Abstract
The biological relevance of the perforin and Fas ligand (FasL) cytolytic pathways of CD8+ T lymphocytes (CTL) for cancer immunotherapy is controversial. We investigated the importance of these pathways in a murine renal cell carcinoma expressing influenza viral hemagglutinin as a defined surrogate antigen (Renca-HA). Following Renca-HA injection, all FasL-dysfunctional FasLgld/gld mice (n = 54) died from Renca-HA tumors by day 62. By contrast, perforin−/− (51%, n = 45) and Faslpr/lpr (55%, n = 51) mice remained tumor-free at day 360. Blocking FasL in vivo inhibited tumor rejection in these mice. Moreover, established Renca-HA tumors were cleared more efficiently by adoptively transferred HA518–526-specific T cell receptor-transgenic CTL utilizing FasL rather than perforin. Strikingly, a range of mouse tumor cells presenting low concentrations of immunogenic peptide were all preferentially lysed by the FasL but not the Pfp-mediated effector pathway of CTL, whereas at higher peptide concentrations the preference in effector pathway usage by CTL was lost. Interestingly, a number of human renal cancer lines were also susceptible to FasL-mediated cytotoxicity. Therefore, the FasL cytolytic pathway may be particularly important for eradicating Fas-sensitive tumors presenting low levels of MHC class-I-associated antigens following adoptive T cell therapy.
Keywords: CTL, Perforin, Fas Ligand, Cytotoxicity, Cancer Immunotherapy
Introduction
Cytotoxic T lymphocytes form the basis of adoptive T cell therapy of cancer. Despite various optimizations of adoptive T cell therapy (1), the majority of patients still fail to show objective cancer regression even in melanoma (1) and renal cell carcinoma (2), the two cancers most responsive to immunotherapy to date. To further improve T cell immunotherapy against cancer, we thus need to understand the molecular basis of tumor eradication by CTL in vivo.
Two direct mechanisms of CTL cytotoxicity have been defined in vitro (3). One involves the perforin (Pfp)-dependent exocytosis of lytic granules (4, 5), whereas the second mechanism utilizes various apoptosis-inducing death ligands of the TNF superfamily (6). Decreased rejection of induced sarcomas and papillomas (7, 8) or spontaneous lymphomas (9) in Pfp−/− mice suggested that Pfp may be important for CTL cytotoxicity in vivo. Nonetheless, the adoptive transfer of Pfp-deficient CTL to tumor-bearing mice demonstrated substantial antitumor therapeutic efficacy (10–12). From these studies, no definite conclusions, however, could be drawn concerning the molecular basis of tumor elimination following T cell transfer, the importance of any alternative cytotoxic pathways in Pfp-replete mice, functional dominance or redundancy of direct cytotoxic pathways and the role of the biological characteristics of the tumor cells in determining the nature of the CTL effector functions.
Studies using the influenza viral hemagglutinin (HA) expressed as a model tumor Ag in a renal cell adenocarcinoma (Renca-HA), as well as a self Ag in pancreatic islet β cells in insulin-HA transgenic mice, showed that host HA-reactive CTL could eliminate HA-expressing tumor cells in the absence of diabetes (13). Taking advantage of this Renca-HA model and T cell receptor (TCR)-transgenic Cln-4 (Tg) mice (14) to generate CTL reactive to MHC class-I-restricted HA518–526 epitope, we investigated the significance of the CTL effector mechanisms involving Pfp or Fas ligand (FasL) pathways for tumor rejection in vivo. The study shows that CTL using FasL are efficient at causing regression of established Renca-HA tumors, most likely because FasL-mediated lysis can still be triggered by the low immunogenic peptide density presented by Renca-HA cells.
Materials and Methods
Mice
All BALB/c mice (7–12-week) were bred at the National Cancer Institute (NCI)-Frederick. NCI-Frederick is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and follows the Public Health Service Policy for the care and use of laboratory animals under pathogen-free conditions. Cln-4 mice (courtesy Linda A. Sherman, The Scripps Research Institute, La Jolla, CA) were established on Pfp−/− (TgPfp−/−), FasLgld/gld (Tggld/gld) backgrounds, and genotyped according to the methods described earlier (14, 15). The Faslpr/lpr genotyping was performed according to the method standardized by the Jackson Laboratory (Bar Harbor, Maine). Mice were cared for in accordance with the procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell lines
The Renca-HA line (14) (courtesy Hyam I. Levitsky, John Hopkins University, Baltimore, MD), Renca (RHW, NCI–Frederick), 4T1 (courtesy Suzanne Ostrand-Rosenberg, University of Maryland Baltimore County, Baltimore, MD), C-26 and A-20 (ATCC, Manassas, VA) and ACHN, TK-10 and UO-31 (DTP Molecular Targets Program, NCI–Frederick) were maintained in FCS-supplemented standard RPMI-1640 culture medium.
Tumor monitoring
Tumor size following subcutaneous injection of Renca-HA cells was monitored every week, and is represented as multiplication of the two perpendicular diameters. Tumor metastatic nodules following intravenous injection with Renca-HA cells were counted on the surgically-removed lungs under a dissection microscope.
Flow cytometry
Cells following FcγR-blocking were stained for surface immunofluorescence staining with various antibodies or control isotypes (BD Biosciences, San Jose, or eBioscience Inc., San Diego, CA). Cells were stimulated for 5 h with 200 ng/ml ionomycin plus 10 ng/ml phorbol 12-myristate 13-acetate (Sigma, St. Louis, MO) in the presence of GolgiStop™ protein transport inhibitor (BD Biosciences) for IFN-γ intracellular staining, or in the presence of matrix metalloproteinase inhibitor BB94 (10µM; HY, Tokyo, Japan) for FasL detection. For measuring effector cell degranulation, CTL were incubated with target cells for 6 h in the presence of monensin (2 µM; eBioscience Inc.) alongwith CD107a mAb (BD Biosciences). Data were acquired using a FACSCalibur or LSR-I flow cytometer (BD Biosciences) and analyzed using CellQuest (BD Biosciences) or FlowJo (Treestar Inc., CA) software.
CTL adoptive transfer
The CTL were generated from the naïve lymph node (LN) cells of Tg, TgPfp−/− and Tggld/gld mice, stimulated in vitro for 3 days with lipopolysaccharide (15 µg/ml)-treated irradiated syngeneic WT red blood cell-depleted splenocytes loaded with 10−6 M HA518–526 (IYSTVASSL) or an irrelevant nucleoprotein (TYQRTRALV) peptide. On day 3, cells were washed and injected intravenously in tumor-bearing recombination-activating-gene-2−/− (RAG2−/−) mice.
ELISA
The IFN-γ concentration was measured by a Quantikine® Mouse IFN-γ Immunoassay kit (R&D Systems, Minneapolis, MI) according to manufacturer’s protocol.
Immunohistochemistry
The Fas (clone M-20) and CD8α immunohistochemical analyses of Renca-HA solid tumors, thymus, or lungs were performed according to standard methods (16). Digital images of the specimens were captured using Spectrum image software (Aperio Technologies, Inc., Vista, CA).
