Summary
The Polycomb repression complex 2 (PRC2) methylates histone H3 lysine 27 at target genes to modify gene expression, and this mark is recognized by PRC1, which ubiquitylates histone H2A. In Caenorhabditis elegans, a complex of the MES-2, MES-3 and MES-6 proteins is functionally analogous to the PRC2 complex, but the functional analog of PRC1, and indeed whether C. elegans has such a complex, has been unclear. We describe here that MIG-32 and SPAT-3A are functional analogs of PRC1 in C. elegans, where they are required for neuronal migrations and during vulval development. mig-32 and spat-3 mutants are defective in H2A ubiquitylation, and have nervous system defects that partially overlap with those of mes mutants. However, unlike the mes mutants, mig-32 and spat-3 mutants are fertile, suggesting that PRC1 function is not absolutely required in the germline for essential functions of PRC2.
Keywords: BMI-1, C. elegans, Histone modification, Polycomb, Neuronal migration, ring1B
INTRODUCTION
The Polycomb group genes encode components of chromatin-modifying complexes, and were initially identified in Drosophila as modifiers of Hox gene expression that antagonize the action of Trithorax group genes (Kennison and Tamkun, 1988; Lewis, 1978; Nusslein-Volhard et al., 1985; Simon et al., 1992). Biochemical and molecular analyses have defined at least three protein complexes, called Polycomb repressive complex 2 (PRC2), PhoRC and PRC1. PRC2 is a histone methyltransferase that methylates Histone 3 lysine 27, and includes the proteins Enhancer of zeste, Extra sex combs, Su(z)12 and Nurf-55 (Cao et al., 2002; Czermin et al., 2002; Kuzmichev et al., 2002; Muller et al., 2002). The H3K27me3 histone mark is in turn recognized by the PRC1 complex (Cao et al., 2002; Czermin et al., 2002; Kuzmichev et al., 2002; Saurin et al., 2001), which has as core components in human cells Ring1, Ring2/Ring1B, Bmi-1 and HPH2 (Wang et al., 2004a). PRC1 is recruited to sites methylated by PRC2, where the Ring1B protein of PRC1 monoubiquitylates H2A at lysine 119 (Wang et al., 2004a; Wang et al., 2004b). In this complex, BMI-1 enhances the stability and enzymatic activity of Ring1B (Cao et al., 2005). PRC1 inhibits gene expression through mechanisms that are as yet not entirely clear but which may involve repressing the initiation of transcription, inhibiting nucleosome remodeling, regulating association of linker histone H1 and/or chromatin compaction (Francis et al., 2004; King et al., 2002; Levine et al., 2002; Shao et al., 1999; Zhu et al., 2007). Recent genome-wide identification of Polycomb group target genes has also shown that some targets are transcribed despite the presence of Polycomb group proteins on the gene, suggesting that repression may not be a universal outcome at all loci (Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006; Tolhuis et al., 2006).
Caenorhabditis elegans orthologs of the PRC2 complex have been identified and well characterized. These include the products of the mes-2, mes-3 and mes-6 genes, mutations in which result in maternal effect sterility (Capowski et al., 1991). The MES proteins form a complex in which the SET domain of MES-2 mediates di- and trimethylation of H3K27 (Bender et al., 2004; Holdeman et al., 1998). The H3K27me3 mark is concentrated on the X chromosome of wild-type animals, and transcription of X chromosome genes is normally silenced in the germline (Fong et al., 2002; Xu et al., 2001b). In mes-2, mes-3 and mes-6 mutants chromatin marks associated with active chromatin are found on the X chromosome and, according to the current model, inappropriate expression of X chromosome genes in the germline is responsible for the degeneration of germ cells and the sterility observed in mes mutants (Fong et al., 2002).
In addition to their role in the germline, mes genes also act in somatic cells. mes-2, mes-3 and mes-6 mutants have weak but reproducible defects consistent with abnormal Hox gene activity, in agreement with the classical role of Polycomb group genes as repressors of Hox gene activity (Ross and Zarkower, 2003). The abnormalities include subtle defects in migration by specific neurons, expansion of the domains of Hox gene expression, and mislocalization of sensory rays in the male tail. The genetic screens that identified the mes genes did not identify genes homologous to PRC1 components, and the C. elegans genome does not encode obvious homologs of many of the components of PRC1. These data suggest that either C. elegans lacks a PRC1 complex, which would suggest that PRC2 function could be uncoupled from PRC1 function, or that the function, composition or amino acid sequences of the proteins in a PRC1-like complex are sufficiently divergent to make recognizing them difficult.
Here we describe the genes mig-32 and spat-3, which encode homologs of the human PRC1 core components Bmi-1 and Ring1B, respectively. Consistent with MIG-32 and SPAT-3 being functionally analogous to PRC1, ubiquitylation of H2A is markedly reduced or absent in mig-32 and spat-3 mutants. Both mutants have defects in their nervous system that are similar those of mes mutants. Surprisingly, unlike mes mutants, mig-32 and spat-3 mutants are fertile, suggesting that H2A ubiquitylation by PRC1 is not required in the germline for MES/PRC2 function.
MATERIALS AND METHODS
Alleles and strains
All strains were maintained at 20°C as described by Brenner (Brenner, 1974).
Mutations and mapped integrated transgenes used were as follows. LGI: mig-1(e1787); mes-3(bn35); unc-73(e936); unc-40(e271); ced-1(e1735). LGII: mes-2(bn11); unc-4(e120); muIs32 [Pmec-7gfp] (Pujol et al., 2000); muIs16 [Pmab-5gfp] (Hunter et al., 1999); nIs128 [Ppkd-2gfp] (Yu et al., 2003). LGIII: pal-1(e2091); mig-10(ct41); unc-119(ed3). LGIV: plx-1(nc37); ced-10(n1993); mes-6(bn38); dpy-20(e1282); mig-32(n4275) (kindly provided by the Horvitz lab, MIT, Cambridge MA, USA), mig-32(tm1807), mig-32(tm1684) (kindly provided by the Mitani lab, Tokyo Women's University, Tokyo, Japan); unc-31(e928). LGV: him-5(e1490). LGX: nIs106 [Plin-11gfp] (Cameron et al., 2002), kyIs4 [Pceh-23gfp] (Zallen et al., 1998); oxIs12 [Punc-47gfp] (McIntire et al., 1997); unc-6(ev400); mig-2(mu28); slt-1(eh15); lin-15(n765ts); spat-3(gk22).
Integrated transgenes not mapped to a chromosome included: kyIs39 [Psra-6gfp] (Troemel et al., 1995); bxIs13 [Pegl-5gfp] (Zhang and Emmons, 2001); mxIs23 [Pmig-32mig-32:gfp].
Extra-chromosomal arrays included: rtEx238 [Pnlp-1gfp] (kindly provided by Leon Avery, University of Texas Southwestern Medical Center, Dallas TX, USA); mxEx53 [Pplx-1mig-32:cfp].
Imaging
Transgenic animals were imaged using a Zeiss Axiophot. To quantitate HSN and ALM migrations, the distance along the body axis between the rectum and the HSN or ALM nuclei was determined using Openlab software and compared to the distance to the vulva.
Construction and analysis of mig-32/Df animals
sDf62 unc-31(e169)/nT1(IV); +/nT1(V) hermaphrodites were mated with him-5(e1490) males and cross-progeny males were mated with mig-32(n4275) unc-31(e928) hermaphrodites. The position of ray-1 in Unc males was determined.
Plasmid constructs and Pmig-32mig-32:gfp construction
To construct Pmig-32 mig-32:gfp, an 8065 bp BamHI/SphI fragment of the F11A10 cosmid was cloned into pUC19. An XmaI fragment of pPD102.33, including the GFP coding sequences, was cloned into the AgeI site in the first exon of mig-32. This construct was injected into lin-15(765ts) worms, integrated and backcrossed three times to mig-32(n4275); him-5(e1490).
To construct Pplx-1mig-32::cfp, an EcoRI-XbaI fragment of the full-length yk1321a11 mig-32 cDNA was cloned into pUC19. CFP coding sequences were amplified by PCR and cloned into mig-32 at the AgeI site. The mig-32 start codon was converted into an NsiI site using site-directed mutagenesis, and 2.6 kb of the plx-1 promoter (Dalpe et al., 2004) was PCR amplified and cloned into the NsiI site. Constructs were injected into unc-119(ed3); mig-32(n4275); him-5(e1490) worms using unc-119 as the co-injection marker. We used the position of Ray 1 as an assay for mig-32 function. Of transgenic mig-32(n4275) mutants carrying the Pmig-32mig-32:gfp reporter (n=50), 28±6% had anterior Ray 1s, compared with 64±5% of mig-32 mutants (n=100).
To construct the spat-3a(RNAi) plasmid, 889 nucleotides of the spat-3a genomic region flanking the sequences that encode the RING domain were amplified by PCR and cloned into the L4440 plasmid as a HindIII-BglII restriction fragment.
