Summary
Drosophila larval skeletal muscles are single, multinucleated cells of different sizes that undergo tremendous growth within a few days. The mechanisms underlying this growth in concert with overall body growth are unknown. We find that the size of individual muscles correlates with the number of nuclei per muscle cell and with increasing nuclear ploidy during development. Inhibition of Insulin receptor (InR; Insulin-like receptor) signaling in muscles autonomously reduces muscle size and systemically affects the size of other tissues, organs and indeed the entire body, most likely by regulating feeding behavior. In muscles, InR/Tor signaling, Foxo and dMyc (Diminutive) are key regulators of endoreplication, which is necessary but not sufficient to induce growth. Mechanistically, InR/Foxo signaling controls cell cycle progression by modulating dmyc expression and dMyc transcriptional activity. Thus, maximal dMyc transcriptional activity depends on InR to control muscle mass, which in turn induces a systemic behavioral response to allocate body size and proportions.
Keywords: Foxo, InR/Tor signaling, Myc, Muscle growth, Endoreplication, Body size, Feeding behavior
INTRODUCTION
Body and organ growth are among the most dramatic processes that a developing organism undergoes (Conlon and Raff, 1999), but an understanding of their inter-regulation remains elusive (Hafen and Stocker, 2003). The Insulin receptor/Target of rapamycin (InR/Tor) pathway regulates cell size and number and hence organ and body growth across evolution (Hafen and Stocker, 2003). Upon Insulin/IGF binding to the Insulin and IGF receptors, a signaling cascade of phosphorylation and docking events, antagonized by Pten, results in the activation of the Ser/Thr kinase Akt. Akt then controls cell survival, cell cycle, cell growth and metabolism through phosphorylation of a number of key substrates, including the Tsc1-Tsc2 complex, and the transcription factors of the Forkhead box O (FoxO) family. Phosphorylation and inhibition of the Tsc1-Tsc2 complex, which has an inhibitory effect on Tor, promotes protein synthesis (Manning and Cantley, 2007). By phosphorylating and sequestering Foxo in the cytoplasm, Akt further promotes cell growth and cell cycle progression (Accili and Arden, 2004; Greer and Brunet, 2008; Puig and Tjian, 2006).
Similar to InR/Tor signaling, Myc has an evolutionarily conserved function in promoting cell growth and proliferation (de la Cova and Johnston, 2006). Myc regulates gene expression by binding to Enhancer box sequences (E-boxes) in promoter regions of target genes, with its dimerization partner Max, but also independently (Steiger et al., 2008). Max also dimerizes with itself and with members of the Mad/Mnt family, opposing Myc-Max transcriptional activity (Eisenman, 2001; Gallant, 2006; Grandori et al., 2000).
Although InR/Tor signaling, Foxo and Myc have been causally associated with the growth of most cell types across species, how organ and body growth are in turn determined is still unclear. Possibly, body size is decided by stereotypical responses of each organ to growth factors, which in turn regulate InR/Tor signaling and Myc activity. Alternatively, InR signaling in some sensor tissues might have a pivotal role in modifying body growth in response to the nutritional status of the organism. Consistent with this model, InR/Tor signaling in the Drosophila fat body, which corresponds to human liver and adipose tissue, and in endocrine glands regulates the growth of other unrelated tissues and, consequently, of the entire body, by modulating the actions of anabolic hormones (Edgar, 2006). However, it is unknown whether other tissues and mechanisms might contribute to the systemic regulation of growth.
Muscles have important metabolic functions, undergo dramatic growth during development, and are continually remodeled throughout life. Despite their importance, it is unclear how muscle growth occurs and whether it contributes to the overall control of body size.
In vertebrates, several stimuli, including those activating InR/Tor signaling and Myc, promote hypertrophy of skeletal muscles and cardiomyocytes by inducing protein synthesis (Glass, 2003b). Conversely, inhibition of InR signaling, which results in Foxo activation, promotes protein degradation and muscle atrophy (Sandri et al., 2004; Stitt et al., 2004). Other processes, in particular an increase in DNA content, either by increasing the number of nuclei or their ploidy, may be involved in muscle growth (Brodsky and Uryvaeva, 1977; Conlon and Raff, 1999). Consistently, satellite cells fuse to pre-existing skeletal muscles, increasing the number of nuclei and supporting hypertrophy (Buckingham, 2006). Further, cardiomyocytes increase their nuclear ploidy during the reparative growth that follows an ischemic injury (Herget et al., 1997; Meckert et al., 2005). However, it is unknown whether nuclear ploidy can sustain muscle growth, whether InR/Foxo signaling and Myc regulate these events, and whether they crosstalk during muscle growth. Studies in epithelial and hematopoietic cells have suggested that Myc might act either upstream (Bouchard et al., 2007), downstream (Bouchard et al., 2004), or in parallel with Foxo (Prober and Edgar, 2002). Thus, the interplay of InR/Foxo signaling and Myc might rely on the specific cellular context and needs to be analyzed in vivo to identify physiologically relevant interactions.
Here, we have used Drosophila muscles to investigate: (1) how InR (Insulin-like receptor)/Tor signaling, Foxo and dMyc (Diminutive) interact in vivo during muscle growth; (2) whether they regulate the nuclear ploidy of muscle cells; (3) whether this is important for cell growth; and (4) whether muscle mass can in turn influence body size.
The Drosophila larval body wall muscles are skeletal muscles, each comprising a single, multinucleated syncytial cell (myofiber) that arises from the fusion of precursor cells (founder cells and fusion-competent myoblasts) during embryonic development. Different degrees of cell fusion account for different numbers of nuclei that are contained within distinct muscle cells (Bate et al., 1999; Beckett and Baylies, 2006). During larval development, body wall muscles (see Fig. 1A) grow dramatically in ∼5 days, via sarcomere assembly and the addition of novel myofibrils, while the number of nuclei remains constant (Bai et al., 2007; Haas, 1950). Muscle growth may also rely on an increase in nuclear ploidy, as previously observed for other Drosophila tissues (Edgar and Orr-Weaver, 2001; Maines et al., 2004), via endoreplication (or endocycle), a modified cell cycle in which DNA replication is not accompanied by mitosis but rather by multiple G–S and S–G transitions (Edgar and Orr-Weaver, 2001).
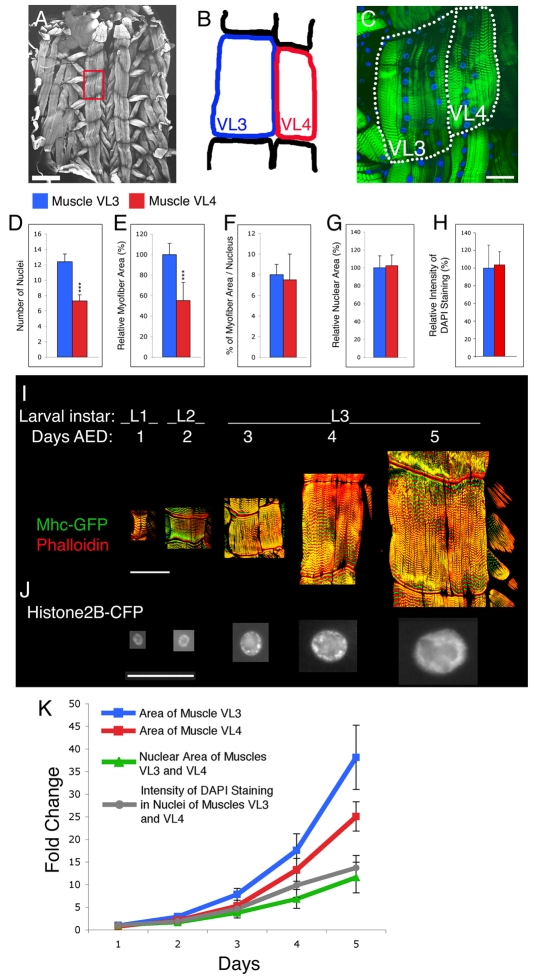
Fig. 1.
Body wall muscles of Drosophila larvae consist of single, syncytial myofibers containing several polyploid nuclei. (A) Phalloidin staining of body wall muscles from a wild-type third instar (L3) larva. Several muscle cells (myofibers) of different sizes can be seen. The red box delineates VL3 and VL4 muscles of an abdominal segment. (B,C) The outline (B) and staining for F-actin (phalloidin, green) and nuclei (DAPI, blue) (C) of body wall muscles VL3 and VL4. Each muscle is composed of a single syncytial cell (myofiber), which differs in size and number of nuclei. (D-H) Quantification of (D) the number of nuclei, (E) myofiber area, (F) ratio of myofiber area/nucleus, (G) nuclear area, and (H) intensity of DAPI staining in VL3 and VL4 muscles. There is no significant difference in the nuclear area between body wall muscles VL3 and VL4, suggesting that ploidy is not a significant cause of differential growth. n(muscles)=10, n(nuclei muscle VL3)=100, n(nuclei muscle VL4)=50; ***P<0.001. (I-K) Variation of (I) myofiber area and (J) nuclear area during larval growth, and (K) quantification of these results. Note the correlation between the extent of muscle growth, the increase in nuclear size, and the intensity of DAPI staining. For statistical analysis in K, n(muscles)=9, n(nuclei)=100 for nuclear size, and n(nuclei)=10 for intensity of DAPI staining. Error bars indicate s.d. Scale bars: 300 μm in A; 47.5 μm in C; 75 μm in I; 22 μm in J.
Here, we find that dMyc and InR/Foxo signaling are key regulators of endoreplication that is necessary, but not sufficient, for muscle growth. Foxo has a pivotal role in this process by regulating dmyc expression and activity downstream of InR signaling. The functional interaction of the transcription factors Foxo and dMyc controls the final muscle mass, which in turn influences body size by regulating larval feeding behavior.
MATERIALS AND METHODS
Drosophila genetics and fly stocks
Fly stocks used are: UAS-foxo (Hwangbo et al., 2004); UAS-InR; UAS-Pten; UAS-Tsc1, UAS-Tsc2 (Potter et al., 2001); UAS-InR DN (Bloomington #8253); UAS-dmyc, UAS-dmyc [second transgene, tr2 (Orian et al., 2007)], UAS-CycE (Bloomington #4781); UAS-dmnt (Loo et al., 2005); chico1/CyO, act-GFP; dm4/FM7i, act-GFP (Pierce et al., 2008); Dmef2-Gal4 (Ranganayakulu et al., 1996); Mhc-Gal4 (Schuster et al., 1996); UAS-Dcr-2 (Dietzl et al., 2007); UAS-dmyc hp (CG10798, VDRC #2947); UAS-Akt1 hp (CG4006, DRSC TR00202A.1); UAS-InR hairpin (hp) [CG18402, DRSC TR00693A.1; courtesy of Dr Jianquan Ni (Ni et al., 2008)]; Mhc-GFP (Wee-P26) (Clyne et al., 2003); and UAS-H2B-CFP (from Dr Shu Kondo, Harvard Medical School, Boston, MA, USA).
The PG157-Gal4 line is a lethal insertion at position 12F7 that drives high transgene expression in ventral internal 1 muscle (VI1, also known as muscle 31 of abdominal segment 1) and muscles of the thoracic segment (see Fig. S5 in the supplementary material). PG157-Gal4 does not drive transgene expression in ventral longitudinal 3 and 4 muscles (VL3 and VL4, also known as muscles 6 and 7). Dmef2-Gal4 and Mhc-Gal4 drive transgene expression in all body wall muscles, but not in other endoreplicating tissues (see Fig. S3 in the supplementary material). For transgene expression with the Gal4-UAS system (Brand and Perrimon, 1993), flies were reared at 25°C (Dmef2-Gal4) or 31°C (Mhc-Gal4). Flies were reared at 29°C for hairpin expression and at 22°C in Fig. 1.
Body size analysis
For analysis of body weight, groups of L3 wandering larvae were weighed on an analytical balance and the average body weight calculated. Larval staging was supported by analysis of mouth hook morphology. Larval and pupal length and diameter were measured manually using AxioVision v4.5 software (Zeiss). Larval and pupal volumes were calculated assuming a prolate spheroid geometry. For analysis of internal organs, dissected organs were stained in a micro-chamber with the lipophilic dye FM4-64 [Molecular Probes (Demontis and Dahmann, 2007)]. Images were acquired with an epifluorescence microscope (Zeiss). Adult flies were analyzed according to Colombani et al. (Colombani et al., 2005), using the Measure Tools of the AxioVision software. Larval feeding behavior was estimated as described previously (Wu et al., 2005).
Histology, laser-scanning confocal microscopy and image analysis
Larvae were dissected in ice-cold Ca2+-free saline buffer (128 mM NaCl, 2 mM KCl, 4 mM MgCl2, 1 mM EGTA, 35 mM sucrose, 5 mM HEPES pH 7.2) using dissection chambers (Budnik et al., 2006). Body wall muscles were fixed for 20-30 minutes in Ca2+-free saline buffer containing 4% paraformaldehyde and 0.1% Triton X-100. After washing, body wall muscles were incubated for 10 hours with DAPI (4′,6-diamidino-2-phenylindole, 1 μg/ml) and Alexa633- or Alexa488-conjugated phalloidin (1:100) to visualize nuclei and F-actin, respectively. To examine biogenesis of nucleoli, an anti-Fibrillarin antibody [EnCore Biotechnology #MCA-38F3 (Grewal et al., 2005)] was applied (1:100), followed by incubation with Alexa546-conjugated secondary antibodies (Molecular Probes). Muscles VL3 and VL4 of abdominal segments 2-5 were imaged using a Leica TCS SP2 confocal laser-scanning microscope. Confocal images were analyzed using the Measure Tools of the AxioVision software. Statistical analysis was performed using Student's t-test and Excel (Microsoft).
Luciferase assays, RNAi treatment and plasmid DNAs
For Luciferase assays, 15×104 S2R+ cells/cm2 were seeded in Schneider's medium (Gibco) containing 10% FCS, and transfected one day later using the Qiagen Effectene Transfection Kit. An actin-Renilla Luciferase reporter was co-transfected as a normalization control.
Double-stranded RNA (dsRNA) synthesis and RNAi treatment were performed according to the DRSC protocols (http://flyRNAi.org), using amplicons DRSC34258 (dmyc) and DRSC31746 (foxo). RNAi treatment was performed for 3 days. foxo and dmyc expression were induced 24 hours prior to Luciferase assay by addition of CuSO4 directly to the culture medium to a final concentration of 500 μM. Serum starvation was also performed for 24 hours. The Luciferase assay was performed in quadruplicate using the Dual-Glo Luciferase Assay (Promega) according to the manufacturer's instructions. Luciferase activity refers to the ratio of firefly to Renilla Luciferase luminescence.
Plasmids used are pMT-foxo (Puig et al., 2003), pMT-dmyc (Orian et al., 2003), actin-Renilla Luciferase, CG4364, CG5033 and CG5033 ΔE-box Luciferase reporters (Hulf et al., 2005).
Quantitative real-time RT-PCR
Total RNA was prepared from L3 wandering larvae using Trizol (Invitrogen), followed by RNA cleanup with the RNAeasy Kit (Qiagen). The RNA QuantiTect Reverse Transcription Kit (Qiagen) was used for cDNA synthesis, and quantitative real-time PCR was performed with the QuantiTect SYBR Green PCR Kit (Qiagen). αTub84B was used as normalization reference. Relative quantitation of mRNA levels was calculated using the comparative CT method.
Immunoprecipitation and immunoblotting
For immunoprecipitation, S2R+ cells were washed with ice-cold PBS, lysed with lysis buffer (20 mM Tris pH 7.6, 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM DTT, 1 mM PMSF and Protease inhibitors), and centrifuged at 10,000 g for 10 minutes at 4°C. Equal amounts of supernatant were incubated with a monoclonal mouse anti-dMyc antibody (Prober and Edgar, 2000), and subsequently with an appropriate amount of protein A-agarose bead slurry (Amersham) in lysis buffer. Immunoprecipitates were washed three times with lysis buffer, boiled in sample buffer, resolved on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Western blotting was performed with a rabbit anti-Foxo (Puig and Tjian, 2005) or, after extensive membrane washing, with a rabbit anti-dMyc antiserum (Maines et al., 2004), and subsequently with anti-rabbit HRP-conjugated secondary antibodies (Amersham). Western blot and densitometric analysis were performed as previously described (Iurlaro et al., 2004; Schlichting et al., 2006).
RESULTS
Correlation of the number and size of nuclei with muscle size
Examination of body wall muscles reveals extensive variability in the size of each muscle cell during larval stages (Bate et al., 1999) (Fig. 1A; note that in Drosophila larvae, one muscle is composed of a single multinucleated cell). Because the DNA content of a cell has been directly correlated with its size in a number of systems (Brodsky and Uryvaeva, 1977; Conlon and Raff, 1999), we have analyzed whether the differences observed in muscle sizes correlate with either the number of nuclei or their level of endoreplication (Edgar and Orr-Weaver, 2001).
We focused our analysis on two body wall muscles, VL3 and VL4, of third instar (L3) wandering larvae, as they are easy to analyze and have distinct sizes (Fig. 1A-C). VL3 muscles possess twice the number of nuclei as VL4 (Fig. 1D), which correlates with an approximate doubling in size (Fig. 1E) and a similar myofiber area/nucleus ratio (Fig. 1F). Further, the nuclear area (Fig. 1G) and the intensity of DAPI staining (Fig. 1H), which are indicators of nuclear ploidy (Maines et al., 2004; Ohlstein and Spradling, 2006; Sato et al., 2008; Shcherbata et al., 2004; Sun and Deng, 2007), did not significantly differ, suggesting that the amount of endoreplication in VL3 and VL4 nuclei is similar.
We next analyzed whether the ploidy of body wall muscle nuclei, as indicated by nuclear size and DNA content, correlates with muscle growth during larval development, similar to other Drosophila tissues (Edgar and Orr-Weaver, 2001; Maines et al., 2004). By scoring muscle size (Fig. 1I) and nuclear size (Fig. 1J) at various developmental stages, a high degree of correlation between these parameters and the intensity of DAPI staining was observed (Fig. 1K). To test whether endoreplication is necessary for growth, we overexpressed in muscles Cyclin E, which has been shown to block endoreplication when present at constant, but not oscillating, levels (Lilly and Spradling, 1996). Dmef2 (Mef2)-Gal4 UAS-CycE animals showed a severe reduction in nuclear size, intensity of DAPI staining and muscle size, demonstrating that muscle growth depends on endoreplication (see Fig. S1 in the supplementary material). Altogether, these results reveal that the number of nuclei is tightly coupled to the differential size of muscles, and that increasing nuclear ploidy is required for the overall growth of the muscles.
Inhibition of InR signaling in muscles regulates muscle growth, body size and the size of unrelated tissues
Since Insulin signaling is a major, evolutionarily conserved regulator of cell size (Hafen and Stocker, 2003), cell cycle progression and endoreplication (Burgering, 2008; Ho et al., 2008), we tested whether this pathway affects muscle growth. To modulate Insulin signaling in muscles, three different approaches were used. First, muscles of wild-type larvae were compared with those from larvae homozygous or heterozygous mutant for chico [also known as Insulin receptor substrate (IRS)] (see Fig. S1 in the supplementary material). Second, the levels of InR and Akt (Akt1) were reduced via RNAi knockdown in muscles using the Gal4-UAS system and Dmef2-Gal4 (see Fig. S2 in the supplementary material), which drives transgene expression in muscles but not in other endoreplicating tissues (see Fig. S3 in the supplementary material). Third, we targeted the expression of activators (InR) (Fig. 2B) and inhibitors of InR signaling in muscles, including a dominant-negative form of InR (InR DN) (Fig. 2C). In all cases, inhibition of InR signaling resulted in decreased nuclear and muscle size, as outlined by DAPI and phalloidin staining, respectively. Conversely, overexpression of wild-type InR resulted in a significant increase in myofiber size and nuclear size (Fig. 2B), suggesting that InR signaling controls muscle growth in part by modulating endoreplication.
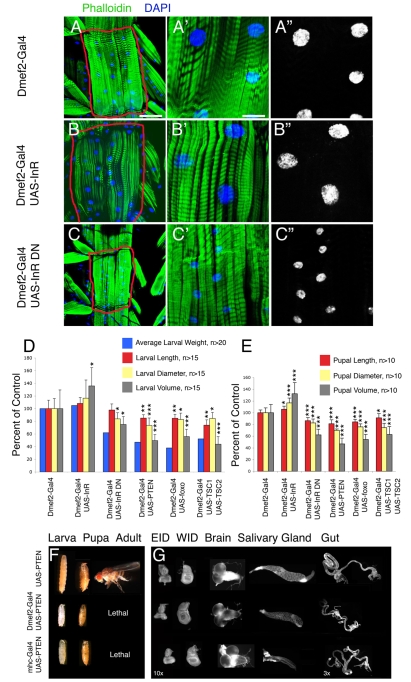
Fig. 2.
Inhibition of muscle growth affects the size of the entire body and of other tissues. (A-C″) Staining of body wall muscles of L3 Drosophila larvae with phalloidin (green) and DAPI (blue). (A-A″) Matched controls (Dmef2-Gal4). (B-C″) Activators [B, Insulin-like receptor (InR)] and repressors [C, Insulin-like receptor dominant-negative (InR DN)] were overexpressed in muscles using the Dmef2-Gal4 muscle driver. (B-B″) Activation of InR signaling results in a significant increase in the area of myofibers VL3 and VL4 (encircled in red) with a concomitant increase in nuclear area. (C-C″) Inhibition of InR signaling exerts converse effects. Scale bars: 75 μm in A-C; 18.7 μm in A′-C′. (D) Quantification of average larval weight (n>20), larval length (n>15), diameter (n>15) and volume (n>15) of larvae in which InR, InR DN, Pten, foxo or Tsc1 and Tsc2 have been overexpressed using Dmef2-Gal4. A decrease in muscle growth (see Fig. 5) always correlates with a reduction in larval body size. Consistent with these results, overexpression of InR, which promotes an increase in muscle growth (B), also increases larval body size. (E) Quantification of length (n>10), diameter (n>10) and volume (n>15) of pupae arising from larvae in which InR signaling has been modulated in muscles (see Fig. 5). Error bars indicate s.d.; *P<0.05, **P<0.01, ***P<0.001. (F) Overexpression of Pten in muscles using Dmef2-Gal4 (Dmef2-Gal4 UAS-Pten) or Mhc-Gal4 (Mhc-Gal4 UAS-Pten) results in smaller larvae and pupae when compared with the control (UAS-Pten). (G)The size of internal tissues and organs, visualized with the lipophilic dye FM4-64, is also affected. Note, especially, the reduction in size of endoreplicating tissues. Magnification: 10×, except for gut (3×). For a full description of genotypes, see Table S1 in the supplementary material.
Interestingly, we noticed that in addition to the autonomous effect on muscle growth, regulation of InR signaling in muscles exerted a systemic effect on body size. In all cases in which InR and Tor signaling were repressed, a significant decrease in weight, length, diameter and volume was observed in larvae (Fig. 2D,F) and pupae (Fig. 2E,F), without substantial developmental delay (not shown). By contrast, activation of InR signaling, following overexpression of InR, resulted in a significant increase in larval and pupal volumes (Fig. 2D,E).
To test whether the size of larval organs and tissues other than muscles are affected when InR signaling is repressed using the Dmef2-Gal4 and Mhc-Gal4 muscle drivers, we examined their size in L3 wandering larvae following staining with the lipophilic dye FM4-64 to outline their dimensions. In addition to a reduction in muscle size (Fig. 2C), the size of other endoreplicating organs, such as the salivary glands, gut, fat body and epidermis, was decreased (Fig. 2G; see Fig. S8 in the supplementary material; data not shown). However, the size of non-endoreplicating tissues, including the brain, wing and eye-antennal imaginal discs was less affected. Further, upon activation of InR signaling in muscles, an increase in muscle size (Fig. 2B) was accompanied by a parallel increase in the size of most other tissues (see Fig. S3 in the supplementary material; data not shown).
Altogether, perturbations of muscle growth through the InR and Tor signaling pathways not only regulate muscle size but also trigger a systemic response that affects body size.
Reduction of muscle size by InR signaling non-autonomously regulates the size of other organs and affects feeding behavior
To characterize how changes in InR signaling in muscles affect the size of other tissues, we analyzed the morphological changes induced in fat body and salivary glands following Pten overexpression in muscles. Strikingly, reduction of Insulin signaling in muscles was accompanied by a reduction of cell size in endoreplicating tissues, via lipid remobilization in fat body cells (Fig. 3A,A′), and activation of catabolic programs possibly related to autophagy in salivary glands (Fig. 3B,B′). Because lipid remobilization and activation of catabolic programs in endoreplicating tissues are common events in response to improper feeding behavior and metabolic regulation (Colombani et al., 2003), we tested whether feeding was affected in larvae with either repressed or activated InR signaling in muscles. Feeding behavior is under strict control in Drosophila and other organisms, as nutrient uptake is crucial for appropriate developmental growth (Saper et al., 2002). To monitor feeding activity, the number of mouth hook contractions, which has been shown to be an indicator of this behavior (Wu et al., 2005), was scored. Interestingly, overexpression of the InR antagonists Pten, Tsc1 and Tsc2 (gigas) or of foxo in muscles resulted in a significant decrease in larval feeding, whereas InR overexpression promoted this behavior (Fig. 3C). Thus, we propose that the levels of InR signaling in muscles somehow modulate larval feeding behavior, which in turn influences body size and tissue growth.
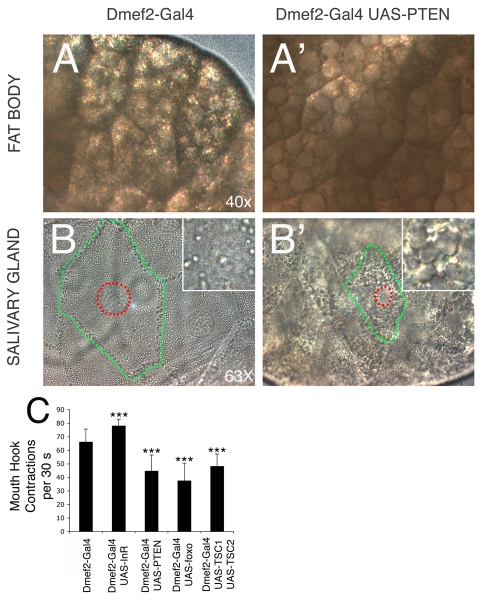
Fig. 3.
Inhibition of InR signaling in muscles induces catabolic programs in endoreplicating tissues and modulates larval feeding behavior. (A-B′) Transmitted light microscopy images of fat body and salivary gland cells from (A,B) control Drosophila larvae (Dmef2-Gal4) and (A′,B′) Dmef2-Gal4 UAS-Pten larvae. Lipid remobilization and catabolic events, possibly related to autophagy, are detected respectively in (A′) fat body and (B′) salivary gland cells of larvae in which Pten is overexpressed in muscles, in comparison with matched controls (A,B). Note the reduction in cell size (encircled in green) and nuclear size (indicative of nuclear ploidy, encircled in red) in salivary gland. Magnification: 40× (fat body) and 63× (salivary gland). (C) Modulation of InR signaling in muscles regulates larval feeding behavior. The number of mouth hook contractions every 30 seconds is significantly reduced in larvae that overexpress Pten, Tsc1 and Tsc2, or foxo in muscles, and is increased upon InR overexpression. n>50; error bars indicate s.d.; ***P<0.001. A similar regulation of feeding behavior was observed in Mhc-Gal4 UAS-Pten larvae (not shown).
To test whether this systemic effect reflects a direct role of InR pathway activity or, rather, a general reduction in muscle mass, we inhibited muscle growth by means distinct from InR signaling, such as by Cyclin E overexpression (see Fig. S1 in the supplementary material). Similar to inhibition of InR signaling, both the larval feeding behavior and the size of most internal organs were affected in Dmef2-Gal4 UAS-CycE larvae (see Fig. S8 in the supplementary material), suggesting that non-autonomous effects might rely principally on muscle size, rather than on InR signaling per se. Consistent with this notion, concomitant overexpression of InR and CycE in muscles was not sufficient to rescue the developmental growth defects associated with CycE overexpression alone (not shown).
Although most manipulations of InR signaling during larval muscle growth result in pupal lethality, we recovered Dmef2-Gal4 UAS-InR and Dmef2-Gal4 UAS-InR DN adult flies, in which InR signaling was activated and inhibited, respectively. As expected, whereas activation of InR during muscle growth resulted in larger flies, smaller flies arose upon inhibition of this pathway (Fig. 4A). To test whether developmental muscle growth regulates, in turn, the size of body parts in adults, we scored the weight, eye size, abdomen length and wing area of these recovered flies. As expected, all these parameters were respectively either increased or decreased upon activation or inhibition of InR signaling in muscles. Changes in tissue and whole-body size occurred by modulating cell size, as observed in the wings of adult flies, whereas cell number barely varied (Fig. 4B). Thus, several tissues, deriving from both endoreplicating and non-endoreplicating tissues, are affected to different extents upon developmental modulation of muscle mass.
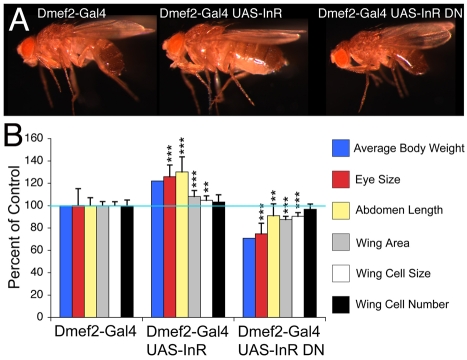
Fig. 4.
Modulation of InR signaling during muscle growth affects the final size of adult flies. (A) Adult flies in which muscle growth has been altered by either activating (InR) or inhibiting (InR DN) InR signaling. (B) Significant changes are correspondingly observed in fly body weight, eye size, abdomen length and wing area. Changes in wing area result from an increase (InR) or decrease (InR DN) in cell size and possibly also cell number. Note that because the growth of distinct body parts is differentially affected, body proportions are also altered. Error bars indicate s.d.; **P<0.01, ***P<0.001; n(weight)>20, n(eye)>12, n(abdomen)>22, n(wing)=10.
dMyc is necessary and sufficient to regulate endoreplication in muscles
Similar to components of InR signaling, the transcription factor dMyc has been implicated in growth events in Drosophila, in part via the induction of endoreplication (Maines et al., 2004; Pierce et al., 2004). To test whether dMyc plays a role in muscle growth, we modulated its function in several ways. First, muscles from wild-type larvae were compared with those from larvae that were homozygous or heterozygous mutant for dmyc (see Fig. S1 in the supplementary material). Second, levels of dMyc were reduced via RNAi knockdown in muscles (see Fig. S2 in the supplementary material). Third, we targeted expression of dmyc in muscles (Fig. 5E), as well as the expression of its inhibitor dmnt (Mnt) (see Fig. S4 in the supplementary material). In all cases, inhibition of dMyc activity resulted in smaller muscles and nuclei and decreased body size. dMyc overexpression was associated with an increase in nuclear size and DAPI staining that was, however, accompanied by only a slight increase in muscle size (Fig. 5E,H-K). Thus, during muscle growth, dMyc is both necessary and sufficient to regulate endoreplication. However, although dMyc and endoreplication are necessary, they are not sufficient to sustain extensive growth.
Fig. 5.
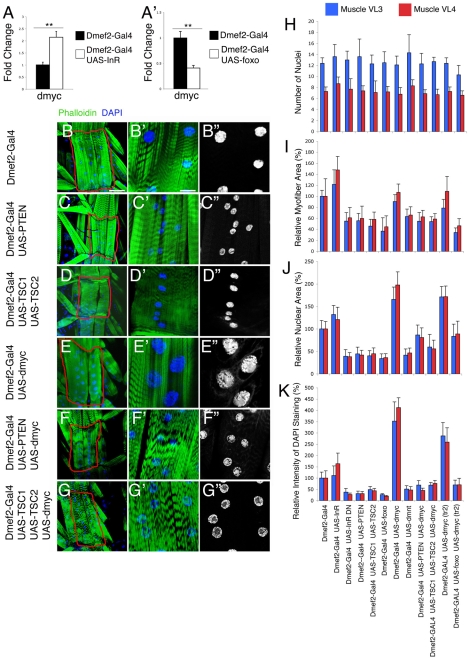
InR/Tor signaling regulates muscle growth and nuclear ploidy by inhibiting dMyc function. (A,A′) Expression of dmyc is promoted by InR and antagonized by Foxo. qRT-PCR analysis of dmyc transcript levels in Drosophila L3 larvae in which either InR or foxo is overexpressed in body wall muscles. (A) A 2-fold increase in dmyc levels is observed upon InR overexpression in muscles, whereas (A′) foxo activation results in a significant 2.5-fold reduction of dmyc transcripts. Error bars indicate s.d. (n=4); **P<0.01. (B-G″) Staining of body wall muscles of L3 larvae with phalloidin (green) and DAPI (blue). (B-B″) Dmef2-Gal4. Overexpression of the InR signaling negative regulators (C) Pten and (D) Tsc1 and Tsc2 in muscles using Dmef2-Gal4. (B-D″) Repression of InR signaling results in all cases in a significant decrease in the area of muscles VL3 and VL4 (encircled in red) with a concomitant reduction in nuclear area. (E) Overexpression of dmyc results in a significant increase in nuclear area without a proportional increase in myofiber area. (F) Co-expression of dmyc with Pten, or (G) with Tsc1 and Tsc2, is sufficient to suppress dMyc-driven polyploidization, indicating that Insulin signaling antagonizes dMyc. For additional examples of muscle phenotypes, generated by overexpressing dmnt and foxo,see Fig. S4 in the supplementary material. Scale bars: 75 μm in B-G; 18.7 μm in B′-G′. (H-K) Quantification of (H) the number of nuclei, (I) myofiber area, (J) nuclear area and (K) intensity of DAPI staining in muscles VL3 (blue) and VL4 (red). Modulation of InR/Tor signaling in muscles is sufficient to promote significant changes in muscle size, which parallel variation in nuclear area and intensity of DAPI staining, but not in the number of nuclei. For statistical analysis, n(myofibers)=10, n(nuclei muscle VL3)=100, n(nuclei muscle VL4)=50; n(nuclei)=10 for intensity of DAPI staining; error bars indicate s.d.
InR, Tor and Foxo are required for optimal dMyc function during muscle growth
Since InR signaling and dMyc loss-of-function elicit similar effects on the control of muscle growth and body size, we further investigated the mechanisms by which InR signaling and dMyc interact. InR overexpression resulted in a 2-fold increase in dmyc expression (Fig. 5A), as estimated by qRT-PCR. Consistent with a previous report (Teleman et al., 2008), overexpression of foxo in muscles resulted in a significant, 2.5-fold reduction in dmyc transcript levels (Fig. 5A′).
Because the regulation of dmyc gene expression by InR/Foxo might only in part account for the regulation of dMyc activity, we tested whether InR and Tor signaling regulate dMyc protein function, as estimated by their ability to control dMyc-driven phenotypes in muscles. When either Pten, or Tsc1 and Tsc2 were co-expressed together with dmyc, dMyc activity was inhibited, resulting in defective myofiber growth and endoreplication (Fig. 5F,G), similar to the expression of Pten (Fig. 5C) or Tsc1 and Tsc2 alone (Fig. 5D). Consistent with being regulated by InR signaling, foxo overexpression also impaired dMyc activity (see Fig. S4 in the supplementary material; Fig. 5H-K). Quantification of muscle phenotypes indicated that significant changes in myofiber area (Fig. 5I), nuclear size (Fig. 5J) and the intensity of DAPI staining (Fig. 5K) occur in concert, without any change in the number of nuclei (Fig. 5H). Thus, maximal dMyc activity relies on optimal InR/Tor signaling and inhibition of Foxo activity. Furthermore, and contrary to previous analyses in fat body cells (Pierce et al., 2004), dMyc overexpression in muscles promoted endoreplication without a proportional increase in cell size.
dMyc is autonomously modulated by InR/Tor signaling
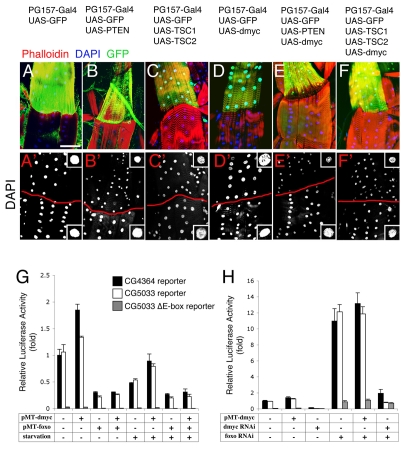
Because muscle growth impacts body growth (Fig. 2), to exclude any potential non-autonomous effect that might bias this analysis, we created genetic mosaics to further test the functional relationship between Pten, Tsc1/2 and dMyc. To this purpose, we used the PG157-Gal4 driver, a previously uncharacterized Gal4 line that we find can drive expression in only a subset of larval muscles (see Fig. S5 in the supplementary material; Fig. 6A; and Materials and methods). Using this method, the expression of Pten, Tsc1/2 and dmyc alone, and dmyc together with Pten or Tsc1/2, was driven in a subset of larval muscles. By examining DAPI staining in adjacent control myofibers, in which no transgene expression is driven, significant decreases or increases in intensity of DAPI staining (see Fig. S6 in the supplementary material) and nuclear size in GFP-expressing myofibers were detected upon Pten (Fig. 6B,B′), Tsc1/2 (Fig. 6C,C′) or dmyc (Fig. 6D,D′) expression. Co-expression of dmyc together with Pten (Fig. 6E,E′) or Tsc1/2 (Fig. 6F,F′) resulted in impaired dMyc-mediated endoreplication. Thus, Pten and Tsc1/2 autonomously control cellular events that are induced by dMyc activity in muscles.
Fig. 6.
dMyc is autonomously antagonized by InR/Tor signaling. (A-F′) Mosaic analysis of Drosophila body wall muscles. (A) PG157-Gal4 drives the expression of transgenes in only a subset of body wall muscles (green for concurrent GFP expression; yellow in merge). Body wall muscles with no GFP expression (red) serve as control. DAPI (blue) and phalloidin (red) staining are used to outline nuclei and muscles, respectively. In A′-F′, only DAPI staining is shown (white) and the red line demarcates transgene-expressing (above) from non-expressing (below) muscles. (A,A′) Expression of GFP alone does not alter muscle and nuclear area. (B,B′) Expression of GFP concomitantly with Pten, or (C,C′) Tsc1 and Tsc2, results in a marked decrease in muscle growth (see Fig. S6 in the supplementary material) and nuclear size (green, above in micrographs), in comparison with neighboring VL3 and VL4 muscles in which no transgene expression occurs (red, below in micrographs). (D) dmyc expression results in a marked increase in nuclear size, in comparison with neighboring control muscles. (E,E′) Concomitant expression of dmyc with Pten, or (F,F′) with Tsc1 and Tsc2, results in decreased muscle and nuclear size, indicating that dMyc function is autonomously controlled by Pten and Tsc. Representative nuclei are shown in the insets. Scale bar: 37.5 μm. (G) Foxo inhibits dMyc transcriptional activity. Luciferase assays performed using three different dMyc transcriptional reporters (CG4364, CG5033 and CG5033 ΔE-box) and overexpression of dmyc and foxo. Transfection of S2R+ cells, with or without subsequent serum starvation, was performed with dmyc (pMT-dmyc), foxo (pMT-foxo), or both in combination. Activation of endogenous Foxo by serum starvation, or following foxo overexpression, suppresses transcription of the dMyc Luciferase reporters. (H) Luciferase assays using dMyc reporters in dmyc and foxo RNAi-treated cells. dmyc RNAi suppresses, whereas foxo RNAi promotes, Luciferase expression. However, in combination with dmyc RNAi, foxo RNAi does not bring about a similar transcriptional regulation. Relative Luciferase activity corresponds to the firefly:Renilla luminescence ratio. The s.e.m. is indicated (n=4).
Foxo inhibits dMyc transcriptional activity
dMyc acts primarily via inducing a transcriptional response, suggesting that InR signaling might regulate dMyc function by modulating its transcriptional activity. Similar to Pten and Tsc, Foxo can regulate dMyc protein function in vivo (see Fig. S4 in the supplementary material; Fig. 5H-K). To test whether Foxo inhibits dMyc by regulating its transcriptional activity, Luciferase assays were performed using CG4364 and CG5033 transcriptional reporters, previously described to be directly regulated by dMyc (Hulf et al., 2005) but not directly regulated by Foxo. The CG5033 ΔE-box reporter is devoid of E-boxes, the dMyc-responsive sequences, and is therefore refractory to dMyc transcriptional regulation.
In S2R+ cells, Luciferase activity of CG4364 and CG5033 reporters was detected in response to endogenous dMyc and was increased by overexpression of dmyc (pMT-dmyc) (for characterization of overexpression, see Fig. S7 in the supplementary material). However, overexpression of wild-type foxo (pMT-foxo) (see Fig. S7 in the supplementary material) or serum starvation, which activates endogenous Foxo, decreased the Luciferase activity of the CG4364 and CG5033 reporters (Fig. 6G) and suppressed the transcriptional response induced by dmyc overexpression. Further, without E-boxes, no substantial Luciferase activity was detected, indicating that it depends on dMyc. Consistently, RNAi treatment against dmyc and foxo respectively attenuated and increased Luciferase activity of the CG4364 and CG5033 reporters (Fig. 6H; see Fig. S7 in the supplementary material), but not of the CG5033 ΔE-box reporter. The increase in Luciferase activity upon foxo RNAi is likely to reflect its ability to inhibit both dmyc gene expression and dMyc protein function. However, upon RNAi treatment of foxo and dmyc, no increase in Luciferase activity was observed (Fig. 6H), further confirming that Foxo regulates transcription of the CG4364 and CG5033 reporters via dMyc. Therefore, Foxo tightly controls dMyc function by modulating its expression (Fig. 5) and its transcriptional activity.
dMyc primes muscle growth via nucleolus biogenesis and expression of growth-promoting genes
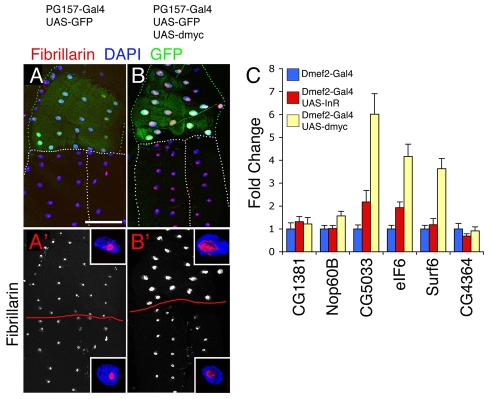
Although dMyc promotes endoreplication, no substantial muscle growth results. To further dissect the role of dMyc in muscle growth, we tested whether dMyc induces (1) nucleolus biogenesis, which is necessary for rRNA transcription and ribosome subunit assembly (Prieto and McStay, 2005), and (2) the expression of genes required for protein translation; both events are necessary for cell growth. By staining with an anti-Fibrillarin antiserum, which outlines nucleoli (Grewal et al., 2005), we observed a dramatic increase in the size of the nucleolus upon dmyc overexpression with the PG157-Gal4 driver, in comparison with controls (Fig. 7A,B). Further, dmyc overexpression with Dmef2-Gal4 increased, to different extents (Fig. 7C), the expression of some dMyc target genes (Grewal et al., 2005; Hulf et al., 2005) that are involved in rRNA processing (Nop60B), ribosome assembly and biogenesis (CG1381, CG5033, Surf6), and translational control (eIF6), but not cell proliferation (CG4364). InR overexpression promoted a modest increase in the expression of dMyc target genes involved in growth (CG1381, CG5033, eIF6, Surf6). Thus, dMyc primes muscles for growth by promoting endoreplication, nucleolus biogenesis and the expression of some genes necessary for protein translation. However, gene expression programs governed by dMyc require concomitant InR/Tor signaling to drive substantial muscle growth (Fig. 8).
Fig. 7.
dMyc primes muscle cells for growth. dMyc promotes biogenesis of nucleoli and expression of genes required for protein synthesis. (A) Fibrillarin immunoreactivity (red) stains the nucleoli of transgene-expressing muscles (green for concurrent GFP expression, above in micrographs; nuclei identified by DAPI staining, blue) and of neighboring control myofibers [outlined in white, based on phalloidin staining (not shown), below in micrographs]. (B) dmyc overexpression promotes nucleolus biogenesis that is, however, insufficient to drive muscle growth. (A′,B′) Fibrillarin immunoreactivity, together with representative nuclei (blue) and nucleoli (red; insets). Scale bar: 37.5 μm. (C)qRT-PCR analysis of dMyc target genes involved in growth. Significant induction of gene expression is observed upon dmyc overexpression and, to a lesser extent, upon overexpression of InR. Error bars indicate s.d. (n=4).
Fig. 8.
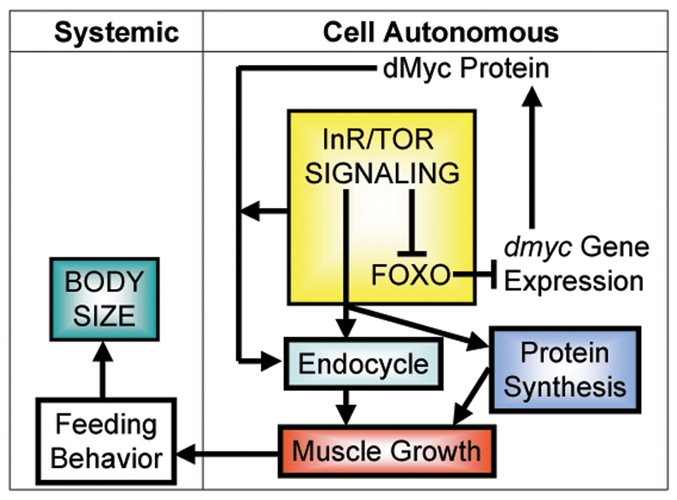
Interplay of growth signals during muscle growth regulates Drosophila body size. Integration of growth signals controls endoreplication, muscle growth and body size. Inhibition of InR/Tor signaling is accompanied by activation of Foxo, which inhibits transcription of dmyc. In turn, dMyc protein requires input from InR/Tor signaling for its maximal function. dMyc promotes endoreplication, biogenesis of nucleoli and expression of genes required for protein synthesis. However, dMyc is necessary, but not sufficient, to sustain extensive muscle growth, which also requires concurrent activation of InR/Tor signaling to drive protein synthesis and other anabolic processes. A decrease or increase in muscle mass in turn perturbs the growth of other tissues and, indeed, the whole body, at least in part by regulating the feeding behavior of the larva.
DISCUSSION
We have examined the mechanisms regulating the extensive muscle growth that occurs during Drosophila larval development. We found that interplay between InR/Tor signaling, Foxo and dMyc activity regulates this process, in part via the induction of endoreplication. Interestingly, the extent of muscle growth is sensed systemically, regulates feeding behavior and, in turn, influences the size of other tissues and indeed the whole body. Thus, the growth of a single tissue is sensed systemically via modulating a whole-organism behavior (Fig. 8).
Foxo regulates endoreplication and dMyc transcriptional activity
We found that dMyc, as well as activation of InR signaling, can promote endoreplication in muscles, whereas Foxo and inhibitors of dMyc and of InR/Tor have the opposite effect. dMyc is likely to regulate the expression of genes required for multiple G–S and S–G transitions during endoreplication (Edgar and Orr-Weaver, 2001; Lilly and Duronio, 2005), similar to vertebrate Myc, which regulates key cell-cycle regulators including cyclin D2, cyclin E, and the cyclin kinase inhibitors p21 and p27 (Cdkn1a and Cdkn1b, respectively) (Grandori et al., 2000). Indeed, aberrant levels of Cyclin E (Lilly and Spradling, 1996) block muscle growth (see Fig. S1 in the supplementary material), indicating that proper muscle growth requires tight control of the expression and activity of endoreplication genes. Further, we found that endoreplication is also modulated by Foxo, which is activated in conditions of nutrient starvation, impaired InR/Tor signaling and by other cell stressors (Arden, 2008). Foxo presumably regulates cell cycle progression at least in part by modulating the expression of evolutionarily conserved Foxo/Myc-target genes, such as dacapo (the Drosophila p21/p27 homolog) and Cyclin E, that regulate the G1–S transition, as previously reported in mammalian systems (Grandori et al., 2000; Salih and Brunet, 2008). Interestingly, Foxo and Myc might control different steps in the activation of common target genes (Bouchard et al., 2004).
In addition, we found that active Foxo can also inhibit dMyc protein activity and regulates dmyc gene expression. Mechanistically, Foxo could influence dMyc activity in several ways. First, it might physically interact with dMyc, although we found no evidence to support this notion (see Fig. S7 in the supplementary material). Second, Foxo could regulate the expression of genes that target dMyc for proteasomal degradation, including several ubiquitin E3 ligases that are induced by Foxo during muscle atrophy in mice and humans (Sandri et al., 2004; Stitt et al., 2004). However, by analyzing dMyc protein levels by western blot, we did not detect significant dMyc protein instability upon Foxo overexpression (see Fig. S7 in the supplementary material). Third, Foxo might promote the expression of transcriptional regulators that oppose dMyc function, including Mad/Mnt (Delpuech et al., 2007), although no substantial increase in dmnt mRNA levels was detected upon Foxo activation in muscles (not shown). Possibly, the expression of other dMyc regulators might be affected by Foxo. Future experiments will be needed to dissect the Foxo-dMyc interaction.
Finally, by manipulating muscle growth and/or endoreplication, we found that in muscles the ratio of cell size to nuclear size is not constant, and increased nuclear size and DNA content, indicative of ploidy, is necessary but not sufficient to drive growth. Usually, an increase in cell size is matched by an increase in nuclear size (Neumann and Nurse, 2007), which commonly parallels increases in nuclear ploidy (Maines et al., 2004; Ohlstein and Spradling, 2006; Sato et al., 2008; Shcherbata et al., 2004; Sun and Deng, 2007). However, our findings indicate that in muscles, dMyc-driven variation in nuclear size and ploidy is permissive but not sufficient for substantial growth, even in the presence of increased biogenesis of nucleoli and expression of genes involved in protein translation. This is different from fat body cells, in which dmyc overexpression induces endoreplication and proportional cell growth (Pierce et al., 2004). Thus, additional instructive signals, possibly modulating protein synthesis, mitochondriogenesis, ribosome biogenesis (Teleman et al., 2008), sarcomere assembly (Bai et al., 2007; Haas, 1950), and other anabolic responses must be concomitantly received to promote maximal muscle growth. Therefore, increases in cell size and nuclear ploidy are surprisingly uncoupled during muscle growth.
Muscle size regulates systemic growth
Little is known about the mechanisms that control and coordinate cell, organ and body size (Edgar, 2006; Mirth and Riddiford, 2007), and in particular how muscle growth is matched with the growth of other tissues and of the entire organism. We found that inhibition of InR/Tor signaling and dMyc activity in muscles impairs, in addition to muscle mass, the size of the entire body and of other internal organs. Similarly, overexpression of Cyclin E in muscles also resulted in autonomous and systemic growth defects (see Figs S1 and S8 in the supplementary material), indicating that, at least in some cases, modulation of muscle growth by means independent from InR signaling can be sensed systemically. In the larva, endoreplicating tissues and organs (gut, salivary glands, epidermis, fat body) were severely affected, whereas non-endoreplicating tissues (brain and imaginal discs) were less affected, indicating distinct tissue responsiveness to this regulation. Similarly, inhibition of Tor signaling in the fat body also primarily affects the size of endoreplicating tissues (Britton et al., 2002; Colombani et al., 2003).
Non-autonomous regulation of tissue size may rely on humoral factors (e.g. hormone-binding proteins, hormones, metabolites) produced by muscles in response to achieving a certain mass (Gamer et al., 2003). However, alternative models are possible. In particular, we observed decreased and increased larval feeding, respectively, upon inhibition and activation of InR signaling in muscles. This whole-organism behavioral adaptation is possibly due to decreased and increased efficiency of smaller and bigger muscles, respectively, and to regulated expression of neuropeptides that hormonally control feeding behavior. As a consequence of the regulation of feeding behavior, nutrient uptake is decreased and larval growth is blocked in the cells of endoreplicating tissues, which are extremely sensitive to poor nutritional conditions, and to a lesser extent in non-endoreplicating tissues, which are more resistant to limited nutritional supply (Bradley and Leevers, 2003; Colombani et al., 2003). In turn, increased or decreased size of non-muscle tissues arise as a consequence of abnormal feeding. Thus, muscle size coordinates with the size of other organs and of the entire body, at least in part via a systemic, behavioral response. Distinct tissues are differently sensitive to this regulation, resulting in altered body proportions.
Drosophila as a disease model of muscle atrophy and hypertrophy
Understanding the mechanisms regulating muscle mass is of special interest because they underline the etiology of several human diseases (Glass, 2003a). Directly relevant to our studies, both MYC and InR (INSR) signaling have been found to regulate muscle growth and maintenance in humans (Sandri et al., 2004; Southgate et al., 2007; Stitt et al., 2004; Zhong et al., 2006). Further, muscle atrophy is triggered by FOXO activation in several pathological conditions (Glass, 2003b; Sandri et al., 2004; Stitt et al., 2004). In addition, MYC function has been implicated in heart hypertrophy (Bello Roufai et al., 2007; Xiao et al., 2001; Zhong et al., 2006), a process that is conversely regulated by FOXO (Evans-Anderson et al., 2008; Skurk et al., 2005).
Our findings that Foxo functionally antagonizes dMyc during the growth of Drosophila muscles suggest that these factors might also interact similarly in humans. Consistent with this hypothesis, FOXO and MYC regulate, in opposite fashions, the atrophic and hypertrophic programs in human skeletal muscles and cardiomyocytes, and display complementary gene expression and activity in these contexts (Lecker et al., 2004; Mahoney et al., 2008; Sandri et al., 2004; Spruill et al., 2008; Stitt et al., 2004).
Finally, our finding that during larval development, inhibition of InR signaling in muscles has profound systemic effects might also reflect physiological conditions found in humans. Indeed, defective responsiveness of muscles to Insulin during type II diabetes has autonomous effects on muscle maintenance that are associated with systemic effects on the metabolism of the entire organism, contributing to the improper control of glycemia and the development of metabolic syndrome (Wells et al., 2008). Here, we have identified feeding behavior as part of the systemic response that in Drosophila senses perturbations in muscle mass. These findings might help further elucidate the signals involved in metabolic and growth homeostasis, which may be conserved across evolution.
Supplementary Material
We thank Bruce Edgar, Robert Eisenman, Peter Gallant, Ernst Hafen, Amir Orian, Susan Parkhurst, Oscar Puig, David Stein, Tian Xu, Alain Vincent, the Drosophila RNAi Screening Center, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center, and members of the Perrimon laboratory for reagents. We thank Jianwu Bai, Mary Packard, Chrysoula Pitsouli and Jonathan Zirin for critically reading the manuscript. This work was supported by the NIH (1P01CA120964-01A1). N.P. is an investigator of the Howard Hughes Medical Institute. Deposited in PMC for release after 6 months.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/cgi/content/full/136/6/983/DC1
References
- Accili, D. and Arden, K. C. (2004). FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell 117, 421-426. [DOI] [PubMed] [Google Scholar]
- Arden, K. C. (2008). FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene 27, 2345-2350. [DOI] [PubMed] [Google Scholar]
- Bai, J., Hartwig, J. H. and Perrimon, N. (2007). SALS, a WH2-domain-containing protein, promotes sarcomeric actin filament elongation from pointed ends during Drosophila muscle growth. Dev. Cell 13, 828-842. [DOI] [PubMed] [Google Scholar]
- Bate, M., Landgraf, M. and Ruiz Gómez Bate, M. (1999). Development of larval body wall muscles. Int. Rev. Neurobiol. 43, 25-44. [DOI] [PubMed] [Google Scholar]
- Beckett, K. and Baylies, M. K. (2006). The development of the Drosophila larval body wall muscles. Int. Rev. Neurobiol. 75, 55-70. [DOI] [PubMed] [Google Scholar]
- Bello Roufai, M., Li, H. and Sun, Z. (2007). Heart-specific inhibition of protooncogene c-myc attenuates cold-induced cardiac hypertrophy. Gene Ther. 14, 1406-1416. [DOI] [PubMed] [Google Scholar]
- Bouchard, C., Marquardt, J., Bras, A., Medema, R. H. and Eilers, M. (2004). Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. EMBO J. 23, 2830-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard, C., Lee, S., Paulus-Hock, V., Loddenkemper, C., Eilers, M. and Schmitt, C. A. (2007). FoxO transcription factors suppress Myc-driven lymphomagenesis via direct activation of Arf. Genes Dev. 21, 2775-2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, G. L. and Leevers, S. J. (2003). Amino acids and the humoral regulation of growth: fat bodies use slimfast. Cell 114, 656-658. [DOI] [PubMed] [Google Scholar]
- Brand, A. H. and Perrimon, N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Britton, J. S., Lockwood, W. K., Li, L., Cohen, S. M. and Edgar, B. A. (2002). Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2, 239-249. [DOI] [PubMed] [Google Scholar]
- Brodsky, W. Y. and Uryvaeva, I. V. (1977). Cell polyploidy: its relation to tissue growth and function. Int. Rev. Cytol. 50, 275-332. [DOI] [PubMed] [Google Scholar]
- Buckingham, M. (2006). Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr. Opin. Genet. Dev. 16, 525-532. [DOI] [PubMed] [Google Scholar]
- Budnik, V., Gorczyca, M. and Prokop, A. (2006). Selected methods for the anatomical study of Drosophila embryonic and larval neuromuscular junctions. Int. Rev. Neurobiol. 75, 323-365. [DOI] [PubMed] [Google Scholar]
- Burgering, B. M. (2008). A brief introduction to FOXOlogy. Oncogene 27, 2258-2262. [DOI] [PubMed] [Google Scholar]
- Clyne, P. J., Brotman, J. S., Sweeney, S. T. and Davis, G. (2003). Green fluorescent protein tagging Drosophila proteins at their native genomic loci with small P elements. Genetics 165, 1433-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani, J., Raisin, S., Pantalacci, S., Radimerski, T., Montagne, J. and Leopold, P. (2003). A nutrient sensor mechanism controls Drosophila growth. Cell 114, 739-749. [DOI] [PubMed] [Google Scholar]
- Colombani, J., Bianchini, L., Layalle, S., Pondeville, E., Dauphin-Villemant, C., Antoniewski, C., Carre, C., Noselli, S. and Leopold, P. (2005). Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310, 667-670. [DOI] [PubMed] [Google Scholar]
- Conlon, I. and Raff, M. (1999). Size control in animal development. Cell 96, 235-244. [DOI] [PubMed] [Google Scholar]
- de la Cova, C. and Johnston, L. A. (2006). Myc in model organisms: a view from the flyroom. Semin. Cancer Biol. 16, 303-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpuech, O., Griffiths, B., East, P., Essafi, A., Lam, E. W., Burgering, B., Downward, J. and Schulze, A. (2007). Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression. Mol. Cell. Biol. 27, 4917-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis, F. and Dahmann, C. (2007). Apical and lateral cell protrusions interconnect epithelial cells in live Drosophila wing imaginal discs. Dev. Dyn. 236, 3408-3418. [DOI] [PubMed] [Google Scholar]
- Dietzl, G., Chen, D., Schnorrer, F., Su, K. C., Barinova, Y., Fellner, M., Gasser, B., Kinsey, K., Oppel, S., Scheiblauer, S. et al. (2007). A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151-156. [DOI] [PubMed] [Google Scholar]
- Edgar, B. A. (2006). How flies get their size: genetics meets physiology. Nat. Rev. Genet. 7, 907-916. [DOI] [PubMed] [Google Scholar]
- Edgar, B. A. and Orr-Weaver, T. L. (2001). Endoreplication cell cycles: more for less. Cell 105, 297-306. [DOI] [PubMed] [Google Scholar]
- Eisenman, R. N. (2001). Deconstructing myc. Genes Dev. 15, 2023-2030. [DOI] [PubMed] [Google Scholar]
- Evans-Anderson, H. J., Alfieri, C. M. and Yutzey, K. E. (2008). Regulation of cardiomyocyte proliferation and myocardial growth during development by FOXO transcription factors. Circ. Res. 102, 686-694. [DOI] [PubMed] [Google Scholar]
- Gallant, P. (2006). Myc/Max/Mad in invertebrates: the evolution of the Max network. Curr. Top. Microbiol. Immunol. 302, 235-253. [DOI] [PubMed] [Google Scholar]
- Gamer, L. W., Nove, J. and Rosen, V. (2003). Return of the chalones. Dev. Cell 4, 143-144. [DOI] [PubMed] [Google Scholar]
- Glass, D. J. (2003a). Molecular mechanisms modulating muscle mass. Trends Mol. Med. 9, 344-350. [DOI] [PubMed] [Google Scholar]
- Glass, D. J. (2003b). Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol. 5, 87-90. [DOI] [PubMed] [Google Scholar]
- Grandori, C., Cowley, S. M., James, L. P. and Eisenman, R. N. (2000). The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu. Rev. Cell Dev. Biol. 16, 653-699. [DOI] [PubMed] [Google Scholar]
- Greer, E. L. and Brunet, A. (2008). FOXO transcription factors in ageing and cancer. Acta Physiol. 192, 19-28. [DOI] [PubMed] [Google Scholar]
- Grewal, S. S., Li, L., Orian, A., Eisenman, R. N. and Edgar, B. A. (2005). Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat. Cell Biol. 7, 295-302. [DOI] [PubMed] [Google Scholar]
- Haas, J. N. (1950). Cytoplasmic growth in the muscle fibers of larvae of Drosophila melanogaster. Growth 14, 277-294. [PubMed] [Google Scholar]
- Hafen, E. and Stocker, H. (2003). How are the sizes of cells, organs, and bodies controlled? PLoS Biol. 1, E86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget, G. W., Neuburger, M., Plagwitz, R. and Adler, C. P. (1997). DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc. Res. 36, 45-51. [DOI] [PubMed] [Google Scholar]
- Ho, K. K., Myatt, S. S. and Lam, E. W. (2008). Many forks in the path: cycling with FoxO. Oncogene 27, 2300-2311. [DOI] [PubMed] [Google Scholar]
- Hulf, T., Bellosta, P., Furrer, M., Steiger, D., Svensson, D., Barbour, A. and Gallant, P. (2005). Whole-genome analysis reveals a strong positional bias of conserved dMyc-dependent E-boxes. Mol. Cell. Biol. 25, 3401-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo, D. S., Gershman, B., Tu, M. P., Palmer, M. and Tatar, M. (2004). Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429, 562-566. [DOI] [PubMed] [Google Scholar]
- Iurlaro, M., Demontis, F., Corada, M., Zanetta, L., Drake, C., Gariboldi, M., Peiro, S., Cano, A., Navarro, P., Cattelino, A. et al. (2004). VE-cadherin expression and clustering maintain low levels of survivin in endothelial cells. Am. J. Pathol. 165, 181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker, S. H., Jagoe, R. T., Gilbert, A., Gomes, M., Baracos, V., Bailey, J., Price, S. R., Mitch, W. E. and Goldberg, A. L. (2004). Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39-51. [DOI] [PubMed] [Google Scholar]
- Lilly, M. A. and Spradling, A. C. (1996). The Drosophila endocycle is controlled by Cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 10, 2514-2526. [DOI] [PubMed] [Google Scholar]
- Lilly, M. A. and Duronio, R. J. (2005). New insights into cell cycle control from the Drosophila endocycle. Oncogene 24, 2765-2775. [DOI] [PubMed] [Google Scholar]
- Loo, L. W., Secombe, J., Little, J. T., Carlos, L. S., Yost, C., Cheng, P. F., Flynn, E. M., Edgar, B. A. and Eisenman, R. N. (2005). The transcriptional repressor dMnt is a regulator of growth in Drosophila melanogaster. Mol. Cell. Biol. 25, 7078-7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney, D. J., Safdar, A., Parise, G., Melov, S., Fu, M., Macneil, L., Kaczor, J., Payne, E. T. and Tarnopolsky, M. A. (2008). Gene expression profiling in human skeletal muscle during recovery from eccentric exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1901-R1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines, J. Z., Stevens, L. M., Tong, X. and Stein, D. (2004). Drosophila dMyc is required for ovary cell growth and endoreplication. Development 131, 775-786. [DOI] [PubMed] [Google Scholar]
- Manning, B. D. and Cantley, L. C. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckert, P. C., Rivello, H. G., Vigliano, C., Gonzalez, P., Favaloro, R. and Laguens, R. (2005). Endomitosis and polyploidization of myocardial cells in the periphery of human acute myocardial infarction. Cardiovasc. Res. 67, 116-123. [DOI] [PubMed] [Google Scholar]
- Mirth, C. K. and Riddiford, L. M. (2007). Size assessment and growth control: how adult size is determined in insects. BioEssays 29, 344-355. [DOI] [PubMed] [Google Scholar]
- Neumann, F. R. and Nurse, P. (2007). Nuclear size control in fission yeast. J. Cell Biol. 179, 593-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, J. Q., Markstein, M., Binari, R., Pfeiffer, B., Liu, L. P., Villalta, C., Booker, M., Perkins, L. and Perrimon, N. (2008). Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat. Methods 5, 49-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein, B. and Spradling, A. (2006). The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470-474. [DOI] [PubMed] [Google Scholar]
- Orian, A., van Steensel, B., Delrow, J., Bussemaker, H. J., Li, L., Sawado, T., Williams, E., Loo, L. W., Cowley, S. M., Yost, C. et al. (2003). Genomic binding by the Drosophila Myc, Max, Mad/Mnt transcription factor network. Genes Dev. 17, 1101-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orian, A., Delrow, J. J., Rosales Nieves, A. E., Abed, M., Metzger, D., Paroush, Z., Eisenman, R. N. and Parkhurst, S. M. (2007). A Myc-Groucho complex integrates EGF and Notch signaling to regulate neural development. Proc. Natl. Acad. Sci. USA 104, 15771-15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, S. B., Yost, C., Britton, J. S., Loo, L. W., Flynn, E. M., Edgar, B. A. and Eisenman, R. N. (2004). dMyc is required for larval growth and endoreplication in Drosophila. Development 131, 2317-2327. [DOI] [PubMed] [Google Scholar]
- Pierce, S. B., Yost, C., Anderson, S. A., Flynn, E. M., Delrow, J. and Eisenman, R. N. (2008). Drosophila growth and development in the absence of dMyc and dMnt. Dev. Biol. 315, 303-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter, C. J., Huang, H. and Xu, T. (2001). Drosophila Tsc1 functions with Tsc2 to antagonize insulin signaling in regulating cell growth, cell proliferation, and organ size. Cell 105, 357-368. [DOI] [PubMed] [Google Scholar]
- Prieto, J. L. and McStay, B. (2005). Nucleolar biogenesis: the first small steps. Biochem. Soc. Trans. 33, 1441-1443. [DOI] [PubMed] [Google Scholar]
- Prober, D. A. and Edgar, B. A. (2000). Ras1 promotes cellular growth in the Drosophila wing. Cell 100, 435-446. [DOI] [PubMed] [Google Scholar]
- Prober, D. A. and Edgar, B. A. (2002). Interactions between Ras1, dMyc, and dPI3K signaling in the developing Drosophila wing. Genes Dev. 16, 2286-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, O. and Tjian, R. (2005). Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 19, 2435-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, O. and Tjian, R. (2006). Nutrient availability and growth: regulation of insulin signaling by dFOXO/FOXO1. Cell Cycle 5, 503-505. [DOI] [PubMed] [Google Scholar]
- Puig, O., Marr, M. T., Ruhf, M. L. and Tjian, R. (2003). Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 17, 2006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganayakulu, G., Schulz, R. A. and Olson, E. N. (1996). Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev. Biol. 176, 143-148. [DOI] [PubMed] [Google Scholar]
- Salih, D. A. and Brunet, A. (2008). FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 20, 126-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri, M., Sandri, C., Gilbert, A., Skurk, C., Calabria, E., Picard, A., Walsh, K., Schiaffino, S., Lecker, S. H. and Goldberg, A. L. (2004). Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper, C. B., Chou, T. C. and Elmquist, J. K. (2002). The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199-211. [DOI] [PubMed] [Google Scholar]
- Sato, M., Kitada, Y. and Tabata, T. (2008). Larval cells become imaginal cells under the control of homothorax prior to metamorphosis in the Drosophila tracheal system. Dev. Biol. 318, 247-257. [DOI] [PubMed] [Google Scholar]
- Schlichting, K., Wilsch-Brauninger, M., Demontis, F. and Dahmann, C. (2006). Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells. J. Cell Sci. 119, 1184-1195. [DOI] [PubMed] [Google Scholar]
- Schuster, C. M., Davis, G. W., Fetter, R. D. and Goodman, C. S. (1996). Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, 641-654. [DOI] [PubMed] [Google Scholar]
- Shcherbata, H. R., Althauser, C., Findley, S. D. and Ruohola-Baker, H. (2004). The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development 131, 3169-3181. [DOI] [PubMed] [Google Scholar]
- Skurk, C., Izumiya, Y., Maatz, H., Razeghi, P., Shiojima, I., Sandri, M., Sato, K., Zeng, L., Schiekofer, S., Pimentel, D. et al. (2005). The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J. Biol. Chem. 280, 20814-20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southgate, R. J., Neill, B., Prelovsek, O., El-Osta, A., Kamei, Y., Miura, S., Ezaki, O., McLoughlin, T. J., Zhang, W., Unterman, T. G. et al. (2007). FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J. Biol. Chem. 282, 21176-21186. [DOI] [PubMed] [Google Scholar]
- Spruill, L. S., Baicu, C. F., Zile, M. R. and McDermott, P. J. (2008). Selective translation of mRNAs in the left ventricular myocardium of the mouse in response to acute pressure overload. J. Mol. Cell. Cardiol. 44, 69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger, D., Furrer, M., Schwinkendorf, D. and Gallant, P. (2008). Max-independent functions of Myc in Drosophila melanogaster. Nat. Genet. 40, 1084-1091. [DOI] [PubMed] [Google Scholar]
- Stitt, T. N., Drujan, D., Clarke, B. A., Panaro, F., Timofeyva, Y., Kline, W. O., Gonzalez, M., Yancopoulos, G. D. and Glass, D. J. (2004). The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395-403. [DOI] [PubMed] [Google Scholar]
- Sun, J. and Deng, W. M. (2007). Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell 12, 431-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman, A. A., Hietakangas, V., Sayadian, A. C. and Cohen, S. M. (2008). Nutritional control of protein biosynthetic capacity by insulin via Myc in Drosophila. Cell Metab. 7, 21-32. [DOI] [PubMed] [Google Scholar]
- Wells, G. D., Noseworthy, M. D., Hamilton, J., Tarnopolski, M. and Tein, I. (2008). Skeletal muscle metabolic dysfunction in obesity and metabolic syndrome. Can. J. Neurol. Sci. 35, 31-40. [DOI] [PubMed] [Google Scholar]
- Wu, Q., Zhang, Y., Xu, J. and Shen, P. (2005). Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc. Natl. Acad. Sci. USA 102, 13289-13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, G., Mao, S., Baumgarten, G., Serrano, J., Jordan, M. C., Roos, K. P., Fishbein, M. C. and MacLellan, W. R. (2001). Inducible activation of c-Myc in adult myocardium in vivo provokes cardiac myocyte hypertrophy and reactivation of DNA synthesis. Circ. Res. 89, 1122-1129. [DOI] [PubMed] [Google Scholar]
- Zhong, W., Mao, S., Tobis, S., Angelis, E., Jordan, M. C., Roos, K. P., Fishbein, M. C., de Alboran, I. M. and MacLellan, W. R. (2006). Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. EMBO J. 25, 3869-3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.