Cytotoxicity
Target tumor cells, pulsed with medium or various concentration of HA peptide for 90 min at 37 °C, radio-labelled with 111In-labeled oxyquinoline (Amersham Health, Medi-Physics Inc., Arlington Heights, IL), and washed, were incubated for 18 h with CTL effectors. After overnight incubation, supernatants were harvested and counted on a 1480 wizard 3 automatic γ-counter (Perkin Elmer Life and Analytical Sciences, Shelton, CT). Percent cytotoxicity was calculated as described previously (11).
Statistics
Comparisons of mean values between the groups were analyzed using GraphPad Instat software (GraphPad Prism). Statistical significance of the differences was analyzed by using unpaired Student t test or by analysis of variance (ANOVA). Comparisons of survival curves estimated by Kaplan-Meïer plots using GraphPad Prizm were performed by the log-rank (Mantel-Cox) test. All statistical tests were two-sided; P-values < 0.05 were considered statistically significant.
Results
Antitumor role of Fas ligand is crucial in vivo
To investigate the roles of perforin, FasL and Fas in the host’s capacity to reject tumors, Renca-HA tumor cells were injected in BALB/c wild type (WT), severe-combined-immunodeficient (SCID), Pfp−/−,FasLgld/gld and Faslpr/lpr mice. Following Renca-HA subcutaneous injection, solid tumors rapidly grew in 100% of SCID and FasLgld/gld mice. In WT, Pfp−/− and Faslpr/lpr mice, tumors grew much more slowly and did not develop in all animals (Supplementary Fig. S1). Concerning long-term survival, all SCID mice (n = 43) died with large Renca-HA tumors with a median survival of 62 days. By contrast, 51% of WT mice (28 of 55) were tumor-free at day 360 (Fig. 1A) most likely due to an adaptive immune response against HA. In Pfp−/− mice, 51% (23 of 45) of Pfp−/− mice were tumor-free at day 360 similar to WT mice. Furthermore, in FasLgld/gld mice (n = 54), which lacked functional FasL protein (17), all mice died with large tumors with a median survival of 62 days. Since FasLgld/gld mice have lymphoproliferative disorders, we compared Renca-HA growth in FasLgld/gld mice with growth in Faslpr/lpr mice (defective Fas but functional FasL) that show similar immunological disorders (17). Strikingly, 55% (28 of 51) of Faslpr/lpr mice were tumor-free at day 360 (Fig. 1A). Therefore, WT, Pfp−/− and Faslpr/lpr mice resisted Renca-HA growth, resulting in long term survival, whereas SCID or FasLgld/gld mice rapidly succumbed to tumor.
Figure 1. Antitumor role of Fas ligand in vivo.
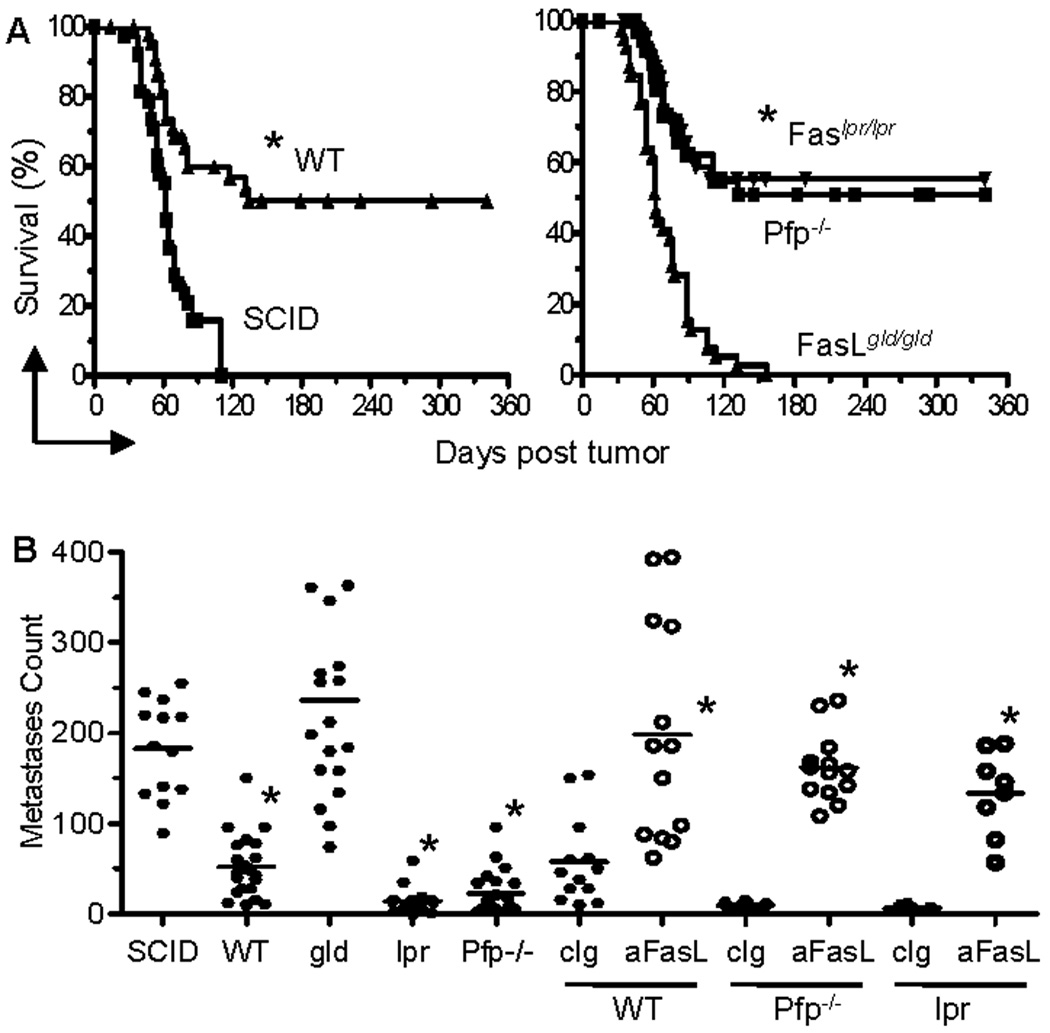
Tumor cells were injected in BALB/c WT, SCID, Pfp−/−, FasLgld/gld and Faslpr/lpr mice. (A) Survival of Renca-HA (1 × 104 subcutaneous) tumor-bearing SCID (n = 43), WT (n = 55), Pfp−/− (n = 45), FasLgld/gld (n = 54) and Faslpr/lpr (n = 51) mice. *P <0.0001 (WT compared to SCID; or Pfp−/− or Faslpr/lpr compared to FasLgld/gld) (two-sided log-rank test). (B) Lung metastases counts on day 14 following tumor (0.5 × 106 intravenous) injection. In some groups, mice were administered intraperitoneally with mAb MFL4 against Fas ligand or its isotype IgG (0.5 mg each) on day 0, 3, 7 and 10. *P < 0.0001 (ANOVA, two-sided) for WT (n = 23), Faslpr/lpr (n = 18) and Pfp−/− (n = 23) compared to SCID (n = 13) or FasLgld/gld (n = 18), or for MFL4-treated WT (n = 13), Pfp−/− (n = 13) or Faslpr/lpr (n = 8) compared to equal numbers of control isotype-treated mice. Data from 7 (A), and 6 (B) independent experiments.