Core histone extraction and western blotting
Packed L1-stage worms (0.5 ml) were harvested from freshly-starved plates. Worms were sonicated in 5 ml NIB buffer [15 mM PIPES (pH 6.8), 5 mM MgCl2, 60 mM KCl, 0.25 M sucrose, 15 mM NaCl, 1 mM CaCl2, 0.8% Triton and protease inhibitors] (Jackson et al., 2004) until the cuticle was completely broken. Lysate was centrifuged at 10,000 g for 10 minutes. The pellet was resuspended in 2 ml of 0.4 N H2SO4 and incubated at 4°C for 1 hour to overnight. The soluble material was dialyzed with PBS or precipitated with 20% TCA. TCA-precipitated histone extract was washed with cold acetone three times and the final pellet dried and dissolved in water. For detection of H2A, rabbit antiserum against histone H2A from Upstate (#07-146) was used at 1/1000 dilution. For detection of ubiquitylated histones, monoclonal anti-ubiquitin (clone P4D1) from Cell Signaling Technology was used at 1/1000 dilution.
RESULTS
MIG-32 is a RING-domain-containing protein homologous to Polycomb group family members, including the core component BMI-1
We identified MIG-32 and SPAT-3A as proteins most similar to core components of the PRC1 complex. As the existence of a PRC1-like complex has been uncertain in C. elegans, the genetic pathways in which mig-32 and spat-3 might participate are unclear. We therefore identified mutations in mig-32 and spat-3 to study their function.
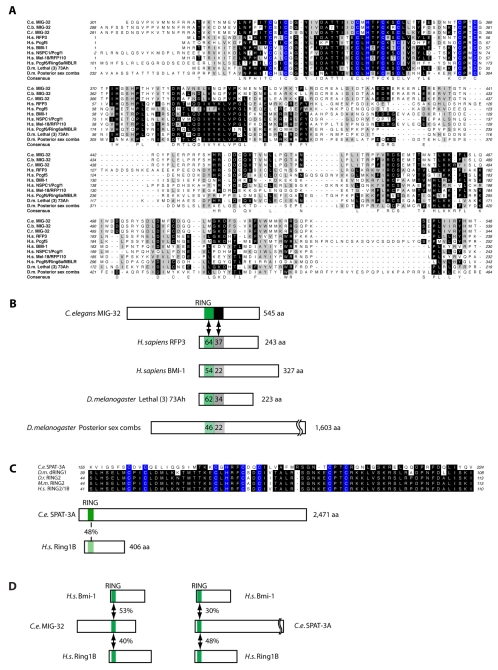
We first confirmed the gene structure predictions for mig-32 by determining the DNA sequences of five full-length mig-32 cDNAs, and found that all were identical in the predicted coding sequences and were SL2-spliced, consistent with genome database predictions that mig-32 is the second gene in a three-gene operon (WormBase, http://www.wormbase.org, release WS193, July, 2008) (Spieth et al., 1993). mig-32 is predicted to encode a 542 amino acid protein with no close homologs in the completely sequenced C. elegans genome, and with a predicted RING domain as the only domain recognized by Pfam (Bateman et al., 2002). Using BLAST searches we identified MIG-32 homologs in vertebrate genomes, including six homologous proteins from humans (Fig. 1). Included in this group are the BMI-1 protein and the related proteins MEL-18/RFP110, NSPc1/PcGRF1 and Pcgf5, which participate in PRC1-related complexes (Alkema et al., 1997; Sanchez et al., 2007; Trimarchi et al., 2001). The Drosophila melanogaster genome contains two homologs: Posterior sex combs, a component of the Drosophila PRC1 complex (Saurin et al., 2001), and Lethal (3) 73 Ah, an essential gene (Belote et al., 1990; Irminger-Finger and Nothiger, 1995). We identified a single MIG-32 homolog in the genomic sequences of each of the nematodes C. briggsae and C. remanei (Fig. 1). These data suggest that MIG-32 is a RING domain protein most similar to core components of human and Drosophila PRC1 and related complexes.
Fig. 1.
MIG-32 and SPAT-3A are RING-domain proteins closely related to Polycomb-group family members. Alignment and comparison of MIG-32 and SPAT-3A with related proteins from other species. (A) Amino acids highlighted in blue indicate the RING domain. (B) MIG-32 compared with related human and Drosophila proteins. The green box indicates the RING domain; the black box indicates a C-terminal region that participates in complex formation with RING1B (Li et al., 2006). Percentage amino acid sequence identities (shown as a number within the compared regions) are indicated in pair-wise comparisons between MIG-32 and the other proteins. (C) Amino acid sequence alignments of the RING domain of SPAT-3A and dRING1 with related proteins. There is little or no additional sequence similarity between SPAT-3A and RING1B/RING2 homologs from these other species. (D) Pair-wise comparisons of the amino acid identities between the RING domains of MIG-32 and SPAT-3A from C. elegans with BMI-1 and RING1B from humans.
We identified three deletion alleles of mig-32 (see Materials and methods). Each of the alleles deletes mig-32 genomic coding sequences and not coding sequences of the upstream or downstream genes in the operon. mig-32(n4275) mutants, and mutants carrying the tm1684 and tm1807 alleles, are homozygous viable and have defects in the male tail and other structures as outlined briefly here and in greater detail below.
The male tail has nine bilateral sensory ray structures (Emmons, 2005). We used the defect in the position of one of these rays to examine the consequences for mig-32 function of the three deletion alleles. All three alleles result in qualitatively and quantitatively similar defects in the position of Ray 1, with 60-80% of the mutants having an anterior Ray 1; all three also result in similar defects in migration of the HSN neurons (see below). The alleles are recessive, and 75% of mutants of genotype n4275 over sDf62, a chromosomal deficiency that deletes the mig-32 region, have defects in Ray 1 position similar to those of mig-32 homozygotes (data not shown).
Three observations suggest that the deletions specifically affect mig-32 function and not the function of F11A10.8 or F11A10.6, the upstream and downstream genes in the operon, respectively. First, F11A10.8 encodes a very-well-conserved homolog of human CPSF4, a splicing factor (WormBase). Inactivation of F11A10.8 by RNAi is lethal, as is a deletion mutation, ok844, which deletes parts of the F11A10.8 and mig-32 coding regions (WormBase), suggesting that the mig-32(n4275), tm1684 and tm1807 deletions do not severely impair F11A10.8 function. Second, RNAi of mig-32, which primarily targets processed mRNA (Fire et al., 1998), results in an anterior position of Ray 1, as we observed in mig-32 mutants. Third, expression using the plx-1 promoter (Dalpe et al., 2004) of a mig-32 cDNA in the male tail rescued the anterior Ray 1 defects of mig-32(n4275) mutants. Specifically, of 50 mig-32(n4275) mutants carrying a Pplx-1mig-32:cfp transgene, 12±5% (standard error of the proportion) had an anterior Ray 1 compared with 64±5% of 100 uninjected controls, and in contrast to mig-32 mutant males the transgenic males mated efficiently (see below and data not shown). These data suggest that the n4275, tm1684 and tm1807 alleles are strong loss-of-function or null alleles of mig-32. Unless otherwise indicated, we used the mig-32(n4275) allele for the experiments described here.
Remarkably, the brood sizes of mig-32 mutants are similar to those of the wild-type strain, N2. Specifically, the brood sizes of n4275, tm1684 and tm1807 mutants are 81, 71 and 63% of the wild type. Eggs laid by mig-32 mutants hatch at rates similar to those of wild-type animals (Table 1 and data not shown).
Table 1.
Brood sizes of mig-32 and spat-3 mutants
| Genotype | Avg brood size | n |
|---|---|---|
| Wild type | 234 | 2,804 |
| mig-32(n4275) | 190 | 2,284 |
| mig-32(tm1807) | 167 | 2,002 |
| mig-32(tm1684) | 148 | 1,775 |
| spat-3(gk22) | 209 | 2,513 |
The average (Avg) number of progeny was determined for twelve hermaphrodites of the indicated genotypes
n, total number of progeny
SPAT-3A is a RING domain protein homologous to the PRC1 core component RING1B
spat-3 was originally identified in RNAi screens as a suppressor of the embryonic polarity gene par-2, although the mechanism of suppression is unknown (Labbe et al., 2006). spat-3 is predicted to encode two large protein products, SPAT-3A and SPAT-3B, generated by alternative promoters and supported by partial cDNA evidence (WormBase). The SPAT-3A protein is 2471 amino acids and contains a RING domain at the N-terminus that is most similar to that of the Ring1B proteins of mammals, although the similarity is very poor (Fig. 1). SPAT-3B lacks the RING domain, and neither protein has additional recognized domains. Using the BLAST algorithm, SPAT-3A is the RING domain protein in the C. elegans genome most similar to Ring1B; the next most similar protein is MIG-32 (Fig. 1). A deletion allele of spat-3, gk22, deletes the genomic sequences common to the spat-3a and spat-3b transcripts, and if transcribed and translated is predicted to truncate the SPAT-3A protein after position 1620. As such, it may represent a loss-of-function but not null allele, as the RING domain is very near the N-terminus (Fig. 1). spat-3(gk22) mutants are viable and healthy, with a brood size that is 90% of wild-type animals (Table 1).
