Abstract
Leukocyte trafficking involves specific recognition between P-selectin and L-selectin and PSGL-1 containing core 2-based O-glycans expressing sialyl Lewis x (SLex) antigen. However, the structural identity of the glycan component(s) displayed by murine neutrophil PSGL-1 that contributes to its P-selectin counter-receptor activity has been uncertain, since these cells express little if any SLex antigen, and because there have been no direct studies to examine murine PSGL-1 glycosylation. To address this uncertainty, we studied PSGL-1 glycosylation in the murine cell line WEHI-3 using metabolic-radiolabeling with 3H-monosaccharide precursors to detect low-abundance O-glycan structures. We report that PSGL-1 from WEHI-3 cells expresses a di-sialylated core 2 O-glycan containing the SLex antigen. This fucosylated O-glycan is scarce on PSGL-1 and essentially undetectable in total leukocyte glycoproteins from WEHI-3 cells. These results demonstrate that WEHI-3 cells selectively fucosylate PSGL-1 to generate functionally important core 2-based O-glycans containing the SLex antigen.
Keywords: Lewis x, murine, O-glycan, P-selectin, PSGL-1
Introduction
Interactions of selectins with their ligands are pivotal to leukocyte recruitment to sites of inflammation (Kobzdej et al. 2002; McEver 2002; Lowe 2003). The binding of human P- and L-selectins to their major leukocyte glycoprotein ligand PSGL-1 relies on the presence of a sialyl Lewis x structure NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAc-R (SLex) on a core 2 O-glycan at the PSGL-1 N-terminus (Sako et al. 1993; Wilkins et al. 1996; McEver and Cummings 1997; Cummings 1999; Leppänen et al. 1999). The SLex determinant is common to core 2 O-glycans and complex-type N-glycans in human neutrophils and other myeloid cells. The importance of SLex-containing core 2 O-glycans was highlighted by genetic studies in mice, demonstrating that its expression is required for murine leukocyte adhesion to P-selectin (Maly et al. 1996; Sperandio et al. 2001).
Studies in mice have also raised questions about the nature of glycan determinants in murine versus human leukocytes. Murine leukocytes and the model murine leukocytic cell line WEHI-3, which has a functional PSGL-1 that is recognized by P-selectin (Yang et al. 1996), express little if any SLex antigen (Thorpe and Feizi 1984; Ito et al. 1994; Kobzdej et al. 2002; Julien et al. 2007). By contrast, human neutrophils and the model human leukocytic cell line HL60 display high amounts of SLex antigen (Fukuda et al. 1985; Phillips et al. 1990; Wilkins et al. 1996). Preliminary mass spectrometry data from the Consortium for Functional Glycomics show the absence of SLex in the major N- or O-glycans from mouse neutrophils and WEHI-3 cells (see http://www.functionalglycomics.org/). Such findings raise questions about the nature of murine glycans on PSGL-1 and their recognition by P-selectin.
To explore murine PSGL-1 glycosylation, we performed detailed studies on O-glycans from purified PSGL-1 and total cellular glycoproteins from WEHI-3 cells. To enhance the sensitivity of detecting and analyzing glycans, we used metabolically radiolabeled glycans and comparisons to synthetic standards. Our results indicate that WEHI-3 cells selectively modify PSGL-1 with a SLex-containing core 2 O-glycan and suggest that α1-3 fucosylation may be glycoprotein specific in murine cells.
Results
Metabolic radiolabeling of WEHI-3 cells and O-glycan preparation
To aid in detecting rare O-glycan structures, we metabolically radiolabeled WEHI-3 cells with 3H-monosaccharide precursors, using procedures that identified SLex antigen in O-glycans of human leukocytes (Wilkins et al. 1996). WEHI-3 cells were labeled with either 3H-glucosamine, which is metabolized into N-acetylglucosamine (GlcNAc), N-acetylgalactosamine (GalNAc), and sialic acid (NeuAc), and thus are incorporated into all glycans, or 3H-fucose, which cannot be metabolized, and thus is incorporated only as fucose into N- and O-glycans of fucosylated glycoproteins.
We purified PSGL-1 from extracts of metabolically radiolabeled WEHI-3 cells and recovered the residual cellular glycoproteins, which we designated the total cellular glycoproteins. Flow cytometry analyses show that PSGL-1 is expressed at similar low levels in both human and murine leukocytes (Kobzdej et al. 2002), which contrasts to expression levels of many other mucin-like glycoproteins, such as CD43, which are expressed at higher levels (Barclay et al. 1993). Autoradiography of purified 3H-fucose-labeled PSGL-1 (Figure 1A) showed a single major glycoprotein of apparent size of ~120 kDa, which matches that predicted from studies on murine PSGL-1 (Yang et al. 1996). We released the O-glycans from PSGL-1 and total glycoproteins by β-elimination. Most radiolabeled O-glycans of PSGL-1 were recovered in anionic, sialylated fractions (−1 and −2 charges), indicating the presence of 1 or 2 sialic acids per O-glycan (Figure 1B), similar to human HL60 cell-derived O-glycans (Wilkins et al. 1996). Neuraminidase treatment caused the loss of all charged species (data not shown), indicating that the glycans are sialylated and not sulfated. Since the di-sialylated species was the major material from PSGL-1 for both radiolabeled samples, we concentrated our analyses on that material.
Fig. 1.
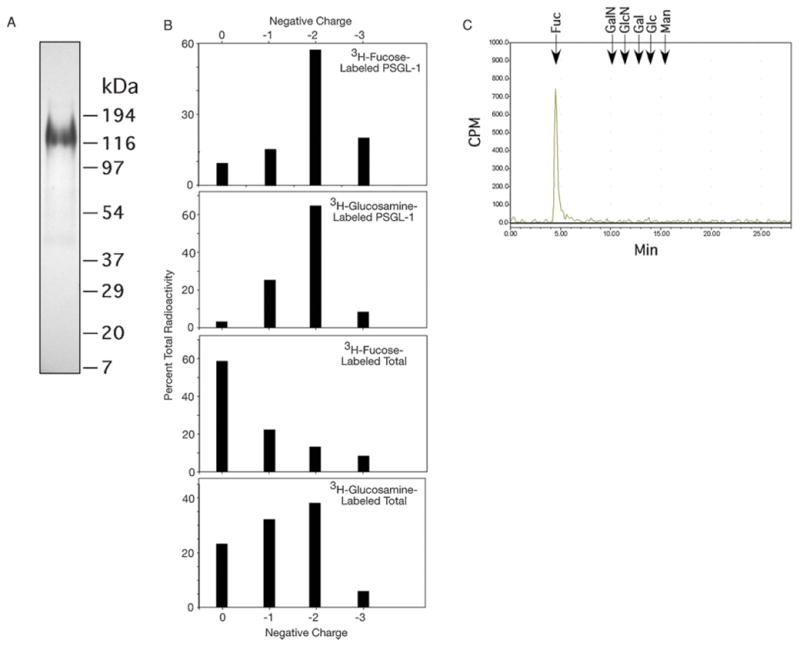
(A) SDS–PAGE and autoradiography of 3H-fucose-labeled PSGL-1 immunoaffinity purified from WEHI-3 cells. (B) The β-eliminated O-glycans from radiolabeled PSGL-1 and total cellular glycoproteins were separated and eluted to collect glycans of neutral structure (0 charge), 1 sialic acid (−1 charge), 2 sialic acids (−2 charge), or >2 sialic acids (−3 charge). The distribution of total radioactivity as a percentage from individual samples in the four major fractions is shown. (C) Monosaccharide analysis of O-glycans from 3H-fucose-labeled WEHI-3 cells. Elution positions of standards are indicated. Fuc, fucose; GalN, galactosamine; GlcN, glucosamine; Gal, galactose; Glc, glucose; Man, mannose.
A portion of O-glycans released from 3H-fucose-labeled cellular WEHI-3 glycoproteins was hydrolyzed and examined by high-pH anion-exchange chromatography (HPAEC). A single radioactive peak was recovered coeluting with the fucose standard, confirming that the incorporated 3H-fucose was restricted to fucose (Figure 1C).
