Abstract
The spinal cord is endogenously capable of several forms of adaptive plasticity and learning, including functional re-training, instrumental, and Pavlovian learning. Understanding the mechanisms of spinal plasticity could lead to improved therapies for spinal cord injury and other neuromotor disorders. We describe and demonstrate techniques for eliciting spinal learning in the adult mouse using the Horridge paradigm. In the Horridge paradigm, instrumental learning occurs when a nociceptive leg stimulus is made to be contingent on leg position and the spinal cord learns to maintain the ankle in a flexed position. Using fine-wire intramuscular stimulating electrodes, an inexpensive real-time video tracking system, and DC current stimulation, we were able to elicit instrumental spinal learning from mouse lumbrosacral spinal cords that were functionally isolated from the brain. This technique makes it more feasible to use the powerful genetic manipulations available in mice to better understand the processes of spinal learning, memory, and plasticity.
Keywords: Instrumental learning, video tracking, Stimulation, spinal cord injury, recovery of function, step training, use-dependent plasticity
Introduction
Spinal cord plasticity is critical for restoring sensorimotor function after spinal cord injury (Edgerton et al., 2008). Even complete, spinal cord transected mammals can be re-trained to perform different tasks with the hind legs, including standing and stepping, and to overcome perturbations to normal locomotion (Heng and de Leon, 2007). Although simple plastic processes such as habituation or sensitization could contribute to re-training, the spinal cord is also capable of more complex instrumental learning (Grau et al., 2006). Consequently, understanding the mechanisms of spinal plasticity and learning is important for improving strategies for restoring function after spinal cord injury.
A useful technique for eliciting spinal learning was developed by Horridge (1962a,b). The legs of headless insects exposed to the noxious stimulus of an electric shock exhibit a flexion withdrawal reflex. If the leg shock was made contingent on leg position by only occurring if the leg dropped below a constant threshold, after 15-30 min the legs held themselves above the threshold. The threshold was implemented by placing a saline solution at a set distance below the leg. When the leg contacted the saline solution, it completed a circuit and received a shock. To verify that the elevation of the leg was due to learning, a second animal was yoked to the first (master) animal, i.e., the leg in the yoked animal was shocked simultaneously with the leg of the master animal regardless of its position. Shock was not contingent on leg position for the yoked animal. Whereas legs receiving contingent leg shock in the master animal showed sustained elevation, the legs of the yoked animals did not, demonstrating that the elevation response was not an artifact of stimulation but reflected learning.
The Horridge paradigm has been used to study spinal learning in many different animals, including insects, rats, and frogs (Buerger and Chopin, 1976; Horridge, 1962a, b). Most recently, the experiments of Grau and colleagues have used the method to better understand the physiological and biochemical mechanisms underlying spinal learning, and to relate these findings to therapies such as step training and functional electrical stimulation (Bigbee et al., 2007; Ferguson et al., 2008; Hook and Grau, 2007).
The laboratory mouse is currently an important model organism for studying the molecular mechanisms of neural function due to the wide array of genetic manipulations that have been developed in mice (Zheng et al., 2006). Consequently, we sought to develop an experimental system to study spinal learning in the mouse using the Horridge paradigm. We show that by using fine-wire electrodes, an inexpensive real-time video point-tracking system, and DC current stimulation, it is possible to elicit spinal learning in adult spinal mice.