MIG-32 and SPAT-3A are required for ubiquitylation of histone H2A
The defined biochemical function of PRC1 is ubiquitylation of histone H2A at position 119. In PRC1, the Ring1B protein serves as the E3 that catalyzes H2A ubiquitylation (Wang et al., 2004a). Other core components, especially BMI-1, stimulate the catalytic activity of Ring1B (Cao et al., 2005; Li et al., 2006; Wei et al., 2006), possibly by promoting folding and stability of Ring1B (Ben-Saadon et al., 2006). We therefore asked whether H2A ubiquitylation was abnormal in mig-32 and spat-3 mutants. We analyzed histone modification using western blots of acid-extracted histones from wild-type, mig-32 and spat-3 mutant C. elegans. Using an H2A-specific antibody and extracts from wild-type animals, we detected H2A and a rare, higher molecular weight band that migrated at the size expected for ubiquitin-modified H2A; this band was not detected in extracts from mig-32 or spat-3 mutants (Fig. 2). Using an antibody that detects ubiquitin with histone extracts from wild-type animals, we detected two bands that correspond to the predicted molecular weights of H2A and H2B, both of which are modified by ubiquitylation (Osley, 2006). H2A, the smaller of these bands, is not detected in extracts from mig-32 mutants and is greatly reduced in spat-3 mutants (Fig. 2). These data suggest that MIG-32 and SPAT-3A are required for ubiquitylation of Histone H2A, the defining biochemical function of PRC1.
Fig. 2.
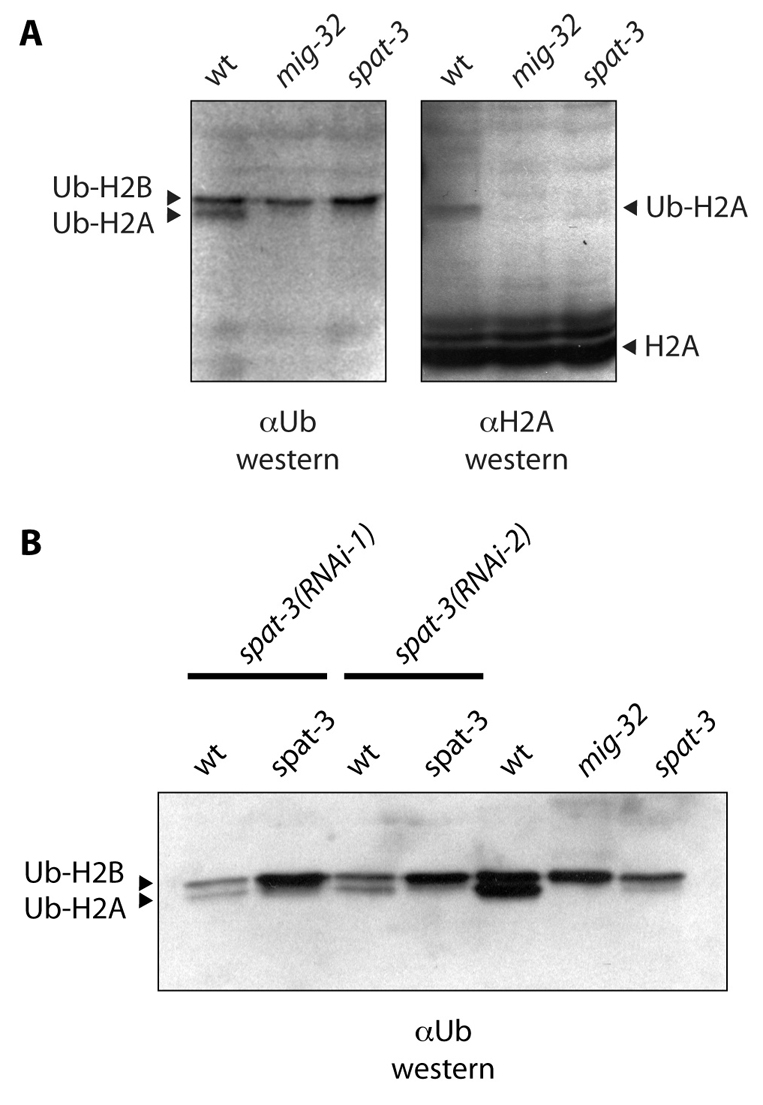
mig-32 is required for ubiquitylation of histone H2A. (A) Western blot analysis of acid-extracted histones from wild-type, mig-32(n4275) and spat-3(gk22) mutant C. elegans. The filter shown in the left panel was probed with anti-ubiquitin antibody. Arrowheads indicate ubiquitylated H2A and H2B. In the right panel, this blot was stripped and probed with anti-H2A antibody. Arrowheads indicate H2A and ubiquitylated H2A. (B) spat-3 mutants have greatly reduced levels of ubiquitylated H2A. A western blot using anti-ubiquitin antibody detects a trace amount of ubiquitylated H2A in most preparations of acid-extracted histones from spat-3(gk22) mutants, consistent with this allele being strong loss-of-function, but not null. A spat-3(RNAi) construct was fed to wild-type animals in two experiments [spat-3(RNAi-1) and spat-3(RNAi-2)]. The RNAi construct reduces, but does not eliminate, ubiquitylated H2A levels. Loading was not equal in all lanes of this blot.
mig-32 is broadly expressed, localized to nuclei and concentrated within nucleoli
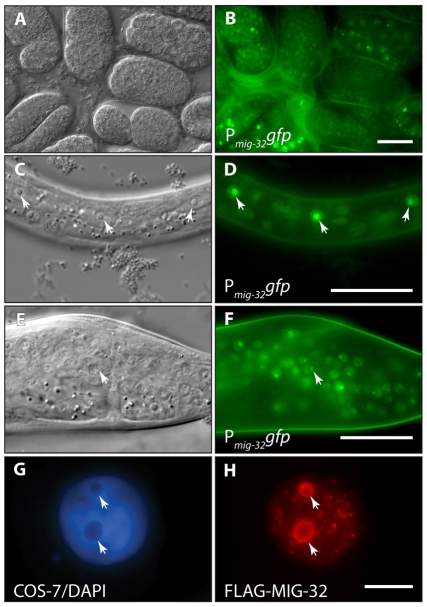
In situ hybridization using a mig-32 cDNA suggested that the gene is expressed prominently in the C. elegans germline (Y. Kohara, personal communication). We constructed a rescuing GFP reporter to determine the expression pattern in somatic cells (see Materials and methods). Consistent with MIG-32 acting as a modifier of chromatin, the Pmig-32mig-32:gfp reporter is expressed broadly in most or all nuclei, beginning early in embryogenesis and continuing in larval development and into adulthood of males and hermaphrodites (Fig. 3 and data not shown). Expression is predominantly nuclear, with relatively bright intranuclear areas of fluorescence that correspond with nucleoli evident as seen with Nomarski optics, particularly in some hypodermal cells where nucleoli are easily identified (Fig. 3). Transfection of mammalian cells with a FLAG-epitope-tagged MIG-32 showed nuclear expression concentrated in a shell around nucleoli, suggesting that the interactions that determine subcellular localization may be evolutionarily conserved (Fig. 3). We considered the possibility that localization of MIG-32 might depend upon mes gene activity. To test this, we examined the expression and localization of the Pmig-32mig-32:gfp reporter in mes-2 mutants and observed that expression of the reporter is brighter in the mes-2 mutant background but that the fusion protein remains localized to nuclei and nucleoli (data not shown).
Fig. 3.
mig-32 is broadly expressed, localized to nuclei, and concentrated in nucleoli. Differential interference contrast (A,C,E) and epifluorescence (B,D,F) images of transgenic C. elegans. (A,B) Mixed-stage embryos. (C,D) L1-stage larva showing the lateral hypodermal cells. Arrowheads highlight nucleoli within hypodermal nuclei. (E,F) L4-stage male tail. Arrowhead highlights a nucleolus within a neuronal nucleus. (G,H) COS-7 cell transfected with FLAG-epitope-tagged C. elegans MIG-32, stained with (G) DAPI to visualize DNA and (H) anti-FLAG antibody. Arrowheads highlight nucleoli in the COS cell nucleus. In C-F, anterior is to the left and ventral down. Scale bars: 10 μm.
mig-32 and spat-3 have similar defects in the anatomy of the male tail, and mig-32 may act with the mes genes to position Ray 1
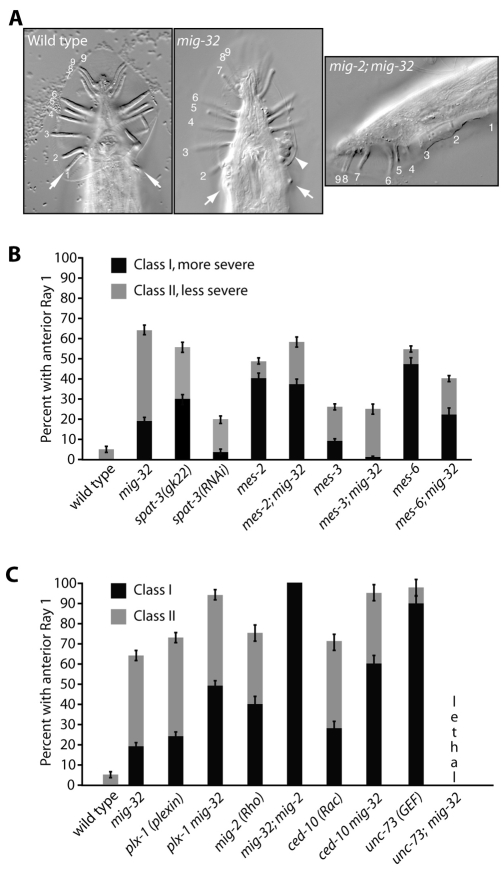
We observed that mig-32 and spat-3 mutant males mate very poorly. Observation of the male tail showed that Ray 1 is located abnormally anterior in mig-32 and spat-3 mutants, and also in mig-32(RNAi) and spat-3a(RNAi) animals (Fig. 4). Several genetic pathways position Ray 1 appropriately in males. These include the mes-2, mes-3 and mes-6 genes, which encode C. elegans homologs of the PRC2 histone methyltransferase complex (Bender et al., 2004; Ross and Zarkower, 2003). We constructed double mutants between mig-32 and putative null alleles of each of the mes genes. Strikingly, the Ray 1 position defects of these double mutants were not enhanced (Fig. 4), suggesting that mig-32 acts in the same genetic pathway as the mes genes to position Ray 1.