Profiling of radiolabeled O-glycans from total glycoproteins of WEHI-3 compared to PSGL-1
Because PSGL-1 is such a minor glycoprotein in human and murine leukocytes, it was not feasible to prepare enough for mass spectrometry analysis. Furthermore, even mass spectrometry of small amounts of material might overlook rare glycans. Metabolic radiolabeling enhances detection of minor glycan species (Varki 1994) and promotes structural analysis when compared to known standards (Wilkins et al. 1996). To aid our analyses, we synthesized several key 3H-labeled O-glycan standards containing a reduced GalNAc (GalNAcol) residue (structures in Figure 2A). These included a di-sialylated core 2 O-glycan, NeuAcα2-3Galβ1-4GlcNAcβ1-6(NeuAcα2-3Galβ1-3)GalNAcol; a fucosylated, sialylated core 2 O-glycan with the SLex antigen, NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-6(NeuAcα2-3Galβ1-3)GalNAcol; and two di-sialylated core 2 O-glycans containing the Sda blood group on one or two branches, NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ1-6(NeuAcα2-3Galβ1-3)GalNAcol or Neu Acα2-3(GalNAcβ1-4)Galβ1-4GlcNAcβ1-6(NeuAcα2-3(GalNAcβ1-4)Galβ1-3)GalNAcol. Previous studies identified the presence of sialylated O-glycans with this antigen in blood cells of humans and other mammals (Blanchard et al. 1983; Morton et al. 1970). Since WEHI-3 O-glycans contained core 2 O-glycan structures, we concentrated on these structures. We attempted to synthesize O-glycans with the SLex antigen in which the β1-4-linked Gal residues were modified by Sda β1-4-N-acetylgalactosaminyltransferase, but we were unsuccessful. Notably, such glycans have not been reported, possibly due to enzymatic blocks in addition to either α1-3Fuc or β1-4-linked GalNAc to the corresponding acceptor core 2 O-glycans.
Fig. 2.
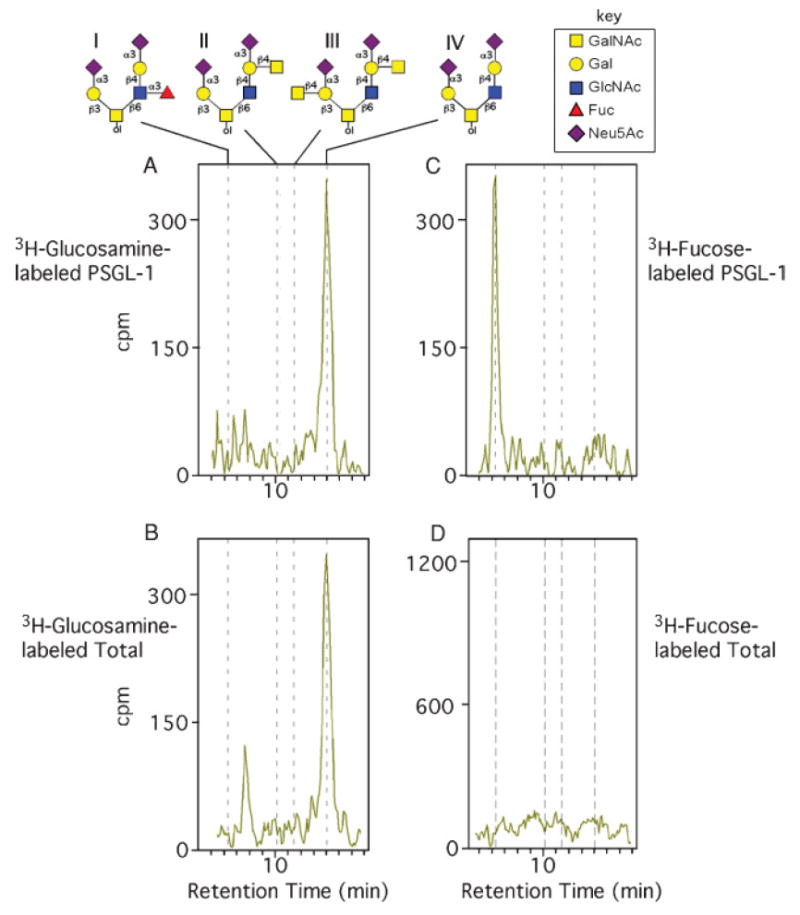
HPAEC-flow scintillation analysis of doubly charged radiolabeled O-glycans from PSGL-1 and total O-glycans from WEHI-3 cells. (A) 3H-glucosamine-labeled PSGL-1 O-glycans. Structures and elution positions of O-glycan standards, labeled I–IV, described in the text, are indicated above the chromatogram. (B) 3H-glucosamine-labeled O-glycans from total glycoproteins of WEHI-3 cells. (C) 3H-fucose-labeled PSGL-1 O-glycans. (D) 3H-fucose-labeled O-glycans from total glycoproteins of WEHI-3 cells.
When 3H-glucosamine was used for metabolic radiolabeling, the HPAEC profiles of the doubly charged O-glycans of both PSGL-1 (Figure 2A) and total cellular glycoproteins (Figure 2B) were similar. The major peak from both samples coeluted with the core 2 structure NeuAcα2-3Galβ1-4GlcNAcβ1-6(NeuAcα2-3Galβ1-3)GalNAcol. Neither chromatogram contained an identifiable peak representing a core 2 with the SLex antigen (NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-6(NeuAcα2-3Galβ1-3)GalNAcol). From these results we conclude that fucose-containing O-glycans in PSGL-1 are rare and based on the recovery of 3H-glucosamine-derived radioactivity in the di-sialylated core 2 O-glycans, >90% of these di-sialylated core 2 O-glycans lack fucose.
To determine whether PSGL-1 contained any fucosylated O-glycans, we prepared 3H-fucose-labeled material and examined O-glycans from PSGL-1 and total glycoproteins. Importantly, the major peak of 3H-fucose-labeled O-glycans in di-sialylated O-glycans from PSGL-1 coeluted with the core 2 structure with the SLex antigen (NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-6(NeuAcα2-3Galβ1-3)GalNAcol) (Figure 2C), whereas this glycan was not detected in 3H-fucose-labeled O-glycans from total cellular glycoproteins (Figure 2D). We observed a major late-eluting peak from the 3H-fucose-labeled sample of total cellular glycoproteins (data not shown), but it represented N-glycans with a core α1,6-linked fucose, which probably arose by their partial release by β-elimination. These results demonstrate that only a minority (<10%) of O-glycans in PSGL-1 contains the core 2 structure with fucosylated SLex antigen and that in total cellular glycoproteins this structure is not detectable.
Discussion
The SLex antigen appears to be common on human neutrophils but expressed poorly on murine neutrophils. Conventional SLex antibodies bind well to human leukocytes, such as neutrophils and monocytes, but they bind poorly to murine leukocytes (Thorpe and Feizi 1984; Fukuda et al. 1985; Phillips et al. 1990; Wilkins et al. 1996; Kobzdej et al. 2002; Julien et al. 2007). These results are consistent with studies showing that human PSGL-1 has α1-3-linked fucose in both N- and O-glycans (Wilkins et al. 1996; Aeed et al. 2001). However, there are no structural data supporting the presence of the SLex antigen or α1-3-linked fucosylation in murine neutrophils. Yet, genetic changes in glycosylation pathways in humans and mice support a role for α1-3-linked fucosylation of PSGL-1 for functional recognition by selectins (Etzioni and Tonetti 2000). Mice lacking expression of myeloid α1-3-fucosyltransferase FucT-VII are deficient in functional selectin ligands, and mice lacking both FucT-VII and FucT-IV exhibit no adhesion to P- and E-selectins (Maly et al. 1996; Lowe 1997). FucT-VII is critical in synthesizing a key determinant for selectin recognition, the SLex antigen, which must be expressed on a core 2 O-glycan at the N-terminus of PSGL-1 for functional recognition by P- and L-selectins (Sako et al. 1993; Wilkins et al. 1996; McEver and Cummings 1997; Cummings 1999; Leppänen et al. 1999). In addition, leukocytes from mice lacking expression of core 2 β1,6-N-acetylglucosaminyltransferase bind poorly to P- and E-selectins (Sperandio et al. 2001). Thus, genetic evidence raised expectation that murine leukocytes might express the SLex antigen, but there has been no structural evidence supporting its presence.
Our studies show that a major O-glycan in total cellular glycoproteins of WEHI-3 cells and in PSGL-1 is a non-fucosylated di-sialylated core 2 O-glycan. However, PSGL-1 also contains a di-sialylated core 2 O-glycan expressing the SLex antigen, but we did not detect this structure in total cellular glycoproteins. These findings suggest that PSGL-1 may preferentially be modified by α1-3-fucosyltransferases, which is consistent with some prior observations. Zollner and Vestweber (1996) observed that recombinant E-selectin ligand ESL-1 in Chinese hamster ovary cells expressing different fucosyltransferases contained SLex. Studies in murine neutrophils deficient in FucT-VII or FucT-IV suggested that FucT-VII may selectively modify PSGL-1, whereas FucT-IV may selectively modify ESL-1 (Huang et al. 2000). The exact mechanism for this specific glycan modification is unknown, but likely involves preferential action of FucT-VII, expressed minutely by WEHI-3 cells (Kobzdej et al. 2002). It has been observed that even very low levels of FucT-VII activity selectively modify PSGL-1 O-glycans, producing core 2 SLex structures (Prorok-Hamon et al. 2005).