Materials and Methods
We closely followed the procedures described by Grau et al. (1998). All surgical and experimental procedures in these experiments were approved by the UCLA Institutional Animal Care and Use Committee. The spinal cords of four-week old Swiss-Webster mice (N = 68) were completely transected at the T7-T8 vertebral level as described previously (Roy et al., 1992). Briefly, under 2% isoflurane anesthesia, a dorsal midline skin incision was made from T6 to T9 and the musculature covering the dorsal vertebral column was removed to expose the spinal lamina. A partial laminectomy of the T7 and T8 vertebrae was performed to expose the spinal cord. The spinal cord, including the dura, was transected completely using stainless-steel microscissors, and the completeness of the lesion was verified by separating the cut ends of the spinal cord with small cotton pellets and passing a fine glass probe through the lesion site. The skin incision was closed using small surgical staples. After surgery, the wound sites were treated with triple antibiotic ointment (Bacitracin) and the mice were given lactated Ringer’s solution (1.5 ml /30 g body weight) sub-cutaneously. Preventing leg extension during recovery has been shown to facilitate subsequent learning in rats. Consequently, the hindlegs were bound with the knee and ankle joints fully-flexed (Grau et al., 1998). The animals recovered in an incubator maintained at 37°C until fully awake and then returned to their home cages. Opioid analgesics (e.g., Buprenex) commonly used following surgeries can negatively affect learning (Hook et al., 2007). Consequently, the mice were given acetaminophen (300 mg/kg body weight, orally) as an analgesic (Jenkins, 1987), and allowed to recover for 20-24 hours before testing.
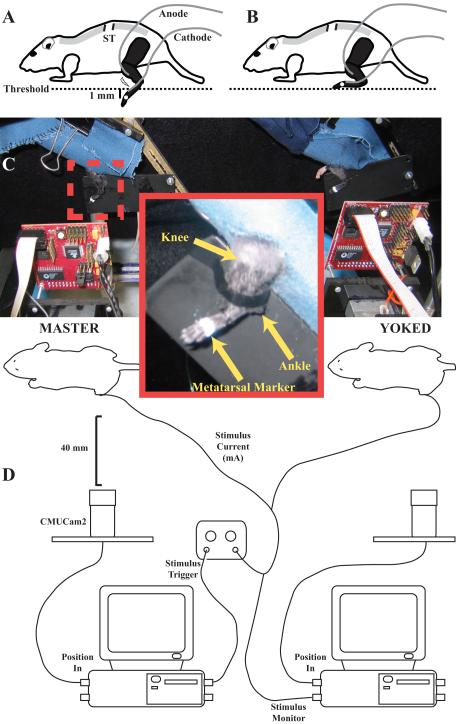
The general design of the experimental setup is shown in Figure 1. Two mice, master and yoked, were tested simultaneously during each trial. The mice were restrained loosely in small cloth harnesses (Fig. 1C). The front of each harness was closed, providing a dark enclosure in which the mice could rest undisturbed. Two slots were cut from the end of the harness, allowing both hind legs to hang freely. To minimize the effects of upper body movements on leg position, the midsection of each mouse was secured gently by using a clip to snugly close the harness. The hind legs were shaved using a small electric clipper (#50 clipper blade) and colored black using a non-toxic marker to optimize camera tracking. A small white mark was placed on the fifth metatarsal-phalangeal (MTP) joint using non-toxic paint (Fig. 1C inset).
Figure 1.
Schematic of instrumental learning technique. A. If the foot is below a pre-determined threshold, fine-wire electrodes deliver a shock to the leg. B. Learning involves ankle dorsiflexion to hold the foot above the threshold. C. Two animals, master and yoked, are placed in front of two cameras to monitor foot position. Inset box shows knee and ankle joints, and metatarsal marker. D. Point tracking information from master mouse is collected by one computer and used to trigger leg shock for both the master and yoked mice. A second computer monitors yoked mouse foot position and stimulation.