Fig. 4.
mig-32 acts parallel to most known pathways that position Ray 1, with the exception of the PRC2 pathway. (A) Ventral views of the tails of male wild-type and mig-32 mutants and a lateral view of a mig-2; mig-32 mutant male. The nine bilateral rays are numbered. Arrows indicate Ray 1 pairs in the wild-type and mig-32 mutants; an arrowhead indicates crumpled Rays 2 and 3 in the mig-32 mutant. (B) The percentage of animals with anterior Ray 1 in wild-type and mutant animals. The severity of the Ray 1 migration defect is categorized as Class I, in which the ray is located anterior to and outside of the normal position of the cuticular fan, or as Class II, in which the ray is anterior but present within the fan (Fujii et al., 2002; Ginzburg et al., 2002). (C) The percentage of animals with anterior Ray 1 in wild-type and mutant animals of the indicated genotypes, scored as in B.
Several additional pathways position Ray 1, including signaling by semaphorins and a Plexin receptor (Fujii et al., 2002; Ginzburg et al., 2002), acting through the unc-73 guanine nucleotide exchange factor and the ced-10/Rac mig-2/Rho GTPases (Dalpe et al., 2004). We constructed double mutants between mig-32 and each of these genes and examined the position of Ray 1 in the male tails of the animals. In each case we identified significantly enhanced defects in the position of Ray 1 (Fig. 4), suggesting that mig-32 acts parallel to these pathways to position Ray 1.
mig-32 and spat-3a are required for HSN neuronal migration and axon extension
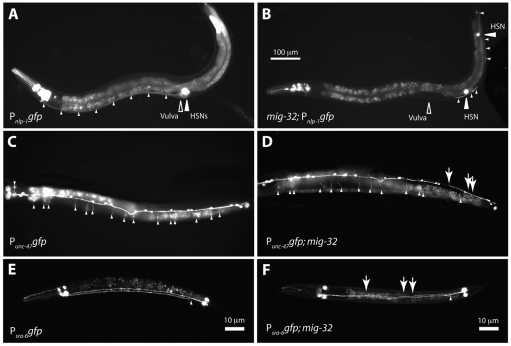
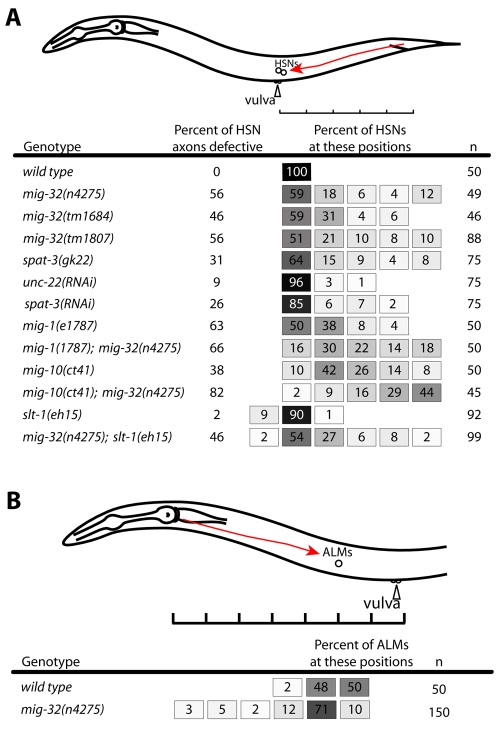
We observed that mig-32 mutants were variably egg-laying defective, with some animals in a population carrying more eggs than wild-type animals. In staged adults the wild-type strain N2 carried an average of 17.5±5 eggs, compared with 28.8±11 eggs in mig-32 mutants (n=55 animals for each genotype, P<0.0001, unpaired two-tailed t-test). Egg-laying requires a vulva through which the eggs are laid, muscles to expel the eggs, and neurons to control the vulval muscles (Trent et al., 1983). We found that the two HSN neurons, which are essential for egg-laying (Trent et al., 1983), are often abnormal in mig-32 mutants (Figs 5 and 6). During embryogenesis the HSN neurons migrate from the tail to the midbody (Sulston et al., 1983). Using the Pnlp-1gfp reporter to identify the HSN neurons (Li et al., 1999), we observed that the HSNs of mig-32(n4275) mutants failed to reach the midbody in 41% of mutants; by comparison, all HSNs migrated to their normal position in otherwise wild-type animals carrying the Pnlp–1gfp reporter (Fig. 6). The axons extended by the HSN neurons were also abnormal. In wild-type animals, each HSN extends an axon from the vulval region ventrally to the ventral nerve cord; the axons then turn anterior and extend to the head. Of the HSN axons of mig-32 mutants, 56% failed to reach the head; by comparison, all HSN axons extended to the head of otherwise wild-type animals carrying the Pnlp-1gfp reporter (Figs 5 and 6). All HSNs of mig-32 mutants expressed the Pnlp-1gfp reporter, suggesting that the HSN neurons correctly establish their identity and that the defects in HSN migration result from a requirement for mig-32 in other processes important for migration and axon extension. The defects in HSN migration and axon extension are likely to account for the variable defects in egg-laying we observed in mig-32 mutants; such variability has been associated with other mutants with defects in HSN migration (Desai et al., 1988).
Fig. 5.
mig-32 mutants have defects in neuronal migration and process extension. Images of wild-type and mig-32 mutant animals carrying gfp reporters. (A) An otherwise wild-type animal expressing Pnlp-1gfp in the HSN neurons. HSN cell bodies are indicated with a large white arrowhead. The position of the vulva is indicated. Small white arrowheads highlight the axons of the two HSN neurons, which proceed along the ventral body wall to the head. (B)A mig-32 mutant expressing Pnlp-1gfp. Large white arrowheads indicate the HSN cell bodies and small arrowheads highlight the HSN axons. (C) Ventral view of a wild-type animal expressing Punc-47gfp in the VD neurons. Small white arrowheads indicate lateral commissures. (D) Ventral view of a mig-32 mutant expressing Punc-47gfp in the VD neurons. Small white arrowheads indicate lateral commissures. Large arrows indicate three commissures on the wrong side. (E) A wild-type animal expressing Psra-6gfp in the PVQL and PVQR neurons. A small white arrowhead indicates the normal separation of the PVQ axons into the right and left sides of the ventral nerve cord. (F)A mig-32 mutant expressing Psra-6gfp in the PVQ neurons. Arrows indicate inappropriate crossing by the PVQ neuronal axons. The scale bar in B applies to A-D, which show adult animals. E and F show L1-stage larvae. Anterior is to the left in all images; ventral is down in A and B, up in C and D, and slightly rotated in E and F.
Fig. 6.
mig-32 and spat-3 act similarly in HSN migration. (A) The embryonic migration path (red arrow) and final positions of the HSN neurons in wild-type and mutant transgenic animals. The Pnlp-1gfp reporter, which is expressed in the HSNs (Li et al., 1999), was used to identify the final positions of the HSNs (see Materials and methods). The shaded boxes indicate the percentage of animals with HSNs in specific regions. The percentage of HSN axons that failed to reach the head is shown. n, number of HSNs assayed. (B) The migration path (red arrow) and final positions of the ALM neurons. The Pmec-7gfp reporter, which is expressed in the ALMs (Hamelin et al., 1992), identified their final positions.
mig-32 could have two distinct roles: modification of H2A to regulate gene expression and a developmental role in neuronal migration that is independent of H2A ubiquitylation. If the defects we observed in the nervous system of mig-32 mutants reflect the role of MIG-32 as part of a PRC1-like complex that includes SPAT-3A, spat-3 mutants should have defects very similar to those of mig-32 mutants. Indeed, the HSN neurons of spat-3 mutants failed to migrate appropriately, and were defective to an extent similar to that observed in mig-32 mutants (Fig. 6). Like mig-32 mutants, all HSNs of spat-3 mutants expressed the Pnlp-1gfp reporter, suggesting that the HSNs of spat-3 mutants also correctly establish their identity.
mig-32 mutants have additional defects in laterality of commissures, process extension and midline crossing by axons
Using gfp reporters, we surveyed mig-32 mutants for defects in neuronal migration and processes extended by other neurons. The Punc-47gfp reporter is expressed in the VD and DD motoneurons of the ventral nerve cord (McIntire et al., 1997). In otherwise wild-type animals carrying the Punc-47gfp reporter, the VD and DD neurons extend commissures from the ventral nerve cord laterally along the body wall to the dorsal nerve cord. In wild-type animals, all but one pair of commissures track along the right side of the animal; only 4% of otherwise wild-type animals carrying the Punc-47gfp reporter had more than one pair of commissures on the wrong side (Fig. 5; Table 2). By contrast, 63% of mig-32 mutants had more than two left-sided commissures, with some animals having as many as five commissures on the wrong side (Fig. 5; Table 2). The number and positions of VD and DD neurons, the total number of commissures and the expression of Punc-47gfp were normal in mig-32 mutants (data not shown).
