IP6K1) not only catalyzes the transfer of a phosphate from ATP to IP6 yielding 5-PP-Ins(1,2,3,4,6)P5 plus ADP, but readily catalyzes the reverse reaction in which a phosphate is transferred from 5-PP-Ins(1,2,3,4,6)P5 to ADP yielding ATP and IP6.42 Thus, synthetic 5-PP-Ins(1,2,3,4,6)P5 was incubated with ADP and recombinant IP6K1, and ATP synthase activity was monitored using the activity of the enzyme luciferase. An increase in ATP, resulting from its production from 5-PP-Ins(1,2,3,4,6)P5 plus ADP, was quantified by measuring the luciferase catalyzed conversion of luciferol to oxyluciferol (Figure 3). Synthetic 5-PP-Ins(1,2,3,4,6)P5 may be used as a substrate for ATP synthesis in a reaction catalyzed by IP6K1.
Figure 3.
Effects of the concentration of ADP and synthetic 5-PP-Ins(1,2,3,4,6)P5 on ATP production by IP6K1.
To ensure IP7 was not directly phosphorylating ADP to ATP without the enzyme IP6K1, the luciferase reaction was repeated without the addition of the enzyme IP6K1. In this case, no increase in luciferase activity was observed, indicating IP6K1 is indeed required for ATP synthase activity (Supporting Information, Figures 1 and 2). Additionally, incubation of 5-PP-Ins(1,2,3,4,6)P5 with ATP and the enzyme luciferase results in decreased conversion of luciferin to oxyluciferin indicating 5-PP-Ins(1,2,3,4,6)P5 may inhibit the luciferase enzyme (Supporting Information, Figure 1).
In conclusion, we have developed an efficient and scalable method for the synthesis of the important IP7 regioisomer 5-PP-Ins(1,2,3,4,6)P5, which will meet the demand of cell signaling scientists in the studies of protein pyrophosphorylation, exocytosis in pancreatic beta cells, and other key cellular processes. In addition, this new synthetic approach method can be adapted to the chemical synthesis of a variety of regioisomers of IP8, the bisdiphosphoinositol tetrakisphosphates (PP2-InsP4), which have recognized importance in cell signaling research.
Supplementary Material
Acknowledgment
We thank the NIH for financial support (NS 29632 to GDP).
Footnotes
Supporting Information Available: Experimental details for synthesis, characterization of new compounds; experimental methods for IP6K1 catalyzed reaction figures for enzyme-free experiment. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.McLaughlin S, Murray D. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 2.Irvine RF, Schell MJ. Nat. Rev. Mol. Cell. Biol. 2001;2:327–338. doi: 10.1038/35073015. [DOI] [PubMed] [Google Scholar]
- 3.Toker A, Cantley LC. Nature. 1997;387:673–676. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 4.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 5.Rohacs T, Lopes CM, Michailidis I, Logothetis DE. Nat. Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- 6.Bennett M, Onnebo SM, Azevedo C, Saiardi A. Cell. Mol. Life. Sci. 2006;63:552–564. doi: 10.1007/s00018-005-5446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. J. Biol. Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagata E, Luo HR, Saiardi A, Bae BI, Suzuki N, Snyder SH. J. Biol. Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 9.Shears SB. Bioessays. 2000;22:786–789. doi: 10.1002/1521-1878(200009)22:9<786::AID-BIES3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F. Curr. Biol. 2006;16:140–149. doi: 10.1016/j.cub.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 11.Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ, Lipp P, Bootman MD. Nat. Rev. Mol. Cell. Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 13.Shears SB. Cell Signal. 2001;13:151–158. doi: 10.1016/s0898-6568(01)00129-2. [DOI] [PubMed] [Google Scholar]
- 14.Bhandari R, Chakraborty A, Snyder SH. Cell. Metab. 2007;5:321–323. doi: 10.1016/j.cmet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 16.Schell MJ, Letcher AJ, Brearley CA, Biber J, Murer H, Irvine RF. FEBS. Lett. 1999;461:169–172. doi: 10.1016/s0014-5793(99)01462-3. [DOI] [PubMed] [Google Scholar]