The fact that different glycoproteins synthesized by the same cells, even different glycosylation sites on the same glycoprotein, can contain distinct glycan structures clearly indicates that glycan synthesis is controlled by protein-specific mechanisms, beyond the simple expression level of various glycosyltransferases. Our results corroborate evidence that PSGL-1 may be a special acceptor for certain glycosyltransferases, especially those involved in SLex antigen synthesis on core 2 O-glycans. Selective PSGL-1 modification with SLex has recently been demonstrated in human monocytes and dendritic cells (Julien et al. 2007), showing discordance between chemical structural analysis and expression of the SLex antigen. Some glycosyltransferases, such as FucT-VII, may have special affinity for unique glycoprotein acceptors such as PSGL-1. Protein acceptor specificity has been seen in a few glycosyltransferases, including β1,4-N-acetylgalactosaminyltransferases (Miller et al. 2008), and collagen UDP-glucose:5-hydroxylysine-collagen glucosyltransferase (Smith et al. 1977), but this issue needs to be addressed in more detail in future studies using glycoprotein, rather than free glycan, acceptors for glycosyltransferases.
Such findings coupled with ours suggest caution in speculating about the repertoire of glycan structures present in specific cells or tissues based solely on general chemical analyses of the glycans. The relatively rare core 2-based SLex antigen we identified on PSGL-1 appears to be absent in total glycan profiles from WEHI-3 cells, even after using metabolic-radiolabeling strategies. Whenever possible, a comprehensive approach to defining cellular glycomes, using function-based assays, immunochemistry, gene expression profiles, and chemical analyses should be utilized. Such approaches are being increasingly used (Comelli et al. 2006; Julien et al. 2007) and will continue to highlight critical roles of minor glycan structures in cellular functions.
Materials and methods
Cell culture and radiolabeling
WEHI-3 cells were cultured in Iscove’s modified Dulbecco’s medium with 10% FBS and 0.05 mM 2-mercaptoethanol at 37°C. For 3H-monosaccharide labeling, cells were grown as above, but with a medium containing 20 μCi/mL 3H-fucose or 3H-glucosamine (MP Biomedicals, Solon, OH) for 72 h. For O-glycan analysis, ~5 × 107 cells were harvested by centrifugation at 1000 × g for 5 min and washed three times with Dulbecco’s PBS containing 25 mM EDTA.
3H-O-glycan standard preparation
We prepared core 2-based O-glycan standards (I–IV) (Figure 2A), using a glycopeptide strategy, releasing each O-glycan from peptide by β-elimination. We synthesized O-glycans containing SLex on the core 2 branch and 3H-sialic acid α2,3-linked to the core 1 galactose (Glycan I) using the glycopeptide GSP-6 as an acceptor. The synthesis of GSP-6 glycopeptide was previously described (Leppänen et al. 1999), containing SLex on the core 2 branch and uncapped galactose on the core 1 branch. The synthesis reaction for Glycan I was performed for 2 h at room temperature in 50 mM sodium cacodylate, pH 7.0, with 1 μCi CMP-3H-sialic acid (ARC), 3 μg GSP-6, and 1 mU α2,3-(O)-sialyltransferase (ST3Gal II, Calbiochem). The reaction was diluted with water, purified using Sep-Pak C-18 cartridges (Waters), eluted in 60% acetonitrile, and dried. Glycan I was released by mild-base β-elimination (Cummings et al. 1983) in 50 mM NaOH, 1 M NaBH4 at 45°C for 16 h. The base was neutralized by glacial acetic acid, and sodium was removed by an AG 50W-X8 column (BIO-RAD). Boric acid was removed by repeated evaporation in methanol/acetic acid, and O-glycans were further purified using a Carbograph-activated charcoal cartridge (Alltech) (Packer et al. 1998) and dissolved in water. This mild-base β-elimination and purification procedure was applied to each synthetic glycopeptide below.
A second core 2-based O-glycan standard (Glycan IV) was prepared as above with ST3Gal II, using the glycopeptide GSP-5 as an acceptor (Leppänen et al. 1999), containing sialyl N-acetyllactosamine on the core 2 branch and uncapped galactose on the core 1 branch.