Table 2.
Quantification of process defects in mig-32 mutants
| Genotype | Cell process | Animals with a defect (%) | n |
|---|---|---|---|
| Pnlp-1gfp | HSN axon | 0 | 50 |
| mig-32(n4275), Pnlp-1gfp | HSN axon | 56 | 49 |
| mig-32(tm1684), Pnlp-1gfp | HSN axon | 46 | 46 |
| mig-32(tm1807), Pnlp-1gfp | HSN axon | 56 | 88 |
| Psra-6gfp | PVQ axons | 8 | 50 |
| mig-32; Psra-6gfp | PVQ axons | 26 | 87 |
| Punc-47gfp | VD commissures | 4 | 25 |
| mig-32; Punc-47gfp | VD commissures | 63 | 100 |
The percentage of animals with a defect in specific neuronal processes is shown. For the HSN axons, animals were scored as defective if the axon failed to reach the head; in general, axons that reached the head followed a normal path from the HSN ventrally into the ventral nerve cord then turned anterior to the head. HSN neurons that failed to migrate to the midbody often had more severe defects in axon pathfinding, with axons that tracked posterior rather than anterior. For the PVQ axons, defective axons included those that crossed the midline inappropriately, as compared with wild-type animals. For the VD commissures, defective commissures included those that tracked on the wrong side of the body wall. mig-32 mutants had 0-5 commissures on the wrong side, with posterior VD neurons being more likely to have defective commissures
We also identified defects in midline crossing by the PVQR and PVQL neurons. The PVQ neurons are located in the lumbar ganglion and each extends an axon anteriorly to the head. Initially, both axons extend along the right side of the hypodermal ridge that divides the ventral nerve cord. The PVQL axon then crosses the midline to the left side and proceeds to the head (White et al., 1986). Using the Psra-6gfp reporter, which is expressed in the PVQ neurons (Troemel et al., 1995), we observed that the PVQ axons of mig-32 mutants did not respect the midline boundary and crossed inappropriately (Fig. 5; Table 2). Of the PVQ axons of mig-32 mutants, 26% crossed the midline inappropriately, compared with 8% of otherwise wild-type animals carrying the Psra-6gfp reporter. All PVQ neurons of mig-32 mutants expressed Psra-6gfp at levels similar to wild-type animals.
Some neurons and axons of mig-32 mutants migrated and extended processes normally, whereas others were slightly abnormal. The CAN neurons originate in the head and migrate posteriorly to a position adjacent to the vulva. Using a Pceh-23gfp reporter, we observed that all 50 CAN neurons of mig-32 mutants migrated appropriately and extended axons as in the wild type (data not shown). Several of the mechanosensory neurons migrate along the anteroposterior body axis during development (Hamelin et al., 1992). The Q cells migrate anteriorly and divide, with the QR cell (right-sided Q cell) generating the AVM mechanosensory neuron and QL (left-sided Q cell) the PVM neuron. The ALM neurons migrate posteriorly from the head. Using the Pmec-7gfp reporter to label the mechanosensory neurons and their axons, we found that the ALM neurons of mig-32 mutants were slightly defective in posterior migration. Of 150 ALM neurons observed in mig-32 mutants, 15 ALMs did not complete their migrations (Fig. 6). The processes of the ALM neurons were similar in mig-32 and wild-type animals (data not shown). The PLM neurons did not migrate, but their posterior processes were often foreshortened in mig-32 mutants. Of 100 PLM neurons of mig-32 mutants, the posterior process terminated prematurely in 55% of animals, extending less than half the normal distance. By contrast, the posterior processes of the PLM neurons terminated prematurely in only 6% of 100 wild-type animals. The locations of the AVM and PVM neurons and the morphology of their processes were similar, comparing 75 mig-32 mutants with 50 wild-type animals (data not shown).
Migration of the distal tip cells, which are somatic cells that lead the anterior and posterior arms of the proliferating germline along the body wall, appeared normal, comparing 23 mig-32 mutants with 18 wild-type animals (data not shown).
In summary, mig-32 is required for normal migration of the HSN neurons and for extension of some neuronal processes. It participates in ensuring that VD neuronal commissures extend along the correct side of the animal, and that the PVQ axons do not cross the midline inappropriately. The defects we observed in mig-32 mutants are unlikely to be a result of markedly altered neuronal differentiation, because expression of all the gfp reporters used for these experiments were expressed in the expected patterns in mig-32 mutants (a list of reporters used is found in Materials and methods).
mig-32 acts parallel to most known pathways that act in HSN migration
Several genetic pathways have been identified that ensure correct migration of the HSN neurons from the tail to the vulva. To determine whether mig-32 acts within one of these pathways, we constructed double mutants between mig-32 and other genes that regulate HSN migration, including mig-1, a Wnt receptor and Frizzled homolog (Pan et al., 2006), mig-10, a cytoplasmic protein that mediates attractive and repulsive guidance signals by unc-6/Netrin and slt-1 (Chang et al., 2006; Quinn et al., 2006), respectively, and slt-1 (Hao et al., 2001). In each case, the double mutants with mig-32 had significantly enhanced defects in HSN migration assayed with the Pnlp-1gfp reporter (Fig. 6). These data suggest that mig-32 acts genetically parallel to these pathways to promote HSN migration.
mig-32 and spat-3 repress ectopic vulval development
The lin-15 locus is an operon that includes two genes, lin-15A and lin-15B, both of which are redundant repressors of a vulval fate for hypodermal descendants of the P cells in the vulval equivalence group (Clark et al., 1994). Mutants carrying the temperature-sensitive allele n765 are morphologically normal when raised at 15°C but show a multiple vulva (Muv) phenotype when raised at 20°C; the lin-15(n765ts) allele has a single mutation that impairs function of both lin-15A and lin-15B (Cui et al., 2008). During strain constructions, we observed that mig-32; lin-15(n765ts) double mutants are 100% Muv at 15°C (data not shown). We used this observation to test whether spat-3 and mig-32 might act similarly to repress vulval fates. We subjected lin-15(n765ts) mutants to mig-32(RNAi) or spat-3a(RNAi) and raised the animals at 15°C; the spat-3a(RNAi) construct specifically targeted the spat-3a transcript that encodes the RING domain. Reducing either mig-32 or spat-3a activity resulted in a highly penetrant Muv phenotype. Seventy-three percent of lin-15(n765ts); mig-32(RNAi) mutants (n=320) and 42% of lin-15(n765ts); spat-3(RNAi) mutants (n=200) were Muv, compared with 3% of lin-15(n765ts); unc-22(RNAi) mutants (n=100). These data suggest that mig-32 and spat-3a act similarly to repress vulval fates in hypodermal cells that do not normally contribute to vulval development.
DISCUSSION
In this manuscript we report the consequences for C. elegans of the loss of two proteins homologous to core subunits of the PRC1 complex, MIG-32 and SPAT-3A. MIG-32 and SPAT-3A are most similar to families of proteins that include Bmi-1 and Ring1B, respectively. In mammals, Ring1B is the E3 ligase in PRC1 that modifies H2A, and BMI-1 is a physical partner of Ring1B that promotes the stability and catalytic activity of Ring1B (Cao et al., 2005; Li et al., 2006; Wang et al., 2004a; Wang et al., 2004b). Consistent with MIG-32 and SPAT-3A acting together in a PRC1-like complex, mig-32 and spat-3 mutants are markedly defective in H2A ubiquitylation and have very similar defects in the anatomy of their nervous systems and as repressors of ectopic vulval fates in hypodermal cells. We therefore propose that MIG-32 and SPAT-3A are core subunits of a PRC1-like complex in C. elegans, although we have not yet demonstrated complex formation by these proteins.
The relationship of MIG-32 and SPAT-3A with PcG complexes of C. elegans
There are at least two complexes in C. elegans that are functionally related to the Drosophila and mammalian Polycomb repression complexes. Strong genetic and biochemical evidence indicates that the MES-2–MES-3–MES-6 complex is functionally analogous to the PRC2 complex (Xu et al., 2001a), which places the histone H3K27me3 mark characteristic of Polycomb repression (Bender et al., 2004; Fong et al., 2002; Holdeman et al., 1998; Xu et al., 2001a). mes mutants are sterile, probably as a consequence of inappropriate expression of genes normally silenced in the developing germ cells. If PRC1 were required for regulation of gene expression by PRC2, mig-32 and spat-3 mutants might be expected to share the Mes phenotype of sterility. However, both mutants are healthy and fertile. mig-32 and spat-3 could be redundant with other proteins or complexes responsible for H2A ubiquitylation in the germline, or H2A ubiquitylation could be dispensable for the silencing function that is thought to be the essential role of the mes genes in ensuring germline integrity. We favor the latter possibility, as western blots of L1-stage wild-type animals with rudimentary germlines have relatively high steady state levels of ubiquitylated H2A and H2B, but we detected little ubiquitylated histone in L4 or young adult animals with proliferative germlines (our unpublished observations).
More recently the sop-2, sor-1 and sor-3 genes have been proposed as components of a distinct Polycomb complex-like repressive mechanism in C. elegans. Mutations affecting these genes result in expanded domains of Hox gene expression (Wang et al., 2004a; Yang et al., 2007; Zhang et al., 2003; Zhang et al., 2004). All are essential genes, and mutants carrying partial loss-of-function alleles have severe defects not observed in mes null mutants, suggesting that the sop and sor genes have many important functions in somatic cells and do not simply maintain the pattern of gene expression established by the mes genes, which have subtle functions in somatic cells (Ross and Zarkower, 2003). The pattern of nuclear fluorescence we observed for a rescuing MIG-32:GFP fusion protein suggests that MIG-32 does not co-localize with the SOP-2 and SOR-1 proteins (Saurin et al., 1998; Yang et al., 2007; Zhang et al., 2006). Given the numerous and severe defects of the sop and sor mutants and the comparatively limited defects of mig-32 and spat-3 mutants, MIG-32 and SPAT-3A are unlikely to be essential components of a putative SOP/SOR Polycomb-like complex.
The roles of Polycomb complexes in nervous system development
The mig-32 homolog Bmi-1 has been intensively studied following its isolation as a target gene upregulated by proviral integration in Eμ-myc-driven lymphomas in mice (Haupt et al., 1991; van Lohuizen et al., 1991). In the mammalian nervous system Bmi-1 is required for the self-renewal of neural stem cells (Molofsky et al., 2005; Molofsky et al., 2003), and epigenetic regulation of the cell cycle is a crucial function of Bmi-1 in the hematopoietic and nervous systems (Jacobs et al., 1999; Molofsky et al., 2005; Molofsky et al., 2003). We have not observed abnormalities in cell numbers in mig-32 mutants that would suggest an essential role in the regulation of the cell cycle, but we have not directly examined this possibility.
Expression of additional MIG-32 homologs in the mammalian nervous system has been reported (Gunster et al., 1997; Jacobs et al., 1999; Kim et al., 2005; Leung et al., 2004; Molofsky et al., 2003; Nunes et al., 2001; Schoorlemmer et al., 1997; Shakhova et al., 2005; van der Lugt et al., 1994), but with the exception of Bmi-1 little is known about what these genes contribute to nervous system function. Our data suggest that epigenetic regulation of gene expression by PRC1 and related complexes will participate in neuronal migration and process extension, but the precise basis for the defects we observe in mig-32 and spat-3 mutants is not yet clear. Specifically, does PRC1 regulate transcription of individual gene targets that are crucial regulators of individual cell migrations or process extensions, or does loss of PRC1 result in a `noisy' pattern of gene expression to which some cells are more sensitive? Recent genomic screens in Drosophila and vertebrates have identified targets of Polycomb repression complexes (Boyer et al., 2006; Bracken et al., 2006; Lee et al., 2006; Negre et al., 2006; Schwartz et al., 2006; Tolhuis et al., 2006). Many of these biochemically defined targets are involved in nervous system patterning, and our data raise the possibility that Polycomb-group complexes regulate these targets in a functionally important way in the developing nervous system. Our observation that most cells appear to adopt fates similar to those of wild-type animals, as suggested by normal expression of the cell-type-specific gfp reporters used in this study, suggests that mig-32 is not crucial for establishing cell fates, but instead acts in a subtle manner to refine cellular phenotypes.
Defects in Hox gene expression are unlikely to be central for the mig-32 or spat-3a mutant phenotypes
Mutations affecting Hox genes result in abnormal neuronal migrations in C. elegans (Baum et al., 1999; Chalfie and Sulston, 1981; Chisholm, 1991; Clark et al., 1993; Harris et al., 1996; Kenyon, 1986; Salser and Kenyon, 1992; Wang et al., 1993), and given the classical role of Polycomb family members as repressors of Hox gene activity we asked whether abnormal Hox gene activity might underlie the defects in the nervous system we observed. However, our data suggest that regulation of Hox gene expression by mig-32 and spat-3 may be subtle. In general, the migration and neuronal process extension defects of mig-32 and spat-3 mutants have little in common with those observed in gain- or loss-of-function Hox mutants. In addition, the expression domains of the Pegl-5gfp Hox and Ppkd-2gfp reporters do not appear to be expanded in mig-32 mutants, and mig-32 mutations do not suppress pal-1 mutations, which reduce Hox activity (our unpublished observations). These data suggest that non-Hox targets of mig-32 and spat-3 are more likely to be responsible for the defects we describe, and are consistent with the Polycomb-group targets of Drosophila and mammalian cells, the vast majority of which are not Hox genes. However, our data do not rule out a role for MIG-32 or SPAT-3A as regulators of Hox gene activity, and further work to define the genetic and biochemical properties of mig-32 and spat-3 in germline and somatic cells will allow comparison to the Polycomb group complexes of other species.
We thank Jean Maines and Leon Avery for comments on the manuscript. We also thank Yuji Kohara for mig-32 cDNAs, the Mitani laboratory for the mig-32(tm1807) and tm1684 deletion alleles, the Horvitz lab for the mig-32(n4275) deletion allele, and the CGC for many nematode strains used in this work (the CGC is funded by the NIH National Center for Research Resources). This work was supported by NIH grant GM069667 to S.C. Deposited in PMC for release after 12 months.
References
- Alkema, M. J., Bronk, M., Verhoeven, E., Otte, A., van't Veer, L. J., Berns, A. and van Lohuizen, M. (1997). Identification of Bmi1-interacting proteins as constituents of a multimeric mammalian polycomb complex. Genes Dev. 11, 226-240. [DOI] [PubMed] [Google Scholar]
- Bateman, A., Birney, E., Cerruti, L., Durbin, R., Etwiller, L., Eddy, S. R., Griffiths-Jones, S., Howe, K. L., Marshall, M. and Sonnhammer, E. L. (2002). The Pfam protein families database. Nucleic Acids Res. 30, 276-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, P. D., Guenther, C., Frank, C. A., Pham, B. V. and Garriga, G. (1999). The Caenorhabditis elegans gene ham-2 links Hox patterning to migration of the HSN motor neuron. Genes Dev. 13, 472-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belote, J. M., Hoffmann, F. M., McKeown, M., Chorsky, R. L. and Baker, B. S. (1990). Cytogenetic analysis of chromosome region 73AD of Drosophila melanogaster. Genetics 125, 783-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Saadon, R., Zaaroor, D., Ziv, T. and Ciechanover, A. (2006). The polycomb protein Ring1B generates self atypical mixed ubiquitin chains required for its in vitro histone H2A ligase activity. Mol. Cell 24, 701-711. [DOI] [PubMed] [Google Scholar]
- Bender, L. B., Cao, R., Zhang, Y. and Strome, S. (2004). The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr. Biol. 14, 1639-1643. [DOI] [PubMed] [Google Scholar]
- Boyer, L. A., Plath, K., Zeitlinger, J., Brambrink, T., Medeiros, L. A., Lee, T. I., Levine, S. S., Wernig, M., Tajonar, A., Ray, M. K. et al. (2006). Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 441, 349-353. [DOI] [PubMed] [Google Scholar]
- Bracken, A. P., Dietrich, N., Pasini, D., Hansen, K. H. and Helin, K. (2006). Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev. 20, 1123-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, S. (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, S., Clark, S. G., McDermott, J. B., Aamodt, E. and Horvitz, H. R. (2002). PAG-3, a Zn-finger transcription factor, determines neuroblast fate in C. elegans. Development 129, 1763-1774. [DOI] [PubMed] [Google Scholar]
- Cao, R., Wang, L., Wang, H., Xia, L., Erdjument-Bromage, H., Tempst, P., Jones, R. S. and Zhang, Y. (2002). Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039-1043. [DOI] [PubMed] [Google Scholar]
- Cao, R., Tsukada, Y. and Zhang, Y. (2005). Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol. Cell 20, 845-854. [DOI] [PubMed] [Google Scholar]
- Capowski, E. E., Martin, P., Garvin, C. and Strome, S. (1991). Identification of grandchildless loci whose products are required for normal germ-line development in the nematode Caenorhabditis elegans. Genetics 129, 1061-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie, M. and Sulston, J. (1981). Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 82, 358-370. [DOI] [PubMed] [Google Scholar]
- Chang, C., Adler, C. E., Krause, M., Clark, S. G., Gertler, F. B., Tessier-Lavigne, M. and Bargmann, C. I. (2006). MIG-10/lamellipodin and AGE-1/PI3K promote axon guidance and outgrowth in response to slit and netrin. Curr. Biol. 16, 854-862. [DOI] [PubMed] [Google Scholar]
- Chisholm, A. (1991). Control of cell fate in the tail region of C. elegans by the gene egl-5. Development 111, 921-932. [DOI] [PubMed] [Google Scholar]
- Clark, S. G., Chisholm, A. D. and Horvitz, H. R. (1993). Control of cell fates in the central body region of C. elegans by the homeobox gene lin-39. Cell 74, 43-55. [DOI] [PubMed] [Google Scholar]
- Clark, S. G., Lu, X. and Horvitz, H. R. (1994). The Caenorhabditis elegans locus lin-15, a negative regulator of a tyrosine kinase signaling pathway, encodes two different proteins. Genetics 137, 987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, M., Allen, M. A., Larsen, A., Macmorris, M., Han, M. and Blumenthal, T. (2008). Genes involved in pre-mRNA 3′-end formation and transcription termination revealed by a lin-15 operon Muv suppressor screen. Proc. Natl. Acad. Sci. USA 105, 16665-16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czermin, B., Melfi, R., McCabe, D., Seitz, V., Imhof, A. and Pirrotta, V. (2002). Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111, 185-196. [DOI] [PubMed] [Google Scholar]
- Dalpe, G., Zhang, L. W., Zheng, H. and Culotti, J. G. (2004). Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development 131, 2073-2088. [DOI] [PubMed] [Google Scholar]
- Desai, C., Garriga, G., McIntire, S. L. and Horvitz, H. R. (1988). A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336, 638-646. [DOI] [PubMed] [Google Scholar]
- Emmons, S. W. (2005). Male development. In WormBook (ed. The C. elegans Research Community). www.wormbook.org.
- Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E. and Mello, C. C. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806-811. [DOI] [PubMed] [Google Scholar]
- Fong, Y., Bender, L., Wang, W. and Strome, S. (2002). Regulation of the different chromatin states of autosomes and X chromosomes in the germ line of C. elegans. Science 296, 2235-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, N. J., Kingston, R. E. and Woodcock, C. L. (2004). Chromatin compaction by a polycomb group protein complex. Science 306, 1574-1577. [DOI] [PubMed] [Google Scholar]
- Fujii, T., Nakao, F., Shibata, Y., Shioi, G., Kodama, E., Fujisawa, H. and Takagi, S. (2002). Caenorhabditis elegans PlexinA, PLX-1, interacts with transmembrane semaphorins and regulates epidermal morphogenesis. Development 129, 2053-2063. [DOI] [PubMed] [Google Scholar]
- Ginzburg, V. E., Roy, P. J. and Culotti, J. G. (2002). Semaphorin 1a and semaphorin 1b are required for correct epidermal cell positioning and adhesion during morphogenesis in C. elegans. Development 129, 2065-2078. [DOI] [PubMed] [Google Scholar]
- Gunster, M. J., Satijn, D. P., Hamer, K. M., den Blaauwen, J. L., de Bruijn, D., Alkema, M. J., van Lohuizen, M., van Driel, R. and Otte, A. P. (1997). Identification and characterization of interactions between the vertebrate polycomb-group protein BMI1 and human homologs of polyhomeotic. Mol. Cell. Biol. 17, 2326-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin, M., Scott, I. M., Way, J. C. and Culotti, J. G. (1992). The mec-7 beta-tubulin gene of Caenorhabditis elegans is expressed primarily in the touch receptor neurons. EMBO J. 11, 2885-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, J. C., Yu, T. W., Fujisawa, K., Culotti, J. G., Gengyo-Ando, K., Mitani, S., Moulder, G., Barstead, R., Tessier-Lavigne, M. and Bargmann, C. I. (2001). C. elegans slit acts in midline, dorsal-ventral, and anterior-posterior guidance via the SAX-3/Robo receptor. Neuron 32, 25-38. [DOI] [PubMed] [Google Scholar]
- Harris, J., Honigberg, L., Robinson, N. and Kenyon, C. (1996). Neuronal cell migration in C. elegans: regulation of Hox gene expression and cell position. Development 122, 3117-3131. [DOI] [PubMed] [Google Scholar]
- Haupt, Y., Alexander, W. S., Barri, G., Klinken, S. P. and Adams, J. M. (1991). Novel zinc finger gene implicated as myc collaborator by retrovirally accelerated lymphomagenesis in E mu-myc transgenic mice. Cell 65, 753-763. [DOI] [PubMed] [Google Scholar]
- Holdeman, R., Nehrt, S. and Strome, S. (1998). MES-2, a maternal protein essential for viability of the germline in Caenorhabditis elegans, is homologous to a Drosophila Polycomb group protein. Development 125, 2457-2467. [DOI] [PubMed] [Google Scholar]
- Hunter, C. P., Harris, J. M., Maloof, J. N. and Kenyon, C. (1999). Hox gene expression in a single Caenorhabditis elegans cell is regulated by a caudal homolog and intercellular signals that inhibit wnt signaling. Development 126, 805-814. [DOI] [PubMed] [Google Scholar]
- Irminger-Finger, I. and Nothiger, R. (1995). The Drosophila melanogaster gene lethal(3)73Ah encodes a ring finger protein homologous to the oncoproteins MEL-18 and BMI-1. Gene 163, 203-208. [DOI] [PubMed] [Google Scholar]
- Jackson, J. P., Johnson, L., Jasencakova, Z., Zhang, X., PerezBurgos, L., Singh, P. B., Cheng, X., Schubert, I., Jenuwein, T. and Jacobsen, S. E. (2004). Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112, 308-315. [DOI] [PubMed] [Google Scholar]
- Jacobs, J. J., Kieboom, K., Marino, S., DePinho, R. A. and van Lohuizen, M. (1999). The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397, 164-168. [DOI] [PubMed] [Google Scholar]
- Kennison, J. A. and Tamkun, J. W. (1988). Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc. Natl. Acad. Sci. USA 85, 8136-8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon, C. (1986). A gene involved in the development of the posterior body region of C. elegans. Cell 46, 477-487. [DOI] [PubMed] [Google Scholar]
- Kim, M. H., Gunnersen, J. M. and Tan, S. S. (2005). Mph2 expression in germinal zones of the mouse brain. Dev. Dyn. 232, 209-215. [DOI] [PubMed] [Google Scholar]
- King, I. F., Francis, N. J. and Kingston, R. E. (2002). Native and recombinant polycomb group complexes establish a selective block to template accessibility to repress transcription in vitro. Mol. Cell. Biol. 22, 7919-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmichev, A., Nishioka, K., Erdjument-Bromage, H., Tempst, P. and Reinberg, D. (2002). Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 16, 2893-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe, J. C., Pacquelet, A., Marty, T. and Gotta, M. (2006). A genomewide screen for suppressors of par-2 uncovers potential regulators of PAR protein-dependent cell polarity in Caenorhabditis elegans. Genetics 174, 285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T. I., Jenner, R. G., Boyer, L. A., Guenther, M. G., Levine, S. S., Kumar, R. M., Chevalier, B., Johnstone, S. E., Cole, M. F., Isono, K. et al. (2006). Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125, 301-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, C., Lingbeek, M., Shakhova, O., Liu, J., Tanger, E., Saremaslani, P., Van Lohuizen, M. and Marino, S. (2004). Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature 428, 337-341. [DOI] [PubMed] [Google Scholar]
- Levine, S. S., Weiss, A., Erdjument-Bromage, H., Shao, Z., Tempst, P. and Kingston, R. E. (2002). The core of the polycomb repressive complex is compositionally and functionally conserved in flies and humans. Mol. Cell. Biol. 22, 6070-6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, E. B. (1978). A gene complex controlling segmentation in Drosophila. Nature 276, 565-570. [DOI] [PubMed] [Google Scholar]
- Li, C., Nelson, L. S., Kim, K., Nathoo, A. and Hart, A. C. (1999). Neuropeptide gene families in the nematode Caenorhabditis elegans. Ann. NY Acad. Sci. 897, 239-252. [DOI] [PubMed] [Google Scholar]
- Li, Z., Cao, R., Wang, M., Myers, M. P., Zhang, Y. and Xu, R. M. (2006). Structure of a Bmi-1-Ring1B polycomb group ubiquitin ligase complex. J. Biol. Chem. 281, 20643-20649. [DOI] [PubMed] [Google Scholar]
- McIntire, S. L., Reimer, R. J., Schuske, K., Edwards, R. H. and Jorgensen, E. M. (1997). Identification and characterization of the vesicular GABA transporter. Nature 389, 870-876. [DOI] [PubMed] [Google Scholar]
- Molofsky, A. V., He, S., Bydon, M., Morrison, S. J. and Pardal, R. (2005). Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev. 19, 1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky, A. V., Pardal, R., Iwashita, T., Park, I. K., Clarke, M. F. and Morrison, S. J. (2003). Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, J., Hart, C. M., Francis, N. J., Vargas, M. L., Sengupta, A., Wild, B., Miller, E. L., O'Connor, M. B., Kingston, R. E. and Simon, J. A. (2002). Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111, 197-208. [DOI] [PubMed] [Google Scholar]
- Negre, N., Hennetin, J., Sun, L. V., Lavrov, S., Bellis, M., White, K. P. and Cavalli, G. (2006). Chromosomal distribution of PcG proteins during Drosophila development. PLoS Biol. 4, e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes, M., Blanc, I., Maes, J., Fellous, M., Robert, B. and McElreavey, K. (2001). NSPc1, a novel mammalian Polycomb gene, is expressed in neural crest-derived structures of the peripheral nervous system. Mech. Dev. 102, 219-222. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard, C., Kluding, H. and Jurgens, G. (1985). Genes affecting the segmental subdivision of the Drosophila embryo. Cold Spring Harb. Symp. Quant. Biol. 50, 145-154. [DOI] [PubMed] [Google Scholar]
- Osley, M. A. (2006). Regulation of histone H2A and H2B ubiquitylation. Brief. Funct. Genomic. Proteomic. 5, 179-189. [DOI] [PubMed] [Google Scholar]
- Pan, C. L., Howell, J. E., Clark, S. G., Hilliard, M., Cordes, S., Bargmann, C. I. and Garriga, G. (2006). Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev. Cell 10, 367-377. [DOI] [PubMed] [Google Scholar]