- 17.Saiardi A, Nagata E, Luo HR, Snowman AM, Snyder SH. J. Biol. Chem. 2001;276:39179–39185. doi: 10.1074/jbc.M106842200. [DOI] [PubMed] [Google Scholar]
- 18.Saiardi A, Erdjument-Bromage H, Snowman AM, Tempst P, Snyder SH. Curr. Biol. 1999;9:1323–1326. doi: 10.1016/s0960-9822(00)80055-x. [DOI] [PubMed] [Google Scholar]
- 19.Illies C, Gromada J, Fiume R, Leibiger B, Yu J, Juhl K, Yang SN, Barma DK, Falck JR, Saiardi A, Barker CJ, Berggren PO. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 20.Nagamatsu S, Ohara-Imaizumi M. Science. 2007;318:1249–1250. doi: 10.1126/science.1151361. [DOI] [PubMed] [Google Scholar]
- 21.Barker CJ, Leibiger IB, Leibiger B, Berggren PO. Am. J. Physiol. Endocrinol. Metab. 2002;283:E1113–E1122. doi: 10.1152/ajpendo.00088.2002. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari R, Juluri KR, Resnick AC, Snyder SH. Proc. Natl. Acad. Sci. USA. 2008;105:2349–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butterfield SM, Tran D, Zhang H, Prestwich GD, Matile S. J. Am. Chem. Soc. 2008;130:3270–3271. doi: 10.1021/ja710186e. [DOI] [PubMed] [Google Scholar]
- 24.Mulugu S, Bai W, Fridy P, Bastidas R, Otto J, Dollins D, Haystead T, Ribeiro A, York J. Science. 2008;316:106–109. doi: 10.1126/science.1139099. [DOI] [PubMed] [Google Scholar]
- 25.Lee Y-S, Mulugu S, York J, O'Shea E. Science. 2008;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin H, Fridy P, Ribeiro A, Choi J, Barma D, Vogel G, Falck J, Shears S, York J, Mayr G. J. Biol. Chem. 2009;284:1863–1872. doi: 10.1074/jbc.M805686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Zhang H, Lee Y-S, O'Shea E, Prestwich GD. 2009 submitted for publication. [Google Scholar]
- 28.Cohen P. Nat. Cell. Biol. 2002;4:E127–E130. doi: 10.1038/ncb0502-e127. [DOI] [PubMed] [Google Scholar]
- 29.Shears SB. Biochem. J. 2004;377:265–280. doi: 10.1042/BJ20031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen P. Nat. Rev. Drug. Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 31.Bhandari R, Saiardi A, Ahmadibeni Y, Snowman AM, Resnick AC, Kristiansen TZ, Molina H, Pandey A, Werner JK, Jr, Juluri KR, Xu Y, Prestwich GD, Parang K, Snyder SH. Proc. Natl. Acad. Sci. USA. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Falck JR, Reddy KK, Ye J, Saady M, Mioskowski C, Shears SB, Tan Z, Safrany S. J. Am. Chem. Soc. 1995;117:12172–12175. [Google Scholar]
- 33.Reddy KM, Reddy KK, Falck JR. Tetrahedron. Lett. 1997;38:4951–4952. [Google Scholar]
- 34.Lee HW, Kishi Y. J. Org. Chem. 1985;50:4402–4404. [Google Scholar]
- 35.Ozaki S, Koga Y, Ling L, Watanabe Y, Kimura Y, Hirata M. Bull. Chem. Soc. Jpn. 1994;67:1058–1063. [Google Scholar]
- 36.Chung S-K, Chang Y-T, Kwon Y-U. J. Carbohydr. Chem. 1998;17:369–384. [Google Scholar]
- 37.Gotfredsen CH, Jacobsen JP, Wengel J. Tetrahedron Lett. 1994;35:6941–6944. [Google Scholar]
- 38.Zhang H, Xu Y, Markadieu N, Beauwens R, Erneux C, Prestwich GD. Bioorg. Med. Chem. Lett. 2008;18:762–766. doi: 10.1016/j.bmcl.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Markadieu N, Beauwens R, Erneux C, Prestwich GD. J. Am. Chem. Soc. 2006;128:16464–16465. doi: 10.1021/ja065002j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang W, Zhang H, Davrazou F, Kutateladze TG, Shi X, Gozani O, Prestwich GD. J. Am. Chem. Soc. 2007;129:6498–6506. doi: 10.1021/ja070195b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laussmann T, Hansen A, Reddy KM, Reddy KK, Falck JR, Vogel G. FEBS. Lett. 1998;426:145–150. doi: 10.1016/s0014-5793(98)00329-9. [DOI] [PubMed] [Google Scholar]
- 42.Voglmaier SM, Bembenek ME, Kaplin AI, Dormán G, Olszewski JD, Prestwich GD, Snyder SH. Proc. Natl. Acad. Sci. USA. 1996;93:4305–4310. doi: 10.1073/pnas.93.9.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.