A third core 2-based O-glycan standard (Glycan II) containing an Sda structure on the core 2 branch and sialic acid α2,3-linked to the core 1 galactose was prepared, using recombinant Sda β1,4-N-acetylgalactosaminyltransferase to add 3H-N-acetylgalactosamine in a β1,4-linkage to the galactose in the sialyl lactosamine unit on the core 2 branch of the GSP-5 O-glycan. The synthesis reaction was performed for 2 h at 37°C in 50 mM sodium cacodylate, pH 7.0, and 25 mM MnCl2 with 1 μCi UDP-3H-N-acetylgalactosamine (ARC) and 3 μg GSP-5. The product was purified using Sep-Pak C-18 cartridge. Non-radioactive sialic acid was added in α2,3-linkage to the core 1 galactose of the latter product using 2 mU ST3Gal II and 1 mM CMP-NeuAc (Sigma) as the donor.
A fourth core 2-based O-glycan standard (Glycan III) containing Sda structures on both the core 1 and core 2 branches was prepared using the same steps to synthesize Glycan II, followed by the addition of nonradioactive N-acetylgalactosamine to galactose on the core 1 branch using recombinant Sda β1,4-N-acetylgalactosaminyltransferase as above and 1 mM UDP-GalNAc (Sigma) as the donor.
O-Glycan preparation from WEHI-3 cells
WEHI-3 cell pellets were prepared as above, lysed with 100 mM Tris–HCl, pH 8.0, 1 mM CaCl2, 1 mM MgCl2, 1.2% Triton X-100, 0.1% NaN3, and 1 × complete protease inhibitor cocktail (Roche) for 5 min in a sonicating ice–water bath, and centrifuged at 1500 × g for 10 min. The supernatant was pre-cleared by applying it to a column of bovine IgG immobilized on an UltraLink matrix (Pierce); PSGL-1 was affinity-purified by applying the flow-through to a column of rabbit polyclonal antibodies raised against a mouse PSGL-1 peptide, immobilized on an UltraLink matrix. These anti-PSGL-1 antibodies were prepared against a synthetic N-terminal peptide of murine PSGL-1 containing the sequence DYTYNTDPPEL, using techniques described previously (Moore et al. 1994). The antisera to this peptide were specific to murine PSGL-1 as shown by immunoblotting and flow cytometry, whereas control antisera showed no binding to PSGL-1 (data not shown). The anti-PSGL-1 column was rinsed with 100 mM Tris–HCl, pH 8.0, 1 mM CaCl2, 1 mM MgCl2, 0.1% Triton X-100, and 0.1% NaN3, and bound PSGL-1 was eluted with 50 mM glycine, pH 2.5, 150 mM NaCl, and 0.05% Triton X-100. Eluted PSGL-1 was concentrated and solution exchanged into water using a Centricon centrifugal filter with a 10 kDa molecular weight cut-off (Millipore), and its O-glycans were β-eliminated and purified as above.
The remaining cellular glycoproteins were precipitated from the anti-PSGL-1 column flow-through by adjusting it to 10% trichloroacetic acid and 1% phosphotungstic acid and centrifugation at 1500 × g for 10 min. The protein pellet was rinsed three times with 90% ethanol and dried, and O-glycans were β-eliminated and purified as above.
O-Glycans released from PSGL-1 and total cellular glycoproteins were separated by charge using a QAE ion-exchange column as described (Wilkins et al. 1996) and desalted using Carbograph cartridges as above.
Monosaccharide analysis
Some O-glycans released from cellular glycoproteins of 3H-fucose-labeled WEHI-3 cells were analyzed for 3H-monosaccharides following hydrolysis in 2 M TFA at 100°C for 4 h. After TFA removal by evaporation in vacuo, monosaccharides were resolved by HPAEC as described (Wilkins et al. 1996). Radiolabeled monosaccharides were detected by Radiomatic 610TR flow-scintillation detection (PerkinElmer).
HPAEC analysis of O-glycans
O-Glycan standards and doubly charged O-glycans from various samples were resolved by HPAEC using a CarboPac PA-10 column and the following running salt gradient: 0–10 min, 100 mM NaOH/70 mM sodium acetate (constant); 10–26 min, linear gradient up to 100 mM NaOH/110 mM sodium acetate; 26–35 min, 100 mM NaOH/500 mM sodium acetate (constant). The resolved O-glycans were detected by flow-scintillation detection.