Similarly, following Renca-HA (0.5 × 106) intravenous injection, WT, Pfp−/− and Faslpr/lpr mice formed significantly fewer lung metastases as compared to SCID or FasLgld/gld mice (Fig. 1B). The requirement of FasL for Renca-HA rejection was further confirmed when administration of a FasL-blocking mAb but not its isotype control in WT, Pfp−/− and Faslpr/lpr mice abrogated the rejection of Renca-HA lung metastases (Fig. 1B). Thus, the expression of functional FasL in the host appeared crucial for resisting the development of Renca-HA solid tumors.
Activation of CTL in tumor-bearing mice
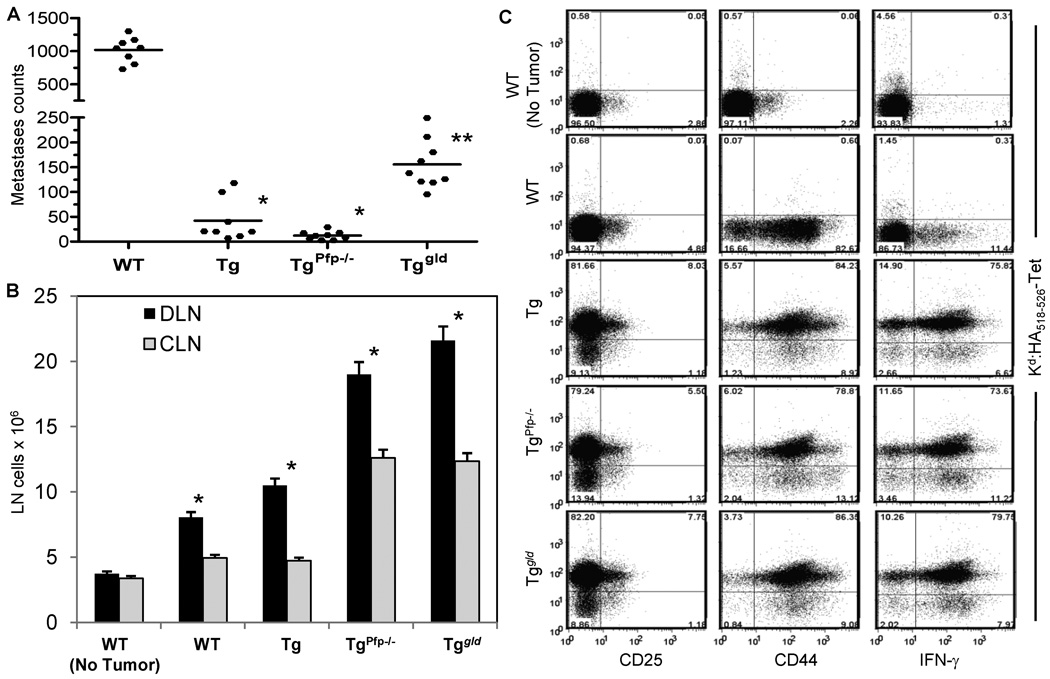
To dissect the mechanisms underlying the preferential effector pathway usage in tumor rejection, we compared tumor metastases in the lungs following Renca-HA (3 × 106) intravenous injection in HA-specific TCR TgPfp−/− and Tggld/gld backgrounds. Reduced pulmonary metastases were found in all Tg mice as compared to WT mice (Fig. 2A). Moreover, TgPfp−/− mice exhibited significantly fewer metastases as compared to Tggld/gld mice, suggesting that HA-specific CTL expressing FasL were more efficient at causing Renca-HA rejection.
Figure 2. Activation of CTL in Renca-HA-bearing mice.
(A) Lung metastases counts on day 14 following Renca-HA (3 × 106 intravenous) in BALB/c WT, Tg, TgPfp−/− and Tggld/gld mice. *P < 0.0001 (ANOVA, two-sided) for Tg (n = 8) and TgPfp−/− (n = 9) compared to WT (n = 8) and Tggld/gld (n = 9) mice (pool of 2 independent experiments). **P < 0.0001 for Tggld/gld compared to WT. (B) Total number of cells ± SD in tumor-draining (axillary and inguinal) and contralateral LN harvested on day 7 post Renca-HA (5 × 106 subcutaneous) injection in WT, Tg, TgPfp−/− and Tggld/gld mice (n = 4). *P < 0.0001 (ANOVA, two-sided) for the draining LN compared to the contralateral LN. (C) Dot plots of Kd:HA-tetramer versus CD25, CD44 and intracellular IFN-γ on gated CD8+ cells in the tumor-draining LN. Numbers in the quadrants represent % positive cells. Plots representative of 2 independent experiments.
We then analyzed the activation of HA-specific CTL in tumor-bearing mice. We injected Renca-HA cells subcutaneously (5 × 106) in WT, Tg, TgPfp−/− and Tggld/gld mice, and evaluated the number of cells in tumor-draining LN (DLN) and contralateral LN (CLN) on day 4. The numbers of cells in the DLN of Renca-HA-bearing mice were significantly increased as compared to the CLN in all of these mice. As expected no differences were seen in non-tumor-bearing mice (Fig. 2B).
The gated CD8+ cells in the DLN of tumor-bearing WT, Tg, TgPfp−/−, and Tggld/gld mice analyzed on day 7 post tumor injection showed an upregulated expression of CD25 and CD44 as well as a capacity to produce high amounts of IFN-γ (Fig. 2C). Moreover, the DLN of Tg, TgPfp−/− and Tggld/gld mice showed a similar percentage of HA518–526-specific Kd:HA-tetramer+ cells with similar levels of T cell activation. Thus, decreased efficiency of Tggld/gld mice to eliminate tumor cannot be attributed to deficiency in activation of HA-reactive CTL.
