Abstract
Objective
Murine embryonic stem (ES) cells can be differentiated into embryoid bodies (EBs), which serve as an in vitro model recapitulating many aspects of embryonic yolk sac hematopoiesis. Differentiation of ES cells deficient in either Gata-4 or Gata-6 results in EBs with disrupted visceral endoderm (VE). While lack of VE has detrimental effects on hematopoiesis in vivo, it is unclear whether lack of VE affects hematopoiesis in EBs. Therefore, we compared Gata-4 null (G4N) and Gata-6 null (G6N) EBs with wild-type (WT) EBs to assess their ability to commit to hematopoietic cells.
Methods
EB VE formation was examined using cell sorting techniques and analysis visceral endoderm gene expression. Hematopoietic progenitor potential of EBs cultured under various conditions was assessed using colony forming assays.
Results
Definitive erythroid, granulocyte-macrophage, and mixed colonies were significantly reduced in G4N and G6N EBs compared to WT EBs. Vascular endothelial growth factor (VEGF) expression and secretion were also reduced in both G4N and G6N EBs, consistent with VE serving as a site of VEGF production. Addition of exogenous VEGF165, to EB cultures completely rescued definitive colony forming cells in G4N and G6N EBs. This rescue response could be blocked by addition of soluble Flk-1 Fc to EB cultures. Similarly, addition of exogenous Indian hedgehog (IHH) to EB cultures also recovers the diminishment in definitive hematopoiesis in a reversible manner.
Conclusion
These results suggest that the absence of VE in G4N and G6N EBs does not prevent the emergence of definitive progenitors from EBs. However, the decreased level of VEGF and IHH production in VE devoid G4N and G6N EBs attenuates definitive hematopoietic progenitor cell expansion.
Keywords: VEGF, Gata-4, Gata-6, Murine Embryoid Bodies, IHH
Introduction
In early mouse embryonic development, the first hematopoietic cells are detected in the extra-embryonic yolk sac [1]. Initially, large nucleated primitive erythrocytes expressing embryonic globins along with early macrophages emerge from the yolk sac mesoderm on embryonic day 7.0 (E7.0); this wave is called primitive hematopoiesis. A second wave of hematopoietic progenitors arises from the yolk sac mesoderm on E8.25 comprising definitive erythroid progenitors, expressing adult globins, as well as other progenitors of the myeloid lineage; referred to as definitive hematopoiesis [2, 3]. In addition to hematopoietic cells, mesoderm derived endothelial cells also first emerge in the extra-embryonic yolk sac region. Together, the hematopoietic progenitors and endothelial cells are organized into blood islands in a region of the proximal yolk sac [4–6]. The lineage commitment of mesoderm to hematopoietic and endothelial cell lineages and their organization into blood islands in the mouse yolk sac requires an overlying layer of visceral endoderm (VE) cells and is influenced by VE gene expression and production of proteins [7–9].
Many aspects of embryonic development are mirrored in vitro by the differentiation of mouse embryonic stem (ES) cells into embryoid bodies (EBs). Particularly, EBs ability to mimic embryonic yolk sac hematopoietic events has undoubtedly led to their widespread use in investigations aiming to detail the process of yolk sac blood island formation, which may be difficult or impossible to address using mouse embryos. Just prior to the onset of primitive hematopoiesis, BL-CFCs (bi-potential precursors of hematopoietic and endothelial cells) are detected in EBs at day ~2.5–4 of differentiation [10]. In a manner that is analogous to mouse embryonic yolk sac hematopoiesis, an early and transient wave of primitive erythrocytes (derived from mesoderm) can be detected from day 4 through 7 of differentiation followed by the emergence of definitive hematopoietic progenitors of multiple lineages. Together with endothelial cells, these hematopoietic progenitors form the blood islands of the EB [11, 12].
Similar to mouse embryonic yolk sacs, EBs have an outer layer of VE [13]. This visceral endoderm layer has characteristic brush border microvilli and phagocytic vesicles and expresses many of the same genes detected in mouse embryonic yolk sac VE cells [14–17]. ES cells can also be selectively differentiated to and maintained as VE when plated at a high density under specific conditions [18]. Differentiation of ES cells with a homozygous null mutation in either Gata-4 or Gata-6 gene results in the formation of EBs devoid of a VE covering [19, 20]. In addition to a block in endoderm formation, a disruption in the organization of blood islands has also been observed in Gata-4−/− EBs grown in suspension culture [21].
In the present study, we aimed to determine if VE is necessary for hematopoietic emergence or expansion in the in vitro ES/EB culture system, given its requirement in vivo for proper mouse yolk sac hematopoiesis. The ES/EB system was particularly attractive because EBs can be quickly generated in large quantities and are frequently used in studies of embryonic hematopoietic processes. To investigate our experimental questions, we differentiated Gata-4−/− and Gata-6−/− ES cells into EBs because of their failure to form a VE covering. After investigating their hematopoietic potential in a series of hematopoietic progenitor colony formation assays, we report that definitive hematopoiesis is impaired in both Gata-4 null (G4N) and Gata-6 null (G6N) EBs. We also demonstrate that among several VE enriched genes, Vegf expression and protein secretion is reduced in G4N and G6N EBs. Furthermore, we show that the defect in definitive hematopoiesis is rescued with the dose dependent addition of exogenous VEGF165 to EB cultures, in a reversible manner upon the addition of a soluble VEGF R2 (Flk-1) Fc chimera to EB cultures. Likewise, definitive hematopoiesis is also rescued upon addition of IHH, another VE derived factor, to EB cultures and can be specifically reversed upon treatment with an antagonist to hedgehog signaling. Thus, cells derived from VE deficient G4N and G6N EBs display diminished definitive hematopoiesis due to insufficient VEGF and/or IHH production.
Materials and Methods
ES Cell Maintenance and Differentiation
Wild-type CCE ES cells, Gata-4−/− ES cells (a kind gift of Bielinska, Margaret) and Gata-6−/− ES cells (a kind gift of Parmacek, Michael) were maintained on 100 mm or 60 mm gelatin coated tissue culture plates in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 15 % bovine growth serum (Hyclone, Logan, UT), 0.055 mM β-mercaptoethanol, leukemia inhibitory factor (LIF; 1000 Units/mL) (ESGRO; CHEMICON, Temecula, CA), 1 mM sodium pyruvate, 2 mM L-glutamine, penicillin and nonessential amino acids. To differentiate ES cells into EBs, ES cells were plated into bacterial grade Petri Dishes at 45,000 to 60,000 cells/mL in differentiation media containing 2 mM L-glutamine, penicