Abstract
Background
The prognosis for patients with breast tumor metastases to brain is extremely poor. Identification of prognostic molecular markers of the metastatic process is critical for designing therapeutic modalities for reducing the occurrence of metastasis. Although ubiquitously present in most human organs, large-conductance calcium- and voltage-activated potassium channel (BKCa) channels are significantly upregulated in breast cancer cells. In this study we investigated the role of KCNMA1 gene that encodes for the pore-forming α-subunit of BKCa channels in breast cancer metastasis and invasion.
Methods
We performed Global exon array to study the expression of KCNMA1 in metastatic breast cancer to brain, compared its expression in primary breast cancer and breast cancers metastatic to other organs, and validated the findings by RT-PCR. Immunohistochemistry was performed to study the expression and localization of BKCa channel protein in primary and metastatic breast cancer tissues and breast cancer cell lines. We performed matrigel invasion, transendothelial migration and membrane potential assays in established lines of normal breast cells (MCF-10A), non-metastatic breast cancer (MCF-7), non-brain metastatic breast cancer cells (MDA-MB-231), and brain-specific metastatic breast cancer cells (MDA-MB-361) to study whether BKCa channel inhibition attenuates breast tumor invasion and metastasis using KCNMA1 knockdown with siRNA and biochemical inhibition with Iberiotoxin (IBTX).
Results
The Global exon array and RT-PCR showed higher KCNMA1 expression in metastatic breast cancer in brain compared to metastatic breast cancers in other organs. Our results clearly show that metastatic breast cancer cells exhibit increased BKCa channel activity, leading to greater invasiveness and transendothelial migration, both of which could be attenuated by blocking KCNMA1.
Conclusion
Determining the relative abundance of BKCa channel expression in breast cancer metastatic to brain and the mechanism of its action in brain metastasis will provide a unique opportunity to identify and differentiate between low grade breast tumors that are at high risk for metastasis from those at low risk for metastasis. This distinction would in turn allow for the appropriate and efficient application of effective treatments while sparing patients with low risk for metastasis from the toxic side effects of chemotherapy.
Background
A significant number of patients in the U.S with metastatic brain tumors face a dismal prognosis and high mortality. Increasing numbers of breast cancer patients are being diagnosed with brain metastases, possibly as a result of the emergence of targeted and aggressive systemic cancer therapy. In overall frequency, breast cancers and lung cancers are by far the most common cancers that metastasize to brain [1,2]. Brain metastasis generally arises in women diagnosed with aggressive breast cancer or in men with advanced lung cancer. The actual incidence of brain metastases is not precisely known, however studies suggest that 6–16% of patients with metastatic breast cancer develop brain metastases during their lifetime. Furthermore, autopsy studies have reported brain metastases in 18–30% of patients dying ofbreast cancer [3]. The majority of women who develop brain metastases have presented with debilitating neurological symptoms, and have undergone aggressive treatment for stage IV disease [4-6]. Although brain metastasis is the leading cause of breast cancer death, its pathogenesis is poorly understood and the predictors of breast metastasis to brain are yet to be characterized.
Accumulating evidence suggests that human epidermal growth factor receptor 2 (HER2) overexpression and consequent trastuzumab (Herceptin)-based therapy might be associated with a higher rate of brain metastases [7]. Retrospective studies in the U.S. in women with HER2 over-expressing breast cancer receiving trastuzumab-based treatment have indicated that approximately one-third of patients had developed brain metastases [7-9]. Palmeri et al. suggested that HER2 overexpression increases metastatic outgrowth of breast cancer cells in the brain [10], and that HER2 overexpression might be a predictor of asymptomatic, occult brain cancer. Therefore, it is extremely important to study the genetic changes in breast cancer cells that lead to brain metastasis and to develop specific targeted molecular agents. Many genetic aberrations are reported in human breast cancers, including altered splice variants and loss of genomic imprinting. Specifically, altered BKCa channels, which respond to changes in intracellular calcium and membrane potential, are described in a wide variety of tumor cell types. For example, Huang [11] reported that p21ras plays a pivotal role in controlling oncogenic transformation [12], and with its immediate downstream target, Raf kinase, is required for the induction of BKCa channels. Although ubiquitously present in most human organs, the pore forming α-subunit of the BKCa channel is encoded by the KCNMA1 gene, and is significantly unregulated and altered in cancer cells. In human glioma cells, up-regulation and constitutive activation of the KCNMA1 gene and its alternate splice variants are correlated with increased malignancy [13]. Up-regulation of BKCa channels was shown to be a novel mechanism for the malignant phenotype of human tumor cells [14]. However, in osteosarcoma, KCNMA1 was shown to have antitumor property, suggesting that KCNMA1 may have diverse roles in different tumor types [15].
In the present study, our results from global exon array analysis showed higher expression of KCNMA1 in metastatic breast cancers located in brain than in metastatic breast cancers in other organs. This observation prompted us to investigate the potential for invasiveness and brain metastasis of KCNMA1 expression in normal breast cells (MCF-10A), non-metastatic breast cancer (MCF-7), non-brain metastatic breast cancer cells (MDA-MB-231), and brain specific metastatic breast cancer cells (MDA-MB-361). Our results clearly show that metastatic breast cancer cells exhibit increased BKCa channel activity, leading to greater invasion and transendothelial migration. We showed that the invasion and transendothelial migration of breast cancer cells can be attenuated by blocking BKCa channel activity. Therefore, determining the relative abundance of KCNMA1 in breast cancer metastases to brain and understanding its role in brain metastasis may allow us to target KCNMA1 for its prognostic and possibly therapeutic potential.