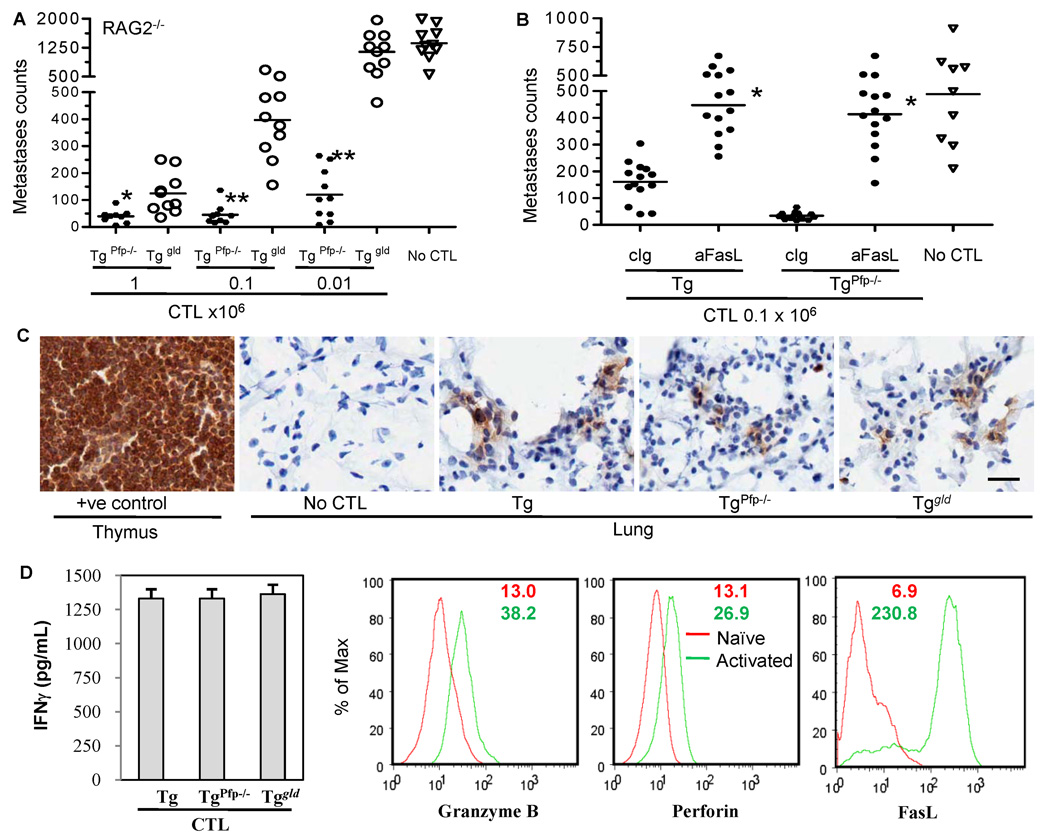
Fas ligand effector pathway is 100-fold more efficient than Pfp in vivo
To more systematically compare the efficiency of Pfp-dependent and FasL-mediated cytolysis, we adoptively transferred a range (0.01 – 1 × 106) of activated TgPfp−/− or Tggld/gld CTL into RAG2−/− mice that lack endogenous T, B and NKT cells and had been injected with Renca-HA cells (2 × 106) intravenously 7 (Fig. 3A) or 10 days earlier (data not shown). Transfer of high numbers of CTL from TgPfp−/− or Tggld/gld mice regressed these established lung metastases without any overt toxicity. However, Tggld/gld CTL were far less efficient at promoting tumor regression when lower numbers of CTL (0.01 × 106) were transferred (Fig. 3A). In addition, the therapeutic benefit following transfer of Tg or TgPfp−/− CTL was abolished by administration of FasL-blocking antibody (Fig. 3B). Activated Tg CTL did express increased granzyme B, perforin and FasL (Fig. 3D).
Figure 3. Fas ligand effector pathway is more efficient than Pfp in vivo.
Lung metastases counts on day 14 following Renca-HA (2 × 106 intravenous) injection in RAG2−/− mice (A) that were transferred with the indicated numbers of the activated TgPfp−/− and Tggld/gld LN CTL intravenously on day 7; *P = 0.004, **P < 0.0001 (ANOVA, two-sided) for Pfp−/− (n = 10) compared to the corresponding gld (n = 10) group; (B) that were administered with FasL-neutralizing MFL4 or its isotype IgG on day 0, 2 and 5 post transfer of the activated Tg and TgPfp−/− CTL (0.1 × 106). *P < 0.0001 (ANOVA, two-sided) for MFL4-treated (n = 9) compared to the corresponding control Ab-treated (n = 9) group. (C) The activated TgPfp−/− and Tggld/gld LN CTL (5 × 106 intravenous) were transferred intravenously in RAG2−/− mice bearing 10-day-established Renca-HA tumor metastases. Representative sections of lungs and WT mouse control thymus harvested a day after CTL transfer and stained with CD8α antibody. Bar = 25 µm, all images. Data representative of 2 independent experiments. (D) Bar graphs depict IFN-γ secretion in the day 4 supernatants from the HA-stimulated Tg, TgPfp−/− and Tggld/gld LN CTL cultures. Values are means ± SD of triplicate samples in a representative of two independent experiments. Histograms represent the % expression of granzyme B, perforin and FasL on gated CD8+ TCR Vβ8.1+ cells in the HA-stimulated Tg LN CTL as compared to naïve Tg LN T cells, with numbers depicting the mean fluorescence intensities for naïve (red) and activated (green) Tg T cells.
Thus, TgPfp−/− CTL that utilize FasL were 100-fold more efficient on a per cell basis at promoting rejection of the Renca-HA tumor in vivo than Tggld/gld CTL, despite the fact that the transferred Tg, TgPfp−/− or Tggld/gld cells showed similar proportions of Kd:HA-Tet+ CD8+ cells (Supplementary Fig. S2), produced equivalent amounts of IFN-γ (Fig. 3D), and trafficked to the site of tumor pulmonary metastases with similar efficacy as shown by the CD8 immunohistochemistry of lung sections from tumor-bearing mice (Fig. 3C). In addition, staining of lungs with H&E revealed punctate infiltration of lymphoid cells into distinct areas of lungs where tumor cells were present (data not shown). These results show that FasL cytolytic effector mechanism is critical in CTL-mediated regression of Renca-HA tumors.
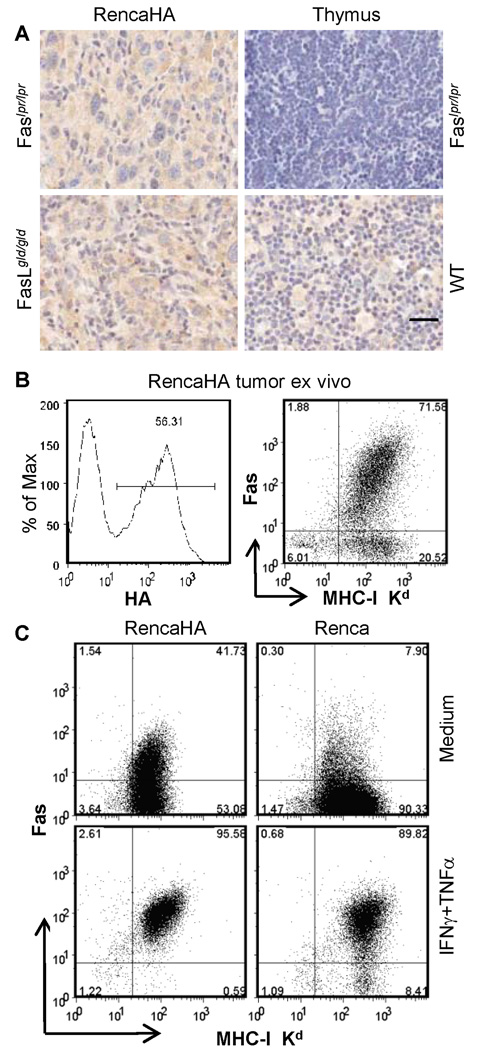
Renal cancer cells express Fas in vivo
Renca-HA cells isolated from the solid tumors in Faslpr/lpr or FasLgld/gld mice inoculated with a high number of Renca-HA cells (5 × 106) expressed Fas (Supplementary Fig. S3). This was confirmed by immunohistochemistry of tumor sections from Faslpr/lpr or FasLgld/gld mice (Fig. 4A). Moreover, 92% of HA-gated tumor cells from established solid tumor in RAG2−/− mice expressed MHC class-I, of which 66% expressed Fas (Fig. 4B). Fas surface expression on MHC class-I+ tumor cells ex vivo was significantly higher than Renca-HA cells grown in vitro. Treatment of Renca-HA or Renca cells with IFNγ and TNFα upregulated their expression of Fas (Fig. 4C and Supplementary Fig. S3). We also examined whether Fas engagement on Renca-HA cells triggered productive apoptotic signaling. Engagement of Fas on Renca-HA cells by FasL expressed on CTL triggered tumor cell apoptosis as it was blocked by a pan-caspase inhibitor zVAD-FMK (Supplementary Fig. S4A).
Figure 4. Renca-HA tumor cells express Fas in vivo.
(A) Fas (M-20)-stained sections (20× magnification) of Renca-HA solid tumors from Faslpr/lpr and FasLgld/gld mice, Faslpr/lpr thymus (negative control) and WT mice (positive control). Bar = 25 µm, all images. (B) Dot plots of Fas (Jo2) versus MHC class-I Kd (right panel) on gated HA+ cells (left panel) in the tumor cells isolated from day-12-established Renca-HA tumors in RAG2−/− mice. Numbers represent % positive cells. (C) Dot plots of Fas versus MHC class-I Kd (right panel) on Renca-HA or Renca tumor cells treated overnight with medium or with 500 U/ml of TNF-α and IFN-γ. Numbers represent % positive cells. Data representative of 2 independent experiments.
Non-redundant roles of FasL and Pfp effector pathways
To investigate why FasL pathway was dominant in CTL-mediated rejection of Renca-HA tumors in vivo, we evaluated the cytolytic activity of stimulated Tg, TgPfp−/− and Tggld/gld CTL against the Renca-HA transfectants and parental Renca tumor cells pulsed with increasing doses of exogenous HA peptide. Renca cells loaded with >10 nM concentration of HA peptide were lysed efficiently by all three types of effector CTLs (Fig. 5A). However, at 10−10 to 10−12 M concentrations of HA, only Tg or TgPfp−/− CTL expressing FasL killed tumor cells, whereas Tggld/gld effectors using Pfp did not. In the same assay, lysis of endogenous HA-expressing Renca-HA cells by Tg or TgPfp−/− CTL was about 40%, whereas by Tggld/gldCTL it was <10%, suggesting that the amount of HA peptide presented by Renca-HA tumor cells was low (Fig. 5A). Lysis of Renca-HA cells by Tggld/gld CTL following loading with 10−6 M HA peptide was increased to 90%. This lysis was blocked by perforin inhibitor concanamycin A (Fig. 5B), suggesting that Renca-HA cells were not intrinsically resistant to perforin-mediated lysis. In addition, FasL-mediated lysis of Renca-HA targets, treated overnight with IFNγ and TNFα, by TgPfp−/− CTL was in the same range as untreated targets, suggesting that presence of IFNγ secreted by CTL in the 18-h CTL assay (Fig. 3D) was sufficient to sensitize targets to FasL (Fig. 5B).
Figure 5. FasL and Pfp effector pathways play non-redundant roles.
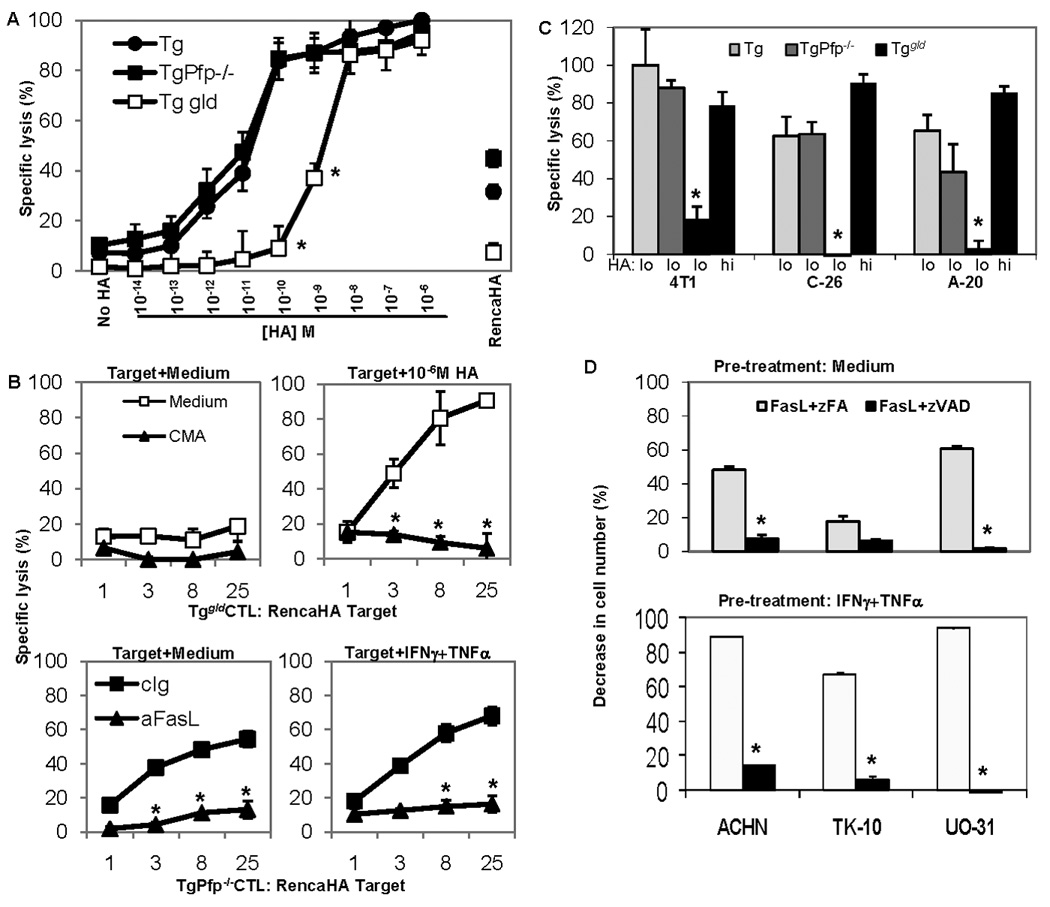
(A) Percent specific lysis of Renca-HA or Renca cells, pre-treated with TNF-α and IFN-γ (500 U/ml each, overnight), pulsed with various concentrations of HA peptide and then incubated for 18 h with activated Tg, TgPfp−/− and Tggld/gld CTL at effector:target ratio 25:1. *P < 0.001 (two-sided unpaired t test) with respect to other CTL. (B) Percent specific lysis of Renca-HA cells pulsed with 10−6 M HA peptide by Tggld/gld CTL pretreated 2 h with medium or with concanamycin A (upper panel). Percent specific lysis of Renca-HA cells pre-treated with medium or with TNF-α and IFN-γ (500 U/ml each, overnight) by TgPfp−/− CTL in the presence of FasL-neutralizing Ab or its isotype control (lower panel). *P < 0.001 (two-sided unpaired t test) with respect to medium or isotype control. (C) Percent specific lysis of 4T1, C-26 and A-20 tumor cells pulsed with 10−10 M (lo) or 10−6 M (hi) HA peptide at effector:target ratio 25:1. *P < 0.001 (two-sided unpaired t test) with respect to the respective lysis values by Tg or TgPfp−/− CTL. (D) Percent decrease in cell number of medium− or human-TNF-α− and IFN-γ (100 U/ml, overnight)−pretreated human renal cancer lines ACHN, TK-10 and UO-31 incubated with pan-caspase inhibitor zVAD-FMK or control analog zFA-FMK (20 µM) one hour before the treatment with FasL (100 ng/ml). *P < 0.001 (two-sided unpaired t test) with respect to the control. Values are means ± SD (n = 3) from a representative of 6 (A), 2 (B, and C) and 3 (D) independent experiments.