- Pujol, N., Torregrossa, P., Ewbank, J. J. and Brunet, J. F. (2000). The homeodomain protein CePHOX2/CEH-17 controls antero-posterior axonal growth in C. elegans. Development 127, 3361-3371. [DOI] [PubMed] [Google Scholar]
- Quinn, C. C., Pfeil, D. S., Chen, E., Stovall, E. L., Harden, M. V., Gavin, M. K., Forrester, W. C., Ryder, E. F., Soto, M. C. and Wadsworth, W. G. (2006). UNC-6/netrin and SLT-1/slit guidance cues orient axon outgrowth mediated by MIG-10/RIAM/lamellipodin. Curr. Biol. 16, 845-853. [DOI] [PubMed] [Google Scholar]
- Ross, J. M. and Zarkower, D. (2003). Polycomb group regulation of Hox gene expression in C. elegans. Dev. Cell 4, 891-901. [DOI] [PubMed] [Google Scholar]
- Salser, S. J. and Kenyon, C. (1992). Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature 355, 255-258. [DOI] [PubMed] [Google Scholar]
- Sanchez, C., Sanchez, I., Demmers, J. A., Rodriguez, P., Strouboulis, J. and Vidal, M. (2007). Proteomics analysis of Ring1B/Rnf2 interactors identifies a novel complex with the Fbxl10/Jhdm1B histone demethylase and the Bcl6 interacting corepressor. Mol. Cell Proteomics 6, 820-834. [DOI] [PubMed] [Google Scholar]
- Saurin, A. J., Shiels, C., Williamson, J., Satijn, D. P., Otte, A. P., Sheer, D. and Freemont, P. S. (1998). The human polycomb group complex associates with pericentromeric heterochromatin to form a novel nuclear domain. J. Cell Biol. 142, 887-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin, A. J., Shao, Z., Erdjument-Bromage, H., Tempst, P. and Kingston, R. E. (2001). A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature 412, 655-660. [DOI] [PubMed] [Google Scholar]
- Schoorlemmer, J., Marcos-Gutierrez, C., Were, F., Martinez, R., Garcia, E., Satijn, D. P., Otte, A. P. and Vidal, M. (1997). Ring1A is a transcriptional repressor that interacts with the Polycomb-M33 protein and is expressed at rhombomere boundaries in the mouse hindbrain. EMBO J. 16, 5930-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, Y. B., Kahn, T. G., Nix, D. A., Li, X. Y., Bourgon, R., Biggin, M. and Pirrotta, V. (2006). Genome-wide analysis of Polycomb targets in Drosophila melanogaster. Nat. Genet. 38, 700-705. [DOI] [PubMed] [Google Scholar]
- Shakhova, O., Leung, C. and Marino, S. (2005). Bmi1 in development and tumorigenesis of the central nervous system. J. Mol. Med. 83, 596-600. [DOI] [PubMed] [Google Scholar]
- Shao, Z., Raible, F., Mollaaghababa, R., Guyon, J. R., Wu, C. T., Bender, W. and Kingston, R. E. (1999). Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98, 37-46. [DOI] [PubMed] [Google Scholar]
- Simon, J., Chiang, A. and Bender, W. (1992). Ten different Polycomb group genes are required for spatial control of the abdA and AbdB homeotic products. Development 114, 493-505. [DOI] [PubMed] [Google Scholar]
- Spieth, J., Brooke, G., Kuersten, S., Lea, K. and Blumenthal, T. (1993). Operons in C. elegans: polycistronic mRNA precursors are processed by trans-splicing of SL2 to downstream coding regions. Cell 73, 521-532. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., Schierenberg, E., White, J. G. and Thomson, J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64-119. [DOI] [PubMed] [Google Scholar]
- Tolhuis, B., de Wit, E., Muijrers, I., Teunissen, H., Talhout, W., van Steensel, B. and van Lohuizen, M. (2006). Genome-wide profiling of PRC1 and PRC2 Polycomb chromatin binding in Drosophila melanogaster. Nat. Genet. 38, 694-699. [DOI] [PubMed] [Google Scholar]
- Trent, C., Tsung, N. and Horvitz, H. R. (1983). Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104, 619-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi, J. M., Fairchild, B., Wen, J. and Lees, J. A. (2001). The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc. Natl. Acad. Sci. USA 98, 1519-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel, E. R., Chou, J. H., Dwyer, N. D., Colbert, H. A. and Bargmann, C. I. (1995). Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83, 207-218. [DOI] [PubMed] [Google Scholar]
- van der Lugt, N. M., Domen, J., Linders, K., van Roon, M., Robanus-Maandag, E., te Riele, H., van der Valk, M., Deschamps, J., Sofroniew, M., van Lohuizen, M. et al. (1994). Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 8, 757-769. [DOI] [PubMed] [Google Scholar]
- van Lohuizen, M., Verbeek, S., Scheijen, B., Wientjens, E., van der Gulden, H. and Berns, A. (1991). Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. Cell 65, 737-752. [DOI] [PubMed] [Google Scholar]
- Wang, B. B., Muller-Immergluck, M. M., Austin, J., Robinson, N. T., Chisholm, A. and Kenyon, C. (1993). A homeotic gene cluster patterns the anteroposterior body axis of C. elegans. Cell 74, 29-42. [DOI] [PubMed] [Google Scholar]
- Wang, H., Wang, L., Erdjument-Bromage, H., Vidal, M., Tempst, P., Jones, R. S. and Zhang, Y. (2004a). Role of histone H2A ubiquitination in Polycomb silencing. Nature 431, 873-878. [DOI] [PubMed] [Google Scholar]
- Wang, L., Brown, J. L., Cao, R., Zhang, Y., Kassis, J. A. and Jones, R. S. (2004b). Hierarchical recruitment of polycomb group silencing complexes. Mol. Cell 14, 637-646. [DOI] [PubMed] [Google Scholar]
- Wei, J., Zhai, L., Xu, J. and Wang, H. (2006). Role of Bmi1 in H2A ubiquitylation and Hox gene silencing. J. Biol. Chem. 281, 22537-22544. [DOI] [PubMed] [Google Scholar]
- White, J. G., Southgate, E., Thomson, J. N. and Brenner, S. (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 311, 1-340. [DOI] [PubMed] [Google Scholar]
- Xu, L., Fong, Y. and Strome, S. (2001a). The Caenorhabditis elegans maternal-effect sterile proteins, MES-2, MES-3, and MES-6, are associated in a complex in embryos. Proc. Natl. Acad. Sci. USA 98, 5061-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L., Paulsen, J., Yoo, Y., Goodwin, E. B. and Strome, S. (2001b). Caenorhabditis elegans MES-3 is a target of GLD-1 and functions epigenetically in germline development. Genetics 159, 1007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Sun, Y., Luo, X., Zhang, Y., Chen, Y., Tian, E., Lints, R. and Zhang, H. (2007). Polycomb-like genes are necessary for specification of dopaminergic and serotonergic neurons in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 104, 852-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H., Pretot, R. F., Burglin, T. R. and Sternberg, P. W. (2003). Distinct roles of transcription factors EGL-46 and DAF-19 in specifying the functionality of a polycystin-expressing sensory neuron necessary for C. elegans male vulva location behavior. Development 130, 5217-5227. [DOI] [PubMed] [Google Scholar]
- Zallen, J. A., Yi, B. A. and Bargmann, C. I. (1998). The conserved immunoglobulin superfamily member SAX-3/Robo directs multiple aspects of axon guidance in C. elegans. Cell 92, 217-227. [DOI] [PubMed] [Google Scholar]
- Zhang, H. and Emmons, S. W. (2001). The novel C. elegans gene sop-3 modulates Wnt signaling to regulate Hox gene expression. Development 128, 767-777. [DOI] [PubMed] [Google Scholar]
- Zhang, H., Azevedo, R. B., Lints, R., Doyle, C., Teng, Y., Haber, D. and Emmons, S. W. (2003). Global regulation of Hox gene expression in C. elegans by a SAM domain protein. Dev. Cell 4, 903-915. [DOI] [PubMed] [Google Scholar]
- Zhang, H., Smolen, G. A., Palmer, R., Christoforou, A., van den Heuvel, S. and Haber, D. A. (2004). SUMO modification is required for in vivo Hox gene regulation by the Caenorhabditis elegans Polycomb group protein SOP-2. Nat. Genet. 36, 507-511. [DOI] [PubMed] [Google Scholar]
- Zhang, T., Sun, Y., Tian, E., Deng, H., Zhang, Y., Luo, X., Cai, Q., Wang, H., Chai, J. and Zhang, H. (2006). RNA-binding proteins SOP-2 and SOR-1 form a novel PcG-like complex in C. elegans. Development 133, 1023-1033. [DOI] [PubMed] [Google Scholar]
- Zhu, P., Zhou, W., Wang, J., Puc, J., Ohgi, K. A., Erdjument-Bromage, H., Tempst, P., Glass, C. K. and Rosenfeld, M. G. (2007). A histone H2A deubiquitinase complex coordinating histone acetylation and H1 dissociation in transcriptional regulation. Mol. Cell 27, 609-621. [DOI] [PMC free article] [PubMed] [Google Scholar]