Acknowledgments
We thank Dr. Jamie Heimburg-Molinaro for help in preparing and editing the manuscript.
Funding National Institutes of Health (AI48075 to R.D.C.); resources from Core C of the Consortium for Functional Glycomics (NIH Grant GM62116).
Abbreviations
- Fuc
fucose
- FucT IV
α1,3-fucosyltransferase IV
- FucT VII
α1,3-fucosyltransferase VII
- Gal
galactose
- GalN
galactosamine
- Glc
glucose
- GlcN
glucosamine
- GSP
glycosulfopeptides
- HPAEC
high-pH anion-exchange chromatography
- Man
mannose
- NeuAc
N-acetyl-d-neuraminic acid
- PSGL-1
P-selectin glycoprotein ligand-1
- Sda
Cad blood group NeuAcα2-3(GalNAcβ1-4)Galβ1-4GlcNAc-R
- SLex
sialyl Lewis x antigen NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAc-R
- TFA
trifluoroacetic acid
Footnotes
Conflict of interest statement None declared.
Publisher's Disclaimer: This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/2.0/uk/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- Aeed PA, Geng JG, Asa D, Raycroft L, Ma L, Elhammer AP. Partial characterization of the N-linked oligosaccharide structures on P-selectin glycoprotein ligand-1 (PSGL-1) Cell Res. 2001;11:28–36. doi: 10.1038/sj.cr.7290063. [DOI] [PubMed] [Google Scholar]
- Barclay AN, Birkeland ML, Brown MH, Beyers AD, Davis SJ, Somoza C, Williams AF. The Leukocyte Antigen Factsbook. New York: Academic Press; 1993. [Google Scholar]
- Blanchard D, Cartron JP, Fournet B, Montreuil J, van Halbeek H, Vliegenthart JF. Primary structure of the oligosaccharide determinant of blood group Cad specificity. J Biol Chem. 1983;258:7691–7695. [PubMed] [Google Scholar]
- Comelli EM, Head SR, Gilmartin T, Whisenant T, Haslam SM, North SJ, Wong NK, Kudo T, Narimatsu H, Esko JD, et al. A focused microarray approach to functional glycomics: Transcriptional regulation of the glycome. Glycobiology. 2006;16:117–131. doi: 10.1093/glycob/cwj048. [DOI] [PubMed] [Google Scholar]
- Cummings RD. Structure and function of the selectin ligand PSGL-1. Braz J Med Biol Res. 1999;32:519–528. doi: 10.1590/s0100-879x1999000500004. [DOI] [PubMed] [Google Scholar]
- Cummings RD, Kornfeld S, Schneider WJ, Hobgood KK, Tolleshaug H, Brown MS, Goldstein JL. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983;258:15261–15273. [PubMed] [Google Scholar]
- Etzioni A, Tonetti M. Leukocyte adhesion deficiency II—from A to almost Z. Immunol Rev. 2000;178:138–147. doi: 10.1034/j.1600-065x.2000.17805.x. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Bothner B, Ramsamooj P, Dell A, Tiller PR, Varki A, Klock JC. Structures of sialylated fucosyl polylactosaminoglycans isolated from chronic myelogenous leukemia cells. J Biol Chem. 1985;260:12957–12967. [PubMed] [Google Scholar]
- Huang MC, Zollner O, Moll T, Maly P, Thall AD, Lowe JB, Vestweber D. P-Selectin glycoprotein ligand-1 and E-selectin ligand-1 are differentially modified by fucosyltransferases Fuc-TIV and Fuc-TVII in mouse neutrophils. J Biol Chem. 2000;275:31353–31360. doi: 10.1074/jbc.M005449200. [DOI] [PubMed] [Google Scholar]
- Ito K, Handa K, Hakomori S. Species-specific expression of sialosyl-Le(x) on polymorphonuclear leukocytes (PMN), in relation to selectin-dependent PMN responses. Glycoconj J. 1994;11:232–237. doi: 10.1007/BF00731223. [DOI] [PubMed] [Google Scholar]
- Julien S, Grimshaw MJ, Sutton-Smith M, Coleman J, Morris HR, Dell A, Taylor-Papadimitriou J, Burchell JM. Sialyl-Lewis x on P-selectin glycoprotein ligand-1 is regulated during differentiation and maturation of dendritic cells: A mechanism involving the glycosyltransferases C2GnT1 and ST3Gal I. J Immunol. 2007;179:5701–5710. doi: 10.4049/jimmunol.179.9.5701. [DOI] [PubMed] [Google Scholar]
- Kobzdej MM, Leppänen A, Ramachandran V, Cummings RD, McEver RP. Discordant expression of selectin ligands and sialyl Lewis x-related epitopes on murine myeloid cells. Blood. 2002;100:4485–4494. doi: 10.1182/blood-2002-06-1799. [DOI] [PubMed] [Google Scholar]
- Leppänen A, Mehta P, Ouyang YB, Ju T, Helin J, Moore KL, van Die I, Canfield WM, McEver RP, Cummings RD. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J Biol Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Selectin ligands, leukocyte trafficking, and fucosyltransferase genes. Kidney Int. 1997;51:1418–1426. doi: 10.1038/ki.1997.194. [DOI] [PubMed] [Google Scholar]
- Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelly RJ, Gersten KM, Cheng G, Saunders TL, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: Lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97–S103. [PubMed] [Google Scholar]
- Miller E, Fiete D, Blake NM, Beranek M, Oates EL, Mi Y, Roseman DS, Baenziger JU. A necessary and sufficient determinant for protein-selective glycosylation in vivo. J Biol Chem. 2008;283:1985–1991. doi: 10.1074/jbc.M708160200. [DOI] [PubMed] [Google Scholar]
- Moore KL, Eaton SF, Lyons DE, Lichenstein HS, Cummings RD, McEver RP. The P-selectin glycoprotein ligand from human neutrophils displays sialylated, fucosylated, O-linked poly-N-acetyllactosamine. J Biol Chem. 1994;269:23318–23327. [PubMed] [Google Scholar]
- Morton JA, Pickles MM, Terry AM. The Sda blood group antigen in tissues and body fluids. Vox Sang. 1970;19:472–482. doi: 10.1111/j.1423-0410.1970.tb01779.x. [DOI] [PubMed] [Google Scholar]
- Packer NH, Lawson MA, Jardine DR, Redmond JW. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj J. 1998;15:737–747. doi: 10.1023/a:1006983125913. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Prorok-Hamon M, Notel F, Mathieu S, Langlet C, Fukuda M, El-Battari A. N-Glycans of core 2 beta(1,6)-N-acetylglucosaminyltransferase-I (C2GnT-I) but not those of alpha(1,3)-fucosyltransferase-VII (FucT-VII) are required for the synthesis of functional P-selectin glycoprotein ligand-1 (PSGL-1): Effects on P-, L- and E-selectin binding. Biochem J. 2005;391:491–502. doi: 10.1042/BJ20050344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako D, Chang XJ, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- Smith DF, Kosow DP, Wu C, Jamieson GA. Characterization of human platelet UDPglucose-collagen glucosyltransferase using a new rapid assay. Biochim Biophys Acta. 1977;483:263–278. doi: 10.1016/0005-2744(77)90055-9. [DOI] [PubMed] [Google Scholar]
- Sperandio M, Thatte A, Foy D, Ellies LG, Marth JD, Ley K. Severe impairment of leukocyte rolling in venules of core 2 glucosaminyltransferase-deficient mice. Blood. 2001;97:3812–3819. doi: 10.1182/blood.v97.12.3812. [DOI] [PubMed] [Google Scholar]
- Thorpe SJ, Feizi T. Species differences in the expression of carbohydrate differentiation antigens on mammalian blood cells revealed by immunofluorescence with monoclonal antibodies. Biosci Rep. 1984;4:673–685. doi: 10.1007/BF01121021. [DOI] [PubMed] [Google Scholar]
- Varki A. Metabolic radiolabeling of glycoconjugates. Methods Enzymol. 1994;230:16–32. doi: 10.1016/0076-6879(94)30004-6. [DOI] [PubMed] [Google Scholar]
- Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL-60 cells. J Biol Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- Yang J, Galipeau J, Kozak CA, Furie BC, Furie B. Mouse P-selectin glycoprotein ligand-1: Molecular cloning, chromosomal localization, and expression of a functional P-selectin receptor. Blood. 1996;87:4176–4186. [PubMed] [Google Scholar]
- Zollner O, Vestweber D. The E-selectin ligand-1 is selectively activated in Chinese hamster ovary cells by the alpha(1,3)-fucosyltransferases IV and VII. J Biol Chem. 1996;271:33002–33008. doi: 10.1074/jbc.271.51.33002. [DOI] [PubMed] [Google Scholar]