Lytic activity of Tg or Tgpfp−/− CTL was also evaluated against Fas-associated death domain-like interleukin-1-converting enzyme inhibitory protein (cFLIPL)-overexpressing Renca (Renca-FLIP) cells resistant to death receptor-mediated apoptosis (18). Tg CTL lysed both control Renca-FLAG and Renca-FLIP cells loaded with 10−6 M HA peptide, consistent with a dominant role for perforin-mediated lysis (Supplementary Fig. S6). By contrast, Tgpfp−/− CTL efficiently lysed Renca-FLAG control cells loaded with either 10−6 or 10−10 M peptide, but not Renca-FLIP cells, consistent with death-ligand mediated apoptosis being the dominant cytolytic pathway. Furthermore, cytolytic activity of Tg CTL against Renca cells loaded with 10−10 M HA peptide or against Renca-HA was blocked by FasL-neutralizing antibody, but not by TNF-related apoptosis-inducing ligand (TRAIL)-neutralizing antibody (Supplementary Fig. S4B and S5). Thus, Tg CTL-mediated lysis of Renca-HA or Renca targets loaded with low concentrations of HA peptide preferentially involved FasL but not TRAIL or Pfp.
Sensitization of various murine and human tumor cells to FasL-mediated lysis
To determine whether the apparent association between tumor peptide concentration and effector pathway usage was a general phenomenon, murine mammary carcinoma 4T1, colon carcinoma C-26 and B cell lymphoma A-20 cells were loaded with different amounts of exogenous HA peptide. Addition of 10−10 M HA sensitized them all to lysis by TgPfp−/− CTL, with minimal cytolysis by Tggld/gldCTL (Fig. 5C). At higher peptide concentrations (10−6 M) preferential effector pathway usage was lost (Fig. 5C). Following FasL-blocking antibody treatment, lysis by TgPfp−/− CTL was reduced in 4T1 targets from 52% to 4.6% and in A20 from 75.9% to 11%.
Interestingly, treatment of human renal cell adenocarcinoma lines ACHN, TK-10 and UO-31 with recombinant FasL triggered apoptosis. This tumor cell apoptosis could be amplified by pre-treatment with IFN-γ and TNF-α, and was blocked by inhibitor zVAD-FMK (Fig. 5D). Thus, FasL pathway of T cell cytotoxicity plays a major role in cytolysis of tumor cells exposed to inflammatory cytokines such as IFN-γ and TNF-α, and becomes particularly dominant under conditions of low tumor peptide presentation.
Antigen presented by tumor targets influences the nature of CTL response and effector cell degranulation
Specific antibodies reactive to cell surface HA allowed us to sort the Renca-HA transfectants that expressed low, intermediate and high surface levels of HA (Fig. 6A), yet similar levels of MHC class-I (data not shown). Renca-HAlow tumor cell lysis by Tg CTL in 18-h CTL assay was mediated predominantly by FasL pathway as it was blocked by FasL-neutralizing Ab but not by perforin inhibitor concanamycin A (Fig. 6B). In contrast, Renca-HAhigh cell lysis by Tg CTL was blocked only partially by FasL-neutralizing Ab but more efficiently by concanamycin A. Lysis of unsorted Renca-HA cells and Renca-HAintermediate cells by Tg CTL showed a contribution from both effector pathways (Fig. 6C).
Figure 6. Antigen density on tumor targets influences CTL response and effector cell degranulation.
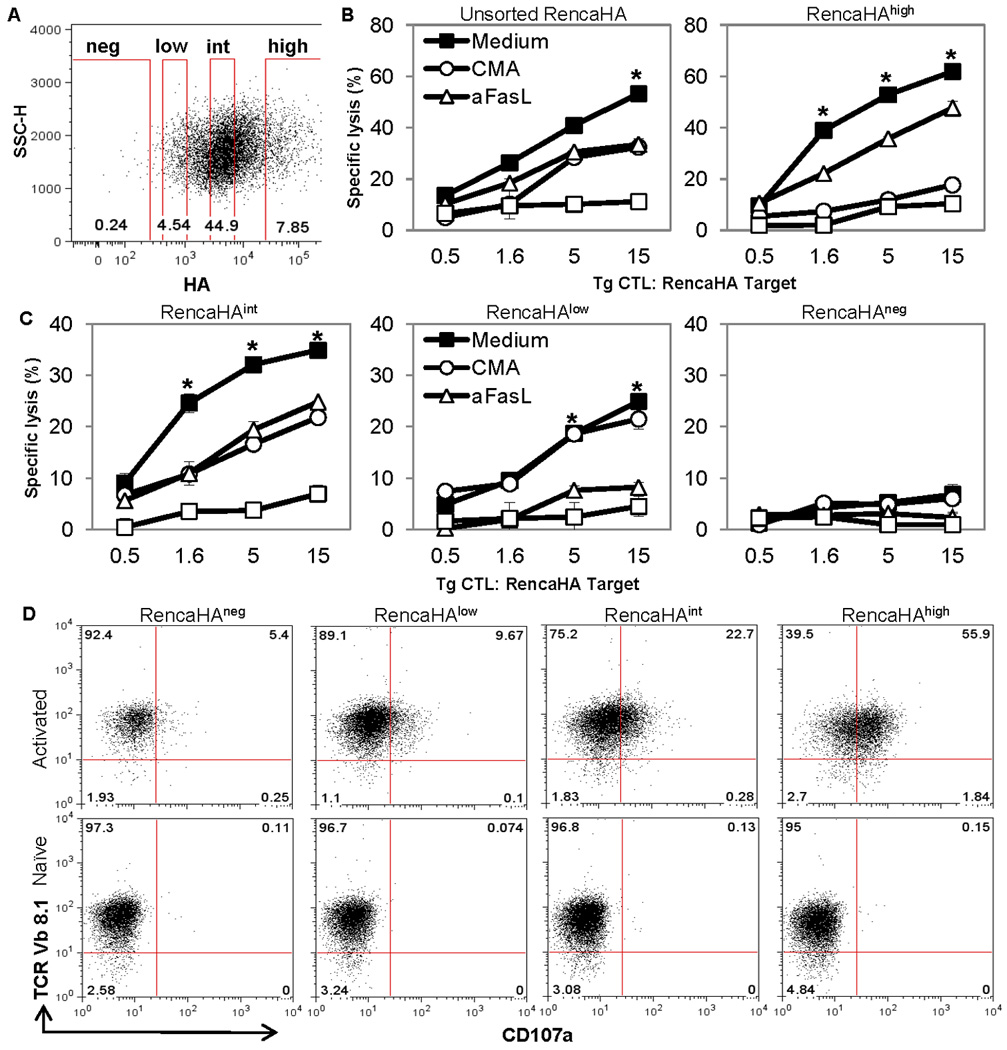
(A) Gating schematic for sorting of HAnegative, HAlow, HAintermediate and HAhigh cells in Renca-HA cells following staining with HA-specific H28-E23 Ab. (B and C) Percent specific lysis of sorted or unsorted Renca-HA cells by activated Tg CTL in the presence of medium, concanamycin A, FasL-neutralizing Ab, MFL4, or a combination of concanamycin A and MFL4. *P < 0.001 (two-sided unpaired t test) with respect to respective control groups. (D) Dot plots of TCR Vβ8.1 versus CD107a on Tg CTL following incubation with sorted Renca-HA tumor cells for 6 h in the presence of monensin. Numbers represent % positive cells. Plots representative of 3 independent experiments.