Fine-wire hook electrodes were constructed by removing the insulation from the tips (∼1 mm) of 50 μm nylon-insulated single-strand stainless steel wires (California Fine Wire Co., Grover City, CA). The skin over the tibialis anterior (TA) muscle was probed with a needle electrode to find the point most sensitive to electrical stimulation, i.e., where a single shock elicited the largest muscle twitch response as assessed visually. This point was typically ∼1 mm distal to the ventral aspect of the knee and laterally adjacent to the tibia. Using a 32-gauge needle, one electrode (anode) was inserted into the TA as close as possible to the identified point. A second electrode (cathode) was attached to the dorsal surface of the foot adjacent to the lateral malleolus (Fig. 1A). To attach this electrode, a small bolus of Spectra 360 electrode gel (Parker Laboratories, Inc., Fairfield, NJ) was placed on the dorsal surface of the paw, and the de-shielded tip of the electrode inserted into the gel. A thin coating of rubber cement was used to cover the gel and electrode, and to secure the electrode wire to the dorsal surface of the foot. The electrodes were attached to a Stimulator (S88, Grass Product Group; W. Warwick, RI), through a stimulus isolation unit (SIU5) and a constant current isolation unit (CCU1). In contrast to the AC current used by Grau et al. (1998), the stimulator used for these experiments used 50 ms square wave direct current pulses.
After the electrodes were implanted, we conducted a series of stimulation trials to establish the relationship between current and force for each animal. Only moderate levels of stimulation have been shown to elicit spinal learning in rats (Grau et al., 1998). Consequently, we sought to stimulate the mice at levels approximately one-half of the amount of current necessary to produce a maximum contraction. The animals were suspended by firmly attaching the harness clip to a horizontal bar in a position where ankle dorsiflexion resulted in nearly vertical movement of the MTP marker. One end of a piece of thread was tied firmly around the distal end of the metatarsals immediately proximal to the MTP joint, and the other end was attached to a force transducer (Dual Mode Muscle Lever 300BLR, Aurora Scientific Inc., Aurora, Ontario, Canada). The position of the transducer was adjusted so that the thread was taut, but the force did not exceed ∼0.1 N. Force resulting from a series of single 50 ms square-pulse stimuli from 0 to 1.0 mA, in increments of 0.1 mA, with a 30 s delay between each stimulus, were recorded and displayed by a custom-written LabVIEW program. When the stimulus series was complete, the thread was cut and removed from the foot.
The two-dimensional position of the foot marker was monitored using an inexpensive video-based point tracking system (CMUCam2; Carnegie Mellon University; Fig. 1D). The CMUCam2 can perform automatic, hardware-based point tracking of region fitting user-specified color and size criteria and report the centroid of the region to the computer. The CMUCam2 communicated with a computer using a serial connection. For these experiments, custom software was written in LabVIEW (National Instruments, Inc.; available at http://www.limblab.org/publications/code/learning.zip) to control the CMUCam2 and collect tracking data from the camera. Full-frame images from the camera were captured and used to correctly position the foot marker in the field of view of the camera. The software then calculated the range of colors associated with the marker. We found that using a high-contrast, (white on a black background) marker produced the best results. By identifying all pixels in the image within the defined color range, we then could remove any regions on the animal or in the background that could interfere with marker tracking.
Once we established the desired parameters, they were used to configure point tracking by the CMUCam2 and the LabVIEW software. The speed of point tracking by the CMUCam2 is limited by the image size, but we found that slightly less than half-size images (125 × 125 pixels) were adequate to measure the foot motions, and could be collected at a sampling frequency of 30 Hz. The camera had a field of view of 15 × 15 mm, or a resolution of 8 pixels/mm with the camera positioned 40 mm from the foot.
After the stimulation parameters were set, a resting position was defined as the foot position following 3 rapid priming stimulations. Muscle stimulation could cause an increase in resting position, and the priming stimuli allowed for determination of a resting position in a post-stimulus state. Assigning a stimulation threshold position, i.e., the vertical distance above the resting position that the foot must exceed to prevent shock, is very important. The threshold determines the difficulty of the task that the spinal cord must learn. Basing the appropriate threshold on the methods used for rats is complicated by two issues. First, mice are approximately one-tenth the weight, and one-half the linear dimensions of rats. Second, Grau et al. (1998) measured the position of a rod attached to the foot of the rat. In this configuration, movements of the rod will be larger than movements of the foot itself. Consequently, we estimated that the appropriate threshold for mice should be at least one-half the 2 mm threshold used for rats to measure comparable learning behavior. We used a threshold of 1 mm above the resting position, which was set for both the master and yoked mice with a threshold parameter in the LabVIEW program.