Methods
Cell lines and patient tissue samples
Established cell lines representing normal breast (MCF-10A), non-metastatic breast cancer (MCF-7, ER positive), non-brain metastatic breast cancer (MDA-MB-231, ER negative), and brain-specific metastatic breast cancer (MDA-MB-361, ER positive) were obtained from the American Type Culture Collection (Manassas, VA) and maintained as per ATCC guidelines. The established human brain microvasculature endothelial cell line (HCMEC/D3) of normal brain endothelial phenotype, kindly provided by Dr. Weksler (Weill Medical College, Cornell University, NY), was used to study transendothelial migration of the breast cancer cell lines [16]. Normal breast and breast tumor tissues were obtained from Memorial Health University Medical Center (MHUMC) and Comprehensive Human Tissue Network, University of Alabama according to the protocol approved by MHUMC Institutional Review Board.
Exon Array
Total RNA from exponentially growing breast cancer cell lines was extracted and purified using TRIzol reagent (Invitrogen) and RNeasy columns (Qiagen). Single-stranded cDNA was generated from the amplified cRNA with the WT cDNA Synthesis Kit (Affymetrix), fragmented and labeled with the WT Terminal Labeling Kit (Affymetrix). Samples were hybridized with GeneChip Human Exon 1.0 ST Arrays (Affymetrix) and scanned at the Memorial Health University Medical Center Genomics Core Facility. Intensity values were deposited with the GEO database http://www.ncbi.nlm.nih.gov/geo under the accession number GSE17019. Data was subjected to background correction and normalized using Expression Console (Affimetrix). Gene level expression values (probeset intensity analysis) were derived using RMA algorithm using core annotation level for transcripts. Exon array data was further analyzed using Partek Genomic Suite 6.4 software (Partek Inc., St. Louis, MO). Gene-level data was then filtered to include only those probesets that are in the 'core' meta-probe list, which represents RefSeq genes and full-length GenBank mRNAs. Analysis of Variance (ANOVA) and multi test correction for P-values in Partek Genomic Suite were used to identify differentially expressed genes, using tissue type (primary versus metastatic tissue) as the candidate variable in the ANOVA model to obtain metastatic specific genes.
RNA extraction and PCR
Total RNA was extracted from cells which were 75% confluent and from the frozen tissues disrupted using a dounce homogenizer and extracted with TRIzol. The concentration of RNA was determined using a NanoDrop Spectrophotometer (NanoDrop Technologies, USA). cDNA for RT-PCR was generated by the SuperScript™ First-Strand Synthesis System according to manufacturer's instructions (Invitrogen). PCR was carried out in a total volume of 20 μl, containing 0.2 mM dNTPs, 1 mM MgCl2 and 1 Unit of AmpliTaq Gold DNA Polymerase (Applied Biosystem). Thirty two amplification cycles were performed (Applied Biosystem Thermal Controller), using a denaturing temperature of 95°C for 20 sec, an annealing temperature of 58°C for 30 seconds, and primer extension at 72°C for 20 sec. Each amplification experiment also included two negative PCR controls, a no-RNA control from reverse transcription procedures and a no-cDNA water control. Following amplification, 20 μl of the samples were separated via electrophoresis on a 2% agarose gel. The primers used for amplification of BKCa channel were – (5'-TGCAAAGGAG GTTATAAAGTTACG-3' and 5'-ATTTCACAAAAGTTTTCACAAGGAC-3').
BKCa channel expression and localization in cell lines
Immunostaining of BKCa channel protein was performed on paraformaldehyde fixed cells, which were permeabilized with 0.05% Triton-X 100 in Phosphate buffer saline (PBS) for 8 min on ice. After washes with PBS, cells were incubated with primary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:400 in PBS containing 1% horse serum, 2% bovine serum albumin (BSA), and 0.05% Triton-X 100 for 30–45 minutes at 4°C. Cells were then washed with PBS containing 1% horse serum and 2% BSA, and incubated with fluorescein isothiocyanate (FITC)-labeled goat anti-rabbit IgG1 secondary antibody (Sigma) diluted 1:500 in PBS containing 1% horse serum, 2% BSA, and 0.05% Triton-X 100 for 60 min at 4°C. BKCa channel green (FITC) fluorescence was recorded for a minimum of 5,000 events using a Guava flow cytometer. Images for localization of BKCa channel protein were acquired using a Nikon Eclipse TE 2000U inverted microscope fitted with a fluorescence filter for FITC, Photometrics coolsnap HQ2 charge couple device camera, and Metamorph software.
BKCa channel expression and localization in tumor tissues
To test immunohistochemical expression of BKCa channel α subunit protein, either fresh-frozen or paraffin-embedded tissues (breast metastasis to brain and one primary breast cancer tissue) were used. Fresh-frozen tumor specimens stored at -80°C were embedded in OCT (Tissue-Tek, Miles Laboratories, Elkhart, IN). Cryostat sections were cut at 10–12 μm thickness, mounted on lysine-coated glass slides, and fixed for 10 min at 4°C in 4% formaldehyde. This step was followed by a 5 minute rinse with PBS at pH 7.3. Slides were then blocked in blocking buffer (1% horse serum, 2% BSA, and 0.05% Triton-X 100) for 1 hour at 4°C to decrease nonspecific IgG binding. The sections were next incubated in a humid chamber at 4°C overnight with the primary antibodies, anti-BKCa channel (rabbit) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:1000 in blocking buffer, or with PBS as a negative control. After 2 washes with PBS, the slides were stained with anti rabbit FITC conjugated secondary antibody.
To study BKCa channel immunoreactivity in paraffin-embedded tumor specimens, sections were deparaffinized with three washes in xylene for 5 minutes each followed by rehydration with decreasing concentrations of ethanol. Following this, sections were heated in a water bath (30 minutes at 80°C) in citrate buffer for antigen retrieval. Then, they were washed in phosphate-buffered saline for 10 minutes and incubated with blocking buffer for 1 hour before the slides were incubated overnight at 4°C with the primary antibody rabbit anti-BKCa channel diluted as above in blocking buffer or with PBS as a negative control. After 2 washes with PBS, the localization of BKCa channels in the tissue sections was studied after staining with anti rabbit FITC conjugated secondary antibody.
Hematoxylin and eosin staining: Cryosections of breast tumors were stained with hematoxylin and eosin and examined by light microscopy.
siRNA studies
The functional activity and biological role of KCNMA1 in breast cancer cells was studied by transiently knocking down the KCNMA1 gene using an Ambion Silencer siRNA transfection II kit. Transfection complexes were prepared in Opti-MEM serum-free medium (Invitrogen) by mixing 0.3 μL of siPORT NeoFX Transfection Reagent (Ambion) and 30 nM of siRNA (silencer pre designed siRNA AM16708A from Ambion). Breast cancer cells (8000 cells/well) were plated in 96-well plates simultaneously with addition of transfection complexes for membrane potential assays while 6-well plates (45,000 cells/well) were used for other studies. Cells were incubated for 72 h, and subsequently used for various assays.
Membrane Potential Assay
The functional activity of BKCa channels in breast cancer cells was measured using the FLIPR Membrane Potential Assay Kit on the FLEXstation (Molecular Devices, Sunnyvale, CA) as described previously [17]. This kit provided a fast, simple and consistent mix-and read procedure and has the potential to examine the functional characteristics of ion channels using changes in fluorescence. The BKCa channel is one such ion channel, whose activation results in increase of membrane potential due to the opening of a K+-permeant, cation-selective channel leading to a decrease in fluorescence. Briefly, the cells were seeded in sterile, clear flat bottom, 96-well plates (Corning Inc., MA) at a density of 4 × 104 cells/well and cultured to achieve a monolayer within 48 hr. The monolayer of cells was incubated with the membrane potential assay reagents for 30 min before loading the test compounds. The anionic potentiometric dye that traverses between cells and extracellular solution in a membrane potential-dependent manner serves as an indicator of voltage changes across the cell membrane. Dose response studies were performed with 0 to 100 μM 1-(5-chloro-2-hydroxyphenyl)-5-trifluoromethyl-1,3-dihydro-2-benzimidazol-2-one (NS-004) (Neurosearch NA, Denmark) with or without Iberiotoxin (IBTX; 10 nM) (Sigma, St. Louis, MO). The FLEXstation was set up using the following parameters: excitation: 530 nm, emission: 565 nm and emission cut off: 550 nm wavelenghs. Observations and recordings were made for 300 seconds after adding NS-004 and IBTX.
Matrigel Invasion
BD BioCoat™ growth factor reduced insert plates (Matrigel™ Invasion Chamber 12 well plates) were prepared by rehydrating the BD Matrigel™ matrix coating in the inserts with 0.5 ml of culture medium for two hours at 37°C. The rehydration solution was carefully removed from the inserts, 0.75 ml DMEM containing chemo-attractant (1%FBS) was added to the lower wells of the plate, and 0.5 ml of cell suspension (2.5 × 104 cells, in serum-free medium containing 0.1%BSA) was added to each insert well. For invasion inhibition assays IBTX (10 nM) was added to the cell culture medium in both upper and lower chambers along with cells and chemo-attractant solution. Cells transiently transfected with siRNA for the KCNMA1 gene was also plated to confirm our results obtained with IBTX. Untreated cells were included as migration controls, were used without rehydration. Invasion assay plates were incubated for 20–22 hours at 37°C. Following incubation, the non-invading cells were removed by scrubbing the upper surface of the insert. The cells on the lower surface of the insert were stained with crystal violet and each transwell membrane mounted on a microscopic slide for visualization and analysis. The number of tumor cells that had migrated from the upper to the lower side of the filter was counted by an individual blinded for the study using a phase-contrast microscope in the central area of the filter. Data are expressed as the percent invasion through the membrane relative to the migration through the control membrane.
% invasion = Mean number of treated cells invading through the matrigel insert membrane/Mean number of untreated cells migrating through membrane
Transendothelial Migration
The invasion of cancer cells through a monolayer of brain endothelial cells is considered to represent the trans-cellular migration activity of the cancer cells. Basic insights into metastasis to brain have been obtained through experiments using human umbilical vein endothelial cells (HUVECs). However, endothelial cells from different vascular beds are uniquely adapted to meet the demands of the underlying tissues [18], and data from HUVECs are not directly applicable to breast tumor cell interaction with endothelial cells at the blood brain barrier (BBB). In this regard, differences between HBMVECs (human brain microvscular endothelial cells) and HUVECs have been reported [18]. We therefore used HCMEC/D3 cells, which are derived from human brain microvasculature to study transendothelial migration of breast tumor cells to brain. HCMEC/D3 cells were seeded at a density of 2 × 105 cells/dish on 35-mm plastic culture dishes coated with collagen and allowed to grow for 4 days to form a confluent monolayer. After the monolayer of HCMEC/D3 has been rinsed once with fresh culture medium, NS-004, IBTX and siRNA- treated breast cancer cells were seeded on top of the HCMEC/D3 at a density of 5 × 104 cells/dish. Following co-incubation for 16 hourat 37°C, non-adherent cells were removed by aspiration and the remaining cell layer was fixed in 10% formalin in PBS. The number of cancer cells that had penetrated the monolayer as well as cancer cell colonies (collectively called invasion foci) was counted under a phase-contrast microscope in 16 different visual fields. Data were expressed as percentage migration of treated breast cancer cells compared with migration of untreated controls, presumably metastatic to brain since the cancer cells were co-cultured with human brain microvascular endothelial cells.
Results
Exon array and PCR
The data obtained by the GeneChip Exon array (Affymetrix) demonstrated that the KCNMA1 gene was over-expressed in breast cancer cell lines derived from human breast cancer metastases to brain (MDA-MB-361) as compared to metastatic breast cancer cells derived from systemic organs (MDA-MB-231) or primary breast cancer (MCF-7) (Figure 1). To study if differential expression of KCNMA1 occurs in vivo in different tissues, a series of specimens of fresh frozen and paraffin-embedded primary breast cancer, breast tumor metastases to different organs of the body and brain were analyzed by semi-quantitative RT-PCR. The PCR results were consistent with the microarray findings (Figure 2). Expression of the KCNMA1 gene was higher in breast tumor specimens from brain metastases compared to specimens from primary breast cancer or metastases to other organs. Similar results were also obtained using breast tumor cell lines of known metastatic predilection. These results imply that overexpression of KCNMA1 gene may likely be associated with breast cancer cells that metastasize to brain.
Figure 1.
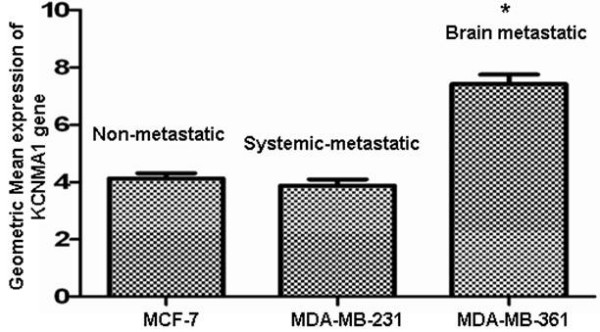
Expression of KCNMA1 gene using Affymetrix Genechip Exon Array. mRNA expression of KCNMA1 was significantly enhanced in the MDA-MB-361 (brain metastatic) cancer cell line as compared to the MCF-7 (non metastatic) and MDA-MB-231 (systemic metastatic) cell lines. MCF-7 (P < 0.0002) and MDA-MB-361(*P < 0.0001), (n = 3 per cell line).
Figure 2.
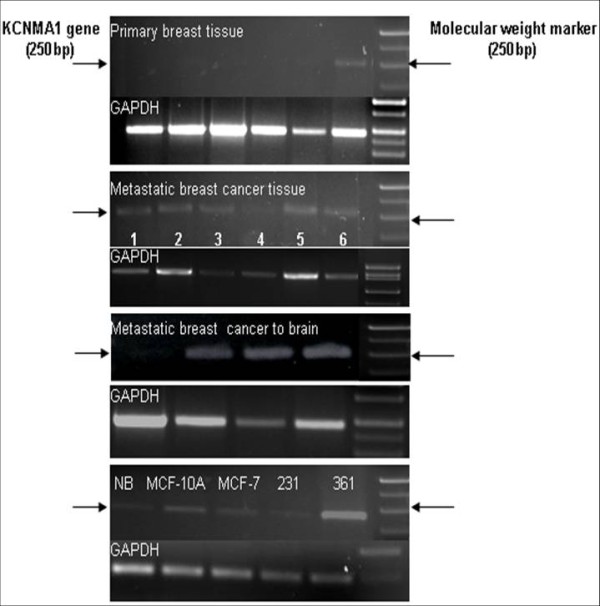
Analysis of KCNMA1 gene expression by RT-PCR. Semiquantitative RT-PCR analyses of human primary breast cancer (n = 6), metastatic breast cancer {n = 6, sample 1 (metastasized to ovary), 2 and 3 (metastasized to liver), 4–6 (metastasized to lymph node)}, metastasized to brain (n = 4) and breast cancer cell lines reveals differential expression of KCNMA1 gene expression (α-subunit, 250 bp). GAPDH was used as the loading control. NB: Normal breast tissue, MCF-10A (normal breast cell line), MCF-7 (non mets), 231: MDA-MB-231 (Mets) and 361: MDA-MB-361 (Brain Mets). Note: Only one of six primary breast cancer tissues showed detectable amount of KCNMA1 RNA (see arrow).
Localization and expression of BKCa channel protein
Immunostaining was performed to localize BKCa channel protein in breast cancer cells. Expression of the BKCa channel was primarily confined to the plasma membrane (Figure 3A). Staining with affinity-purified anti-BKCa channel antibody also revealed higher expression of BKCa channel protein in MDA-MB-361 cells compared to MCF-10A, MCF-7 or MDA-MB-231 cells (Figure 3A). Control experiments with cells negative for BKCa channels demonstrated lower protein signal intensity, indicating the specificity of the antibody (data not shown). In other supporting experiments, the mean fluorescence intensity (MFI) of BKCa channel protein was 187 in MDA-MB-361 cells compared to 13 in MCF-10A, 46 in MCF-7, and 79 in MDA-MB-231 cells (Figure 3B). These results demonstrate the presence of different levels of BKCa channel protein expression in different breast cancer cell lines evaluated.
Figure 3.
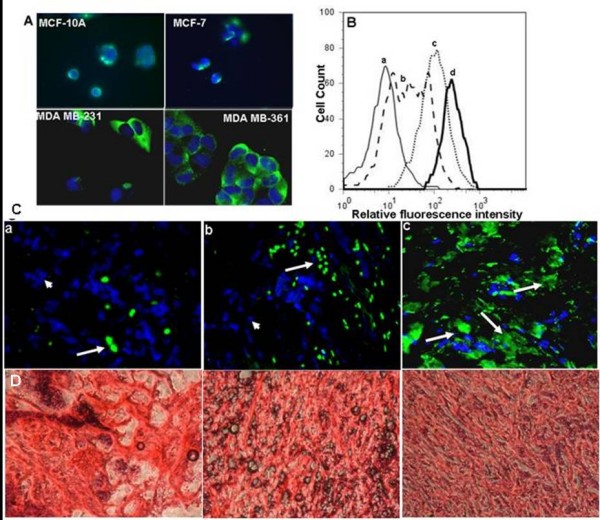
Localization and expression of BKCa channel protein. A: Immunofluorescence of breast cancer cell lines stained for BKCa channel (green) and nuclei with DAPI (blue). Expression of BKCa channel was strong in MDA-MB-231 and MDA-MB-361, weak in MCF-7 and indiscernible in MCF-10A. Stains were repeated twice. B: Flow cytometric detection of BKCa channel protein expression in various breast cell lines. Representative histograms from (a) MCF-10A, (b) MCF-7, (c) MDA-MB-231, and (d) MDA-MB-361 showing expression of BKCa channel protein. C: Immunofluorescence of anti-BKCa channels antibody on human breast cancer tissue. Antibody labeled in green (FITC), nuclei counterstained in blue (DAPI). (a) Very few cells show BKCa channels staining in the primary breast cancer tissue (n = 6, See large arrow). Note: Absence of green staining in most of the cells also indicates specificity of the antibody (See small arrows). (b) Systemic metastatic breast cancer (lymph node) shows an increased number of cells stained with BKCa channel antibody (n = 6; See large arrows). (c) All breast cancer tissue metastatic to the brain are strongly positive for the BKCa channels (n = 4). Magnification = 400×. D: Representative H&E staining of breast tumor tissue sections.
Expression of BKCa channel protein is not only restricted to cell lines but also present in human breast cancer tissues. Immunofluorescence performed on human breast cancer tissue revealed very few cells expressing BKCa channel on primary breast cancer tissue (Figure 3C:a), while a moderate number of cells expressed BKCa channel protein in breast cancer metastatic to different organs (Figure 3C:b). In agreement with the previous reports almost all the breast cancer cells metastatic to brain robustly expressed BKCa channel protein (Figure 3C:c).
Membrane potential
Immunolocalization and protein expression studies showed increased BKCa channel expression in MDA-MB-361 cells as compared to MCF-7 or MDA-MB-231. We performed membrane potential studies to determine whether the overexpressed BKCa channels show increased functional activity using the FLIPR Membrane Potential Assay kit on the FLEX station. Hyperpolarization is reflected as decrease in fluorescence at 565 nm. A specific BKCa channel activator, NS-004, was added to the cells in a dose-dependent manner, and an optimum concentration of 50 μM was established (data not shown), for further studies. All the four breast cancer cell lines listed above showed hyperpolarization after the addition of NS-004 and the effect lasted for more than 300 seconds. The hyperpolarizing action of NS-004 was most marked in MDA-MB-361 cell lines (Figure 4) compared to other breast cancer cell lines. These results demonstrate that BKCa channels in MDA-MB-361 and other breast cancer cell lines were functional. IBTX (10 nM), a BKCa channel antagonist, blocked the membrane hyperpolarization in all the breast cancer cells tested. In addition, transient silencing of functional BKCa channels by siRNA was performed. After testing different concentrations of siRNA (10–75 nM) 30 nM was determined to be optimum (>30 nM was toxic to cells, data not shown). Exposure of the breast cancer cells lines to 30 nM siRNA inhibited NS-004 induced activity of BKCa channels by 65–70% in MCF-10A and MCF-7, and 75–85% reduction MDA-MB-231 and MDA-MB-361 cells (Figure 4, dark bars). These data indicate that BKCa channel activity is functionally distinct in the four breast cancer cell lines studied, being highest in the line that metastasizes preferentially to brain, and can be silenced by a specific BKCa channel inhibitor such as IBTX or by treatment with siRNA.
Figure 4.
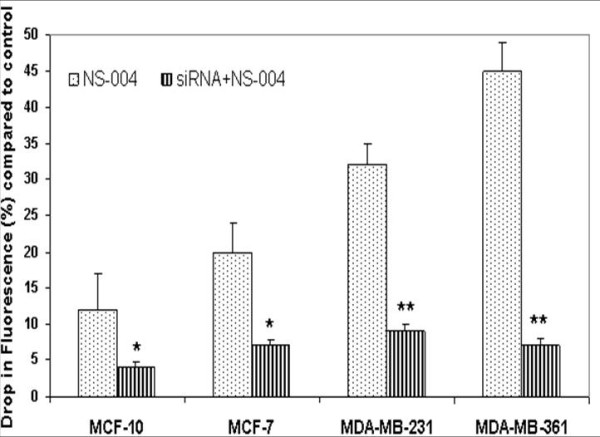
Differential BKCa channel activity measured by membrane potential in breast cancer cell lines. The BKCa channel activity was measured in the presence of its activator NS-004 with and without siRNA for KCNMA1 gene. Pretreatment of breast cancer cell lines with siRNA significantly (*p < 0.046; **p < 0.001) inhibited the BKCa channel activity following addition of NS-004. Note: Decreased drop in fluorescence (indicator of BKCa channel opening/activity) of siRNA + NS-004- treated cells compared to NS-004 treated cells demonstrates that BKCa channel activity was inhibited by addition of siRNA to cells, as fluorescence quenching is an indirect measure of K+ ion-mediated hyperpolarization (hyperpolarization is caused by efflux of K+ through K+ channels), shown in the Y-axis. Bars represent the mean and standard error of the mean obtained from three different experiments in triplicate (n = 9).
BKCa channel inhibition and effect on breast cancer cell invasiveness
BKCa channels are known to enhance the invasion of tumor cells. Therefore, we studied the effect of increased activity of BKCa channels on the invasion of breast cancer cells into Matrigel-coated membranes in a Transwell chamber. No invasion was observed with MCF-10A cells, while MDA-MB-361 cells were more invasive than MCF-7 or MDA-MB-231 cells (Figure 5A). Interestingly the enhanced invasiveness of MDA-MB-361 cells was inhibited by only 50% by IBTX while invasion of MCF-7 was inhibited by 80% by IBTX (Figure 5B) suggesting that BKCa channels may play a greater role in the invasion of MCF-7 cells. Furthermore, treating cells with siRNA that targeted the KCNMA1 gene inhibited the invasion of breast cancer cell lines to a similar extent as did treating them with IBTX (Figure 5B). These results clearly suggest that BKCa channels may play a role in invasion of breast cancer cells because inhibition of BKCa channels significantly attenuated the invasion process.
Figure 5.