To understand the reason for the insensitivity of Renca-HAlow targets to perforin-mediated lysis, we investigated effector cell degranulation by measuring CD107a, a lysosomal-associated membrane glycoprotein (19). Interestingly, a significant upregulation of CD107a expression was observed in HA-specific clonotypic TCR Vβ8.1+ Tg CTL following incubation for 6 h with Renca-HAhigh targets as compared with Renca-HA targets presenting lower or no HA (Fig. 6D). Thus, Renca-HAhigh tumor cell targets triggered degranulation in activated Tg CTL. In contrast, Renca-HAlow targets failed to trigger degranulation, explaining why they are poor targets for perforin-mediated cytolysis.
Discussion
We addressed the contribution of the two direct mechanisms of CD8+ T cell cytotoxicity in the control of subcutaneous tumor and pulmonary metastases. In the murine model of Renca-HA cancer, where limiting levels of HA peptide are presented by tumor cells, the FasL pathway plays a dominant role in the antitumor response. Our findings provide the first clear evidence of the non-redundant antitumor roles of the Pfp and FasL effector mechanisms in vivo.
Interestingly, following the adoptive transfer of Tg CTL identical to those used in our current study, the perforin pathway was found to be 30-fold more efficient than FasL in inducing HA-reactive autoimmune diabetes in mice with HA-expressing pancreatic β cells (15). Our study suggests that Renca-HA tumors are probably much more sensitive to the cytotoxic effects of FasL than pancreatic β cells. Endogenous cytokines may upregulate Fas expression on tumor cells in vivo (20, 21) and further sensitize them to FasL-mediated lysis. Thus, the changes in the tumor microenvironment produced by an influx of activated CTL, coupled to the intrinsic biological characteristics of the tumor cells, seem to be crucially important in determining whether this tumor progresses or regresses. Consequently, our study explains why Pfp can be dispensable for antitumor activities of adoptively transferred CTL (10–12, 22), in certain mouse tumor models.
Previous in vitro studies have demonstrated that self peptides differing by one amino acid (23, 24), chemically-modified peptides (25), or low concentrations of peptides (26) only act as partial agonists for cytotoxic T cells as they lead to a reduced TCR signaling. This weaker intracellular signaling triggers FasL-mediated cytotoxicity but not Pfp-mediated cytolysis (27). Our study using TCR-transgenic CD8+ T cells is in agreement with these findings. More importantly, our adoptive CTL transfer studies suggest that low levels of tumor peptide determine the characteristics of the CTL effector response and the subsequent therapeutic outcome. We would speculate that many tumors will not trigger engagement of the Pfp-mediated cytotoxic pathway in vivo, even if the T cells have all the relevant lytic machinery available to them.
We found no role of FasL-mediated cytotoxicity in the “bystander” lysis of Ag-deficient Renca targets by TgPfp−/− CTL (data not shown), suggesting that the eradication of tumor by Pfp−/− CTL seemingly requires a direct tumor:effector contact. Antitumor effects involving tumor stroma are unlikely, as evident from the efficient FasL-dependent rejection of Renca-HA tumor in Faslpr/lpr mice where the stroma should be resistant to FasL. However, the role of stromal cells in tumor rejection may be dependent on the tumor site or type. Cytokines such as IFN-γ or TNF have been reported to have effects on tumor stroma in promoting tumor rejection in some models (28, 29). We found also no role for TRAIL in Renca-HA lysis by HA-reactive CTL, although Renca cells are known to be sensitive to TRAIL (18, 30).
The FasL-mediated tumor eradication may also serve as an immunoselective pressure to select Fas-deficient/low and/or Ag-negative tumor cells. This immunoselection has been reported in the context of CD4+ T cell-mediated response against Renca-HA tumor (31) and in other tumor models (32). We have also observed that the subcutaneous Renca-HA tumors growing out in WT mice become HA and/or Fas negative (unpublished data). The ultimate success of the antitumor T cell response may thus rely not only on the efficacies of Pfp and FasL pathways to control the continuum of high Ag to low antigenic targets but also on various other mechanisms including activation of innate immune cells that infiltrate the tumor. Recently, the antitumor CTL response was shown to activate local natural killer cells to eliminate Ag-loss tumor variants (33). Tumor destruction in vivo, by mechanisms other than direct CTL-mediated cytotoxicity, is worthy of further investigation.
This study suggests that biological characteristics of the tumors, including their FasL sensitivity and the quantity of antigen presented in association with MHC class-I, will play a major role in determining the efficacy of immunotherapy. Interestingly, some human renal carcinoma lines are susceptible to the apoptotic effects of FasL in vitro. In addition, pharmacological agents such as the proteasome inhibitor bortezomib can sensitize tumor cells to apoptosis (30, 34). It is thus tempting to speculate that certain human renal carcinomas that are/can be made sensitive to FasL-mediated apoptosis would be more responsive to adoptive T cell therapy. Promotion of FasL-mediated T cell cytotoxicity in combination with other therapeutic strategies that sensitize tumor cells to apoptosis may thus offer significant future potential for effective cancer immunotherapy.
Supplementary Material
Acknowledgments
We thank D. Haines for the immunohistochemistry, W. Gillette and R. Hopkins for antibody purification and L. Sherman, H. Levitsky, J. Yewdell, J. Ortaldo, S. Durum, A. Hurwitz, V. Singh and R. de Kluyver for helpful feedback.