Data collection began once the 1 mm thresholds were established for both the master and yoked mice. First, at least 2 min of data were collected to measure and verify baseline positions for both animals. Subsequently, stimulation contingent on the leg position of the master mouse was enabled, and the animals were left undisturbed for 30 min. The LabVIEW software collected vertical and horizontal foot position from both animals, the threshold level, and the times when the master and yoked animals were stimulated. Once the trial was complete, data were post-processed using custom scripts written in MATLAB (The Math Works, Inc., Natick, MA, USA). Response duration was calculated as (time above threshold) / (number of stimulations + 1) for each 60 sec bin (Grau et al., 1998). We compared the response durations of both the master and yoked mice using repeated-measures ANOVA (JMP 4.0.2; SAS Institute, Inc.).
Results
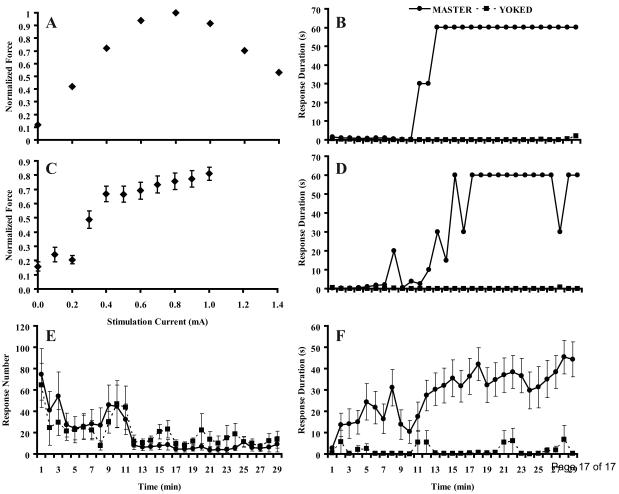
Stimulating the TA muscle with increasing current resulted in increasing dorsiflexion force up to ∼1 mA, followed by decreasing force at higher current levels (Fig. 2A). Decreased forces at the higher currents were likely due to current spread recruiting muscles other than the TA, i.e., activating antagonists. Consequently, after establishing this relationship in pilot studies, we used 1.0 mA as the maximum stimulation level. Mice showed increases in force with increasing current between 0.1 mA and 1.0 mA (Fig. 2C). Forces were often small and variable at low current levels, however, and many trials showed a plateau above 0.5 mA. Consequently, for each animal we used a current level one 0.1 mA step over the current yielding at least 50% of the maximum force, as determined visually. Over all animals, the average current used was 0.61±0.07 mA.
Figure 2.
Results from instrumental learning experiments. A. Typical recording from one animal shows dorsiflexion force increasing with increasing current until reaching a peak at 0.8 mA followed by a decrease in force. C. Force recordings normalized to maximum force across all currents for each animal, then averaged across all master and yoked animals (N=22). Below 0.3 mA, little force is observed. Above 0.4 mA, dorsiflexion force increases with increasing current. B, D and F. Response durations for master and yoked animals over time. B. Trial from a single master-yoked pair. The master animal shows low response durations for ∼10 min, followed by a rapid transition to a flexed position associated with instrumental learning. D. Occasionally the foot position of the master rat decreased below threshold and elicited a leg shock. F. Response duration over time averaged across all animals (N=11 for both master and yoked conditions). E. Response number (number of ankle flexions) over time averaged across all animals (N=11 for both master and yoked conditions). Bars in C, E, and F, SEM.