KCNMA1 expression promotes breast cancer cell invasion. A: Representative images of breast cancer cells that have migrated through the Matrigel coated transwell and adhered to the lower surface of the filters. The migrated cells were stained with crystal violet (see arrows). B: Breast cancer cells pretreated with IBTX or siRNA to silence the KCNMA1 gene demonstrated significantly (*P < 0.03; **P < 0.001) reduced BKCa channel mediated invasion compared to untreated breast cancer cell lines. Data are expressed as percentage of migration in the untreated controls (100%). Bars represent mean ± the standard error of the mean obtained from three different experiments (n = 3).
Transendothelial migration (TEM) of breast cancer cells
The effect of IBTX and siRNA on the capacity of breast cancer cells to invade a human brain microvascular endothelial cell monolayer (HCMEC/D3) in vitro was measured by a TEM assay as described by Kusama et al [19]. Non-brain metastatic breast cancer cells (MDA-MB-231) showed 65–70% less TEM than did brain metastatic breast cancer cells (MDA-MB-361) (Figure 6A), while normal breast epithelial cells (MCF-10A) did not show detectable TEM. IBTX inhibited the TEM of MCF-7 cells by 48% and migration of MDA-MB-361 cells by 69% (Figure 6B). The inhibition of TEM was similar when siRNA was used to suppress expression of the KCNMA1 gene (Figure 6B). These results clearly demonstrate that either chemical or biological inhibition of BKCa channels might significantly decrease breast cancer metastasis to brain.
Figure 6.
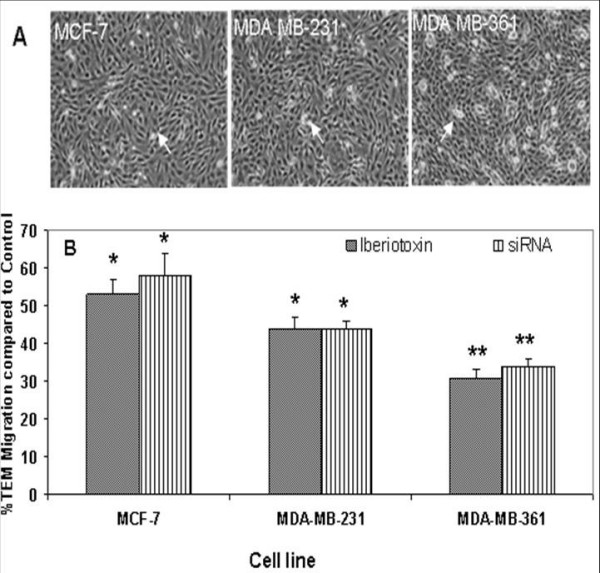
Effects of IBTX and siRNA on transendothelial migration of breast cancer cells. A: Representative images of breast cancer cells that adhered to monolayer of the human brain microvasculature endothelial cell line HCMEC/D3. The adherent breast cancer cells appear white in color against the background of HCMEC/D3. (See arrows). B: Breast cancer cells pretreated with IBTX or siRNA to silence the KCNMA1 gene demonstrated significantly (*P < 0.05; **P < 0.001) reduced BKCa channel mediated-TEM of breast cancer cells across HCMEC/D3 monolayer compared to untreated control. Data is presented as percentage migration of treated breast cancer cells compared with migration of untreated controls (100%); Bars represent the mean ± standard error of the mean obtained from three different experiments (n = 3).
Discussion
Genetic damage or alteration of primary tumor characteristics potentiates cancer metastasis to distant organs including brain. Tumor metastasis is a clonally selective process that occurs as part of tumor progression, a concept that has been supported by experimental [10] and clinical research. Generally, breast cancer metastasis is the hallmark of an aggressive tumor, resistant to treatment. Molecular factors that may contribute to organ-specific metastasis of breast cancer to lung and bone have been identified [20,21]. However, it is not clear how and why certain patients with primary breast cancers develop brain metastases, while in other patients tumors remain confined to the primary sites. Recent investigations in patients with metastatic lung and breast cancers reported alarming rates of brain metastases in both instances. For example, among metastatic breast cancer patients with HER2 positive tumors, the incidence of brain metastases varied from 26% to 48% (8–12). The brain and its supporting structures represent a unique environment for metastatic growth. Molecular factors contributing to the survival of tumor cells in metastatic lesions may be organ specific and could further influence the tumor cells with respect to their gene and protein expression, growth dynamics, and non-responsiveness to treatment. Unraveling the mechanism that drives the metastatic machinery in these cancer cells may offer insights into how to limit or potentially prevent this lethal process.
We showed that the biochemical opening of BTB with BKCa channel modulators increases the BTB permeability and enhance delivery of hydrophilic therapeutic drugs or small- to large-sized molecules, anti-tumor compounds, therapeutic proteins and viral vectors in vivo to brain tumors selectively with little or no drug delivery to normal brain [17,22-24].