Financial support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. This Research was supported [in part] by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JC, Childs R. Immunotherapy for renal cell cancer. J Clin Oncol. 2006;24:5576–5583. doi: 10.1200/JCO.2006.08.3774. [DOI] [PubMed] [Google Scholar]
- 3.Sayers TJ, Brooks AD, Lee JK, et al. Molecular mechanisms of immune-mediated lysis of murine renal cancer: differential contributions of perforin-dependent versus Fas-mediated pathways in lysis by NK and T cells. J Immunol. 1998;161:3957–3965. [PubMed] [Google Scholar]
- 4.Smyth MJ, Kelly JM, Sutton VR, et al. Unlocking the secrets of cytotoxic granule proteins. J Leukoc Biol. 2001;70:18–29. [PubMed] [Google Scholar]
- 5.Henkart PA, Catalfamo M. CD8+ effector cells. Adv Immunol. 2004;83:233–252. doi: 10.1016/S0065-2776(04)83007-4. [DOI] [PubMed] [Google Scholar]
- 6.Sayers TJ, Brooks AD, Seki N, et al. T cell lysis of murine renal cancer: multiple signaling pathways for cell death via Fas. J Leukoc Biol. 2000;68:81–86. [PubMed] [Google Scholar]
- 7.Kagi D, Ledermann B, Burki K, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 8.van den Broek ME, Kagi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng L, Krauss JC, Plautz GE, Mukai S, Shu S, Cohen PA. T cell-mediated tumor rejection displays diverse dependence upon perforin and IFN-gamma mechanisms that cannot be predicted from in vitro T cell characteristics. J Immunol. 2000;165:7116–7124. doi: 10.4049/jimmunol.165.12.7116. [DOI] [PubMed] [Google Scholar]
- 11.Seki N, Brooks AD, Carter CR, et al. Tumor-specific CTL kill murine renal cancer cells using both perforin and Fas ligand-mediated lysis in vitro, but cause tumor regression in vivo in the absence of perforin. J Immunol. 2002;168:3484–3492. doi: 10.4049/jimmunol.168.7.3484. [DOI] [PubMed] [Google Scholar]
- 12.Winter H, Hu HM, Urba WJ, Fox BA. Tumor regression after adoptive transfer of effector T cells is independent of perforin or Fas ligand (APO-1L/CD95L) J Immunol. 1999;163:4462–4472. [PubMed] [Google Scholar]
- 13.Morgan DJ, Kreuwel HT, Fleck S, Levitsky HI, Pardoll DM, Sherman LA. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 14.Morgan DJ, Liblau R, Scott B, et al. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 15.Kreuwel HT, Morgan DJ, Krahl T, Ko A, Sarvetnick N, Sherman LA. Comparing the relative role of perforin/granzyme versus Fas/Fas ligand cytotoxic pathways in CD8+ T cell-mediated insulin-dependent diabetes mellitus. J Immunol. 1999;163:4335–4341. [PubMed] [Google Scholar]
- 16.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 17.Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159:1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seki N, Hayakawa Y, Brooks AD, et al. Tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis is an important endogenous mechanism for resistance to liver metastases in murine renal cancer. Cancer Res. 2003;63:207–213. [PubMed] [Google Scholar]
- 19.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 20.Lee JK, Sayers TJ, Brooks AD, et al. IFN-gamma-dependent delay of in vivo tumor progression by Fas overexpression on murine renal cancer cells. J Immunol. 2000;164:231–239. doi: 10.4049/jimmunol.164.1.231. [DOI] [PubMed] [Google Scholar]
- 21.Peshes-Yaloz N, Rosen D, Sondel PM, Krammer PH, Berke G. Up-regulation of Fas (CD95) expression in tumour cells in vivo. Immunology. 2007;120:502–511. doi: 10.1111/j.1365-2567.2006.02521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobrzanski MJ, Reome JB, Hollenbaugh JA, Hylind JC, Dutton RW. Effector cell-derived lymphotoxin alpha and Fas ligand, but not perforin, promote Tc1 and Tc2 effector cell-mediated tumor therapy in established pulmonary metastases. Cancer Res. 2004;64:406–414. doi: 10.1158/0008-5472.can-03-2580. [DOI] [PubMed] [Google Scholar]
- 23.Brossart P, Bevan MJ. Selective activation of Fas/Fas ligand-mediated cytotoxicity by a self peptide. J Exp Med. 1996;183:2449–2458. doi: 10.1084/jem.183.6.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao W, Tykodi SS, Esser MT, Braciale VL, Braciale TJ. Partial activation of CD8+ T cells by a self-derived peptide. Nature. 1995;378:295–298. doi: 10.1038/378295a0. [DOI] [PubMed] [Google Scholar]
- 25.Kessler B, Hudrisier D, Schroeter M, Tschopp J, Cerottini JC, Luescher IF. Peptide modification or blocking of CD8, resulting in weak TCR signaling, can activate CTL for Fas- but not perforin-dependent cytotoxicity or cytokine production. J Immunol. 1998;161:6939–6946. [PubMed] [Google Scholar]
- 26.Kojima H, Toda M, Sitkovsky MV. Comparison of Fas- versus perforin-mediated pathways of cytotoxicity in TCR- and Thy-1-activated murine T cells. Int Immunol. 2000;12:365–374. doi: 10.1093/intimm/12.3.365. [DOI] [PubMed] [Google Scholar]
- 27.Esser MT, Krishnamurthy B, Braciale VL. Distinct T cell receptor signaling requirements for perforin- or FasL-mediated cytotoxicity. J Exp Med. 1996;183:1697–1706. doi: 10.1084/jem.183.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wigginton JM, Gruys E, Geiselhart L, et al. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J Clin Invest. 2001;108:51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shanker A, Brooks AD, Tristan CA, et al. Treating Metastatic Solid Tumors With Bortezomib and a Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Receptor Agonist Antibody. J Natl Cancer Inst. 2008;100:649–662. doi: 10.1093/jnci/djn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou G, Lu Z, McCadden JD, Levitsky HI, Marson AL. Reciprocal changes in tumor antigenicity and antigen-specific T cell function during tumor progression. J Exp Med. 2004;200:1581–1592. doi: 10.1084/jem.20041240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu K, Caldwell SA, Greeneltch KM, Yang D, Abrams SI. CTL adoptive immunotherapy concurrently mediates tumor regression and tumor escape. J Immunol. 2006;176:3374–3382. doi: 10.4049/jimmunol.176.6.3374. [DOI] [PubMed] [Google Scholar]
- 33.Shanker A, Verdeil G, Buferne M, et al. CD8 T cell help for innate antitumor immunity. J Immunol. 2007;179:6651–6662. doi: 10.4049/jimmunol.179.10.6651. [DOI] [PubMed] [Google Scholar]
- 34.Hallett WH, Ames E, Motarjemi M, et al. Sensitization of tumor cells to NK cell-mediated killing by proteasome inhibition. J Immunol. 2008;180:163–170. doi: 10.4049/jimmunol.180.1.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.