Using fine-wire electrodes and video tracking of foot position, we were able to elicit spinal learning from adult mice. A typical time course of response duration shows initially low values associated with many leg shocks (Fig. 2B). After a period of position-contingent leg shocks, master mice showed sustained ankle dorsiflexion, resulting in large response duration values. Ankle dorsiflexion could be sustained or exhibit occasional periods where the foot dropped below threshold and triggered a shock (Fig. 2D). The mean values of 11 successful master and yoked pairs show that master animals have longer response durations than yoked animals (ANOVA; P< 0.001; Fig. 2F). This difference was not due to an inability of yoked animals to respond to the stimuli, as master and yoked animals did not show significantly different response numbers (ANOVA; P> 0.9; Fig. 2E).
Discussion
Our experiments show that by using fine-wire electrodes, a commercially available image-tracking system, and DC current stimulation, it is possible to use the Horridge paradigm to elicit spinal learning in adult mice. Mouse spinal cords can show instrumental learning similar to that observed in rats (Grau et al., 1998). Moreover, these techniques offer several advantages over those commonly used in the Horridge paradigm. First, it was not necessary to use stiff wires attached to the feet of the mice to contact a solution and trigger a stimulus. Given the small mass of mouse leg muscles, any added weight could potentially diminish the ability of the animals to maintain ankle flexion and demonstrate learning. The video tracking system also allowed easy adjustment of stimulation thresholds, and recording of instantaneous foot position throughout the entire trial. Second, we demonstrated that learning could be evoked using DC current stimulation without requiring custom-built AC stimulators (Grau et al., 1998).
Although we were able to elicit learned flexion responses from spinal mice, developing the techniques described here resulted in many failed experiments during the initial stages of development. Of the 34 pairs of master/yoked mice used in the study, only 11 pairs, i.e., 32% of experiments, resulted in spinal learning in the master mice. We considered a successful trial as a trial during which the master mouse showed a response duration of 60 sec for at least two one-min-interval response duration bins. Experiments using 10 pairs of mice were conducted before we achieved a successful trial. After these 10 experiments, the number of successful trials increased, but our success rate did not reach 100%. In the final six experiments conducted, we experienced one failure, i.e., a 17% failure rate.
It is likely that several factors contributed to the initial high failure rate. First, proper electrode placement in the mouse TA muscle was difficult. The region of the muscle that elicited maximum flexion was very small (∼1 mm diameter). Even using fine-wire electrodes, it was often difficult to ensure that the electrodes maintained proper placement during and after insertion. Second, due to the small size of the mouse, we frequently observed current spread resulting in unintended stimulation of other leg muscles, including antagonists. Current spread was most evident at higher levels of stimulation (Fig. 2A), but could also potentially result from improper electrode placement. Third, as observed by Grau et al. (1998) in the spinal rat, we observed habituation of the muscle response if the selected stimulation intensities were too low. Selecting intensities lower than approximately one-half of the maximum resulted in habituation of the TA response and an absence of learning. For the mouse, therefore, both electrode placement and selected current level must be very carefully controlled to successfully elicit learning.
Using a master-yoked paradigm, as we have here, demonstrates learning by testing animals under common conditions. Care must be taken, however, when interpreting the results of experiments using a master-yoked design. If the average time for leg extension is long relative to the latency for leg withdrawal (i.e., 10 s vs. 0.2 s), differences in the time the leg spends below threshold between master and yoked groups can emerge as artifacts of contingent stimulation without requiring learning (Church and Lerner, 1976). The time for leg extension for the mice, however, was short (∼0.3 s), and the response duration metric used provides a substantially more stringent test for learning than time spent below threshold alone. Consequently, it is unlikely that the observed differences in response duration emerged from artifacts of contingent stimulation in a purely reactive model.
Our initial goal of establishing a working instrumental mouse model was successful, but these initial experiments did not include some tests that could elaborate the extent or mechanisms of learning (Grau et al., 2006). For example, our experiments did not address whether training animals with an instrumental relationship results in behavioral modification that extends beyond the training period, transfers to other tasks, or facilitates learning by the contralateral leg. Also unexplored are whether non-contingent stimuli can disrupt learning. Experiments using separate training and testing stages are necessary to evaluate these questions, and will be the topic of future studies.
In conclusion, the instrumental learning paradigm offers a relatively rapid, effective method for eliciting spinal learning. Whereas other methods, such as step training, may require weeks or months of effort, experiments using these methods can be completed in two days. The ability to elicit instrumental learning in spinal mice will provide unprecedented opportunities to study the molecular and genetic aspects of spinal learning. Transgenic and knock-out mouse models allow for simple and effective manipulations of single genes which regulate specific cellular markers involved in the processes of learning, memory, and plasticity. The roles of these genes in the spinal cord may be important not only in understanding the basic forms of learning, but also may allow for increased understanding of other types of spinal learning. Better understanding of spinal learning will ultimately facilitate efforts to restore neuromotor function after a spinal cord injury or other debilitating conditions.
Acknowledgments
We thank Dr. Adam Ferguson for helpful discussions, Maynor Herrera, Yevgenia Faynerman, Sajini Sasthri-Rajaputrage and Rusvelda Cruz for providing excellent animal care, and Sharon Zdunowski for technical assistance. This work was supported by NIH grants NS40917 (NJKT) and NS16333 (VRE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bigbee AJ, Crown ED, Ferguson AR, Roy RR, Tillakaratne NJ, Grau JW, Edgerton VR. Two chronic motor training paradigms differentially influence acute instrumental learning in spinally transected rats. Behavioural brain research. 2007 doi: 10.1016/j.bbr.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerger AA, Chopin SF. Instrumental avoidance conditioning in spinal vertebrates. Advances in psychobiology. 1976;3:437–61. [PubMed] [Google Scholar]
- Church RM, Lerner ND. Does the headless roach learn to avoid? Physiological Psychology. 1976;4:439–42. [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain research reviews. 2008;57:241–54. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Bolding KA, Huie JR, Hook MA, Santillano DR, Miranda RC, Grau JW. Group I metabotropic glutamate receptors control metaplasticity of spinal cord learning through a protein kinase C-dependent mechanism. J Neurosci. 2008;28:11939–49. doi: 10.1523/JNEUROSCI.3098-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Barstow DG, Joynes RL. Instrumental learning within the spinal cord: I. Behavioral properties. Behavioral neuroscience. 1998;112:1366–86. doi: 10.1037//0735-7044.112.6.1366. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behavioral and cognitive neuroscience reviews. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. The rodent lumbar spinal cord learns to correct errors in hindlimb coordination caused by viscous force perturbations during stepping. J Neurosci. 2007;27:8558–62. doi: 10.1523/JNEUROSCI.1635-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Grau JW. An animal model of functional electrical stimulation: evidence that the central nervous system modulates the consequences of training. Spinal Cord. 2007;45:702–12. doi: 10.1038/sj.sc.3102096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW. The impact of morphine after a spinal cord injury. Behavioural brain research. 2007;179:281–93. doi: 10.1016/j.bbr.2007.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horridge GA. Learning of leg position by headless insects. Nature. 1962a;193:697–8. doi: 10.1038/193697a0. [DOI] [PubMed] [Google Scholar]
- Horridge GA. Learning of leg position by the ventral nerve cord in headless insects. Proc. Roy. Soc. B. 1962b;157:33–52. [Google Scholar]
- Jenkins WL. Pharmacologic aspects of analgesic drugs in animals: an overview. Journal of the American Veterinary Medical Association. 1987;191:1231–40. [PubMed] [Google Scholar]
- Roy RR, Hodgson JA, Lauretz SD, Pierotti DJ, Gayek RJ, Edgerton VR. Chronic spinal cord-injured cats: surgical procedures and management. Lab Anim Sci. 1992;42:335–43. [PubMed] [Google Scholar]
- Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends in neurosciences. 2006;29:640–6. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]