Increasingly, targeted molecular therapy is gaining popularity, especially the use of humanized monoclonal antibodies. Although targeting oncogenes expressed on tumor cells is one viable cancer treatment strategy, oncologists often need to target additional molecules related to tumor stroma that promote tumor metastasis and growth. We used gene expression profile study to identify increased KCNMA1 expression in a breast cancer cell line metastatic to brain (MDA-MB-361) (Figure 1) as compared to expression in a primary, breast cancer cell line (MCF-7) or breast cancer cell line metastatic to other organs (MDA-MB-231) of the body but not to brain. Others have shown an up-regulation of BKCa channels as a novel mechanism for producing the malignant phenotype of human tumor cells [14]. Our results clearly demonstrate that the KCNMA1 is upregulated in the MDA-MB-361 cell line (Figure 1). RT-PCR data (Figure 2) from tumor tissues and from breast cancer cell lines further supported these findings, suggesting a close association between level of BKCa channel expression and metastasis to brain. In agreement with the PCR results on breast cancer cell lines, immunocytochemistry results showed strong expression of BKCa channels in MDA-MB-361 cells (Figure 3A) as compared to MCF-10A cells (Figure 3A). The presence of the BKCa channel protein was also confirmed using flow cytometry (Figure 3B). The immuno staining of the sections from human breast cancer tissue indicated that BKCa channel protein expression was pervasive in metastatic brain tumors (Figure 3C:c) compared to primary cancers and those metastatic to other organs (Figure 3C:a&b). In addition, we showed that BKCa channel is pharmacologically functional by knocking down the KCNMA1 gene with siRNA, which has led to a decrease in activity (drop in fluorescence) in all the cell lines studied (Figure 4). The BKCa channel activation was 3-fold higher in breast cancer cells metastatic to brain (MDA-MB-361) than in normal breast cells (MCF-10A) (Figure 4). These results confirm higher expression of BKCa channels on brain specific metastatic breast cells (MDA-MB-361) than in normal breast cells and non-brain metastatic breast cancer cells (MDA-MB-231). These findings corroborate the immunostaining data (Figure 3A) and are consistent with our previous studies showing overexpression of BKCa channels in gliomas and in endothelial cells surrounding malignant brain tumors [17]. Other investigators have also demonstrated over expression of BKCa channels to correlate with increased malignancy of human gliomas [25]. Other type of K+ channel (KCNK9) was also shown to have oncogenic properties in breast cancer [26]. Taken together our data suggest that there may be an association between BKCa channel overexpression and breast cancer metastasis to brain.
The most recent electrophysiological information about BKCa channels came from studies on vertebrate cells with the patch clamp [27]. The cDNAs of BKCa channels have been cloned, and its 7-transmembrane topology has been identified. The N-terminus of BKCa channels is in the extracellular space because an additional transmembrane segment called S0 precedes the canonical S1 through S6 transmembrane segments. With fixed level of intracellular Ca2+ [Ca2+]i, BKCa channels behave like delayed-rectifier K+ channels, activating with depolarization where the gating rates and the resulting probability of being open are also sensitive functions of [Ca2+]i.
Various therapeutic suggestions have been made by investigators based on genome-wide expression analysis of breast cancers, such as adjusting the intensity of current chemotherapy based on cancer subtypes as defined by gene expression signatures [28]. Unfortunately, because a large number of genes with many diverse functions are identified as prognostic signatures, the lists of genes may yield excellent prognostic markers without revealing much of the underlying biological mechanisms thereby providing little guidance for a course of action. In contrast our study showed that inhibition of BKCa channels using either a siRNA or specific chemical inhibitor significantly reduces in vitro the invasive capability of the breast cancer cell lines (Figure 5). This finding further requires validation in biological tumor models. Surprisingly the decrease in the invasive capability of MCF-7 following the addition of BKCa channel inhibitor IBTX was greater than that observed in MDA-MB-361 cells. One possible explanation could be that MDA-MB-361 cells are HER2 positive, which may induce the breast cancer cells to metastasize to brain. Furthermore, HER2 expression may skew the BKCa channel induced invasiveness of MDA-MB-361 cells, while MCF-7 (HER2 negative) cells may depend predominantly on BKCa channels for invasion.
The unique properties of the brain may explain the propensity of certain breast cancer cells to metastasize to the brain. Specifically the brain vascular endothelial cells forming the BBB are significantly different from the systemic endothelial cells [29]. A key event in brain metastasis is TEM of breast cancer cells. We examined whether inhibition of BKCa channels expressed in breast cancer cells metastatic to brain, prevents TEM of MCF-7, MDA-MB-231, and MDA-MB-361 cells across the monolayer of HCMEC/D3 cells. As anticipated, targeting KCNMA1 with siRNA or BKCa channels with IBTX significantly decreased the TEM of MCF-7, MDA-MB-231, and MDA-MB-361 cells across the monolayer of HCMEC/D3 (Figure 6). Most importantly a normal breast cell line (MCF-10A) did not exhibit TEM even when the BKCa channels were activated with NS-004 (data not shown). Our data suggest that increased TEM, at least in part, requires increased BKCa channel expression and/or activity. Thus, blocking BKCa channel function potentially may delay or prevent breast cancer metastasis to the brain. In summary, we showed that BKCa channels are overexpressed in breast cancer cells metastatic to brain. The high expression level correlated with BKCa channel activity, invasion and TEM of the breast cancer cells metastatic to brain.
Conclusion
Tumor metastasis is a complex and highly regulated process involving multiple tumor-host interactions. Accurately determining breast cancer's risk of metastasis to other organs including brain will improve the capacity of clinicians to decide on optimal patient management. Identifying organ-specific KCNMA1 gene involved in metastatic process, and developing targeted therapies against KCNMA1 will hopefully lead to better treatments for this deadly metastatic disease. Furthermore, advances in the identification of contributing factors including the role of KCNMA1 in the metastatic processes, as well as understanding its function and regulation may result in the development of targeted agents to suppress BKCa channel over expression in breast cancers.
Abbreviations
BBB: Blood-brain barrier; BKCa: maxi calcium-activated voltage sensitive potassium; KCNMA1: BKCa channel α-subunit gene; CCD: Charge couple device; PBS: Phosphate-buffered-saline; PCR: Polymerase chain reaction; MgCl2: Magnesium chloride; FITC: Fluorescein isothiocyanate; FLIPR: Fluorescent Imaging Plate Reader; NS-004: 1-(5-chloro-2-hydroxyphenyl)-5-trifluoromethyl-1,3-dihydro-2-benzimidazol-2-one; IBTX: Iberiotoxin; DMEM: Dulbecco's Modified Eagle's Medium; MFI: Mean fluorescence intensity; CNS: Central nervous system; TEM: Transendothelial migration; MHUMC: Memorial Health University Medical Center.
Competing interests statement
The authors declare that they have no competing interests.
Authors' contributions
DK contributed 50% in all aspects of this work. UTS helped in the exon array and PCR studies. BW, IAR and POC provided the human brain microvasculature endothelial cells and helped to draft the manuscript. EAM performed statistical and microarray data analyses. NSN participated in conception, design, and coordination of the study and helped drafting the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Divya Khaitan, Email: divyakh@rediffmail.com.
Umesh T Sankpal, Email: sankpum1@memorialhealth.com.
Babette Weksler, Email: babette@med.cornell.edu.
Edward A Meister, Email: MeistEd1@memorialhealth.com.
Ignacio A Romero, Email: i.romero@open.ac.uk.
Pierre-Olivier Couraud, Email: couraud@cochin.inserm.fr.
Nagendra S Ningaraj, Email: nningaraj@yahoo.com.
Acknowledgements
This work was supported in part by the Georgia Cancer Coalition award and the faculty funding to NSN from Memorial Health University Medical Center, Savannah, GA.
References
- Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003;8(5):398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- Lassman AB, DeAngelis LM. Brain metastases. Neurol Clin. 2003;21(1):1–23. doi: 10.1016/S0733-8619(02)00035-X. vii. [DOI] [PubMed] [Google Scholar]
- Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- Boogerd W, Vos VW, Hart AA, Baris G. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol. 1993;15(2):165–174. doi: 10.1007/BF01053937. [DOI] [PubMed] [Google Scholar]
- DiStefano A, Yong Yap Y, Hortobagyi GN, Blumenschein GR. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44(5):1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::AID-CNCR2820440554>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Sparrow GE, Rubens RD. Brain metastases from breast cancer: clinical course, prognosis and influence of treatment. Clin Oncol. 1981;7(4):291–301. [PubMed] [Google Scholar]
- Bendell JC, Domchek SM, Burstein HJ, Harris L, Younger J, Kuter I, Bunnell C, Rue M, Gelman R, Winer E. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- Lower EE, Drosick DR, Blau R, Brennan L, Danneman W, Hawley DK. Increased rate of brain metastasis with trastuzumab therapy not associated with impaired survival. Clin Breast Cancer. 2003;4(2):114–119. doi: 10.3816/CBC.2003.n.016. [DOI] [PubMed] [Google Scholar]
- Shmueli E, Wigler N, Inbar M. Central nervous system progression among patients with metastatic breast cancer responding to trastuzumab treatment. Eur J Cancer. 2004;40(3):379–382. doi: 10.1016/j.ejca.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, Kurek R, Vega-Valle E, Feigenbaum L, Halverson D. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- Huang Y, Rane SG. Potassium channel induction by the Ras/Raf signal transduction cascade. J Biol Chem. 1994;269(49):31183–31189. [PubMed] [Google Scholar]
- Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, Gutmann DH, Squire JA, Nagy A, Guha A. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Cancer Res. 2001;61(9):3826–3836. [PubMed] [Google Scholar]
- Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. 2002;22(5):1840–1849. doi: 10.1523/JNEUROSCI.22-05-01840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima N, Schonherr K, Niedling S, Kaatz M, Kanno H, Schonherr R, Heinemann SH. Ca2+-activated K+ channels in human melanoma cells are up-regulated by hypoxia involving hypoxia-inducible factor-1alpha and the von Hippel-Lindau protein. J Physiol. 2006;571(Pt 2):349–359. doi: 10.1113/jphysiol.2005.096818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambien B, Rezzonico R, Vitale S, Rouzaire-Dubois B, Dubois JM, Barthel R, Karimdjee BS, Mograbi B, Schmid-Alliana A, Schmid-Antomarchi H. Silencing of hSlo potassium channels in human osteosarcoma cells promotes tumorigenesis. Int J Cancer. 2008;123(2):365–371. doi: 10.1002/ijc.23511. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. Faseb J. 2005;19(13):1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Ningaraj NS, Rao M, Hashizume K, Asotra K, Black KL. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;301(3):838–851. doi: 10.1124/jpet.301.3.838. [DOI] [PubMed] [Google Scholar]
- Man S, Ubogu EE, Williams KA, Tucky B, Callahan MK, Ransohoff RM. Human brain microvascular endothelial cells and umbilical vein endothelial cells differentially facilitate leukocyte recruitment and utilize chemokines for T cell migration. Clin Dev Immunol. 2008;2008:384982. doi: 10.1155/2008/384982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusama T, Nakamori S, Ohigashi H, Mukai M, Shinkai K, Ishikawa O, Imaoka S, Matsumoto Y, Akedo H. Enhancement of in vitro tumor-cell transcellular migration by tumor-cell-secreted endothelial-cell-retraction factor. Int J Cancer. 1995;63(1):112–118. doi: 10.1002/ijc.2910630120. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436(7050):518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/S1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- Black KL, Hoff JT, McGillicuddy JE, Gebarski SS. Increased leukotriene C4 and vasogenic edema surrounding brain tumors in humans. Ann Neurol. 1986;19(6):592–595. doi: 10.1002/ana.410190613. [DOI] [PubMed] [Google Scholar]
- Inamura T, Black KL. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J Cereb Blood Flow Metab. 1994;14(5):862–870. doi: 10.1038/jcbfm.1994.108. [DOI] [PubMed] [Google Scholar]
- Sugita M, Black KL. Cyclic GMP-specific phosphodiesterase inhibition and intracarotid bradykinin infusion enhances permeability into brain tumors. Cancer Res. 1998;58(5):914–920. [PubMed] [Google Scholar]
- Sontheimer H. Ion channels and amino acid transporters support the growth and invasion of primary brain tumors. Mol Neurobiol. 2004;29(1):61–71. doi: 10.1385/MN:29:1:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, Tong JJ, Spiegel L, Nguyen KC, Servoss A. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. 2003;3(3):297–302. doi: 10.1016/S1535-6108(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8(3):321–329. doi: 10.1016/S0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Chang HY, Nuyten DS, Sneddon JB, Hastie T, Tibshirani R, Sorlie T, Dai H, He YD, van't Veer LJ, Bartelink H. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102(10):3738–3743. doi: 10.1073/pnas.0409462102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38(6):323–337. doi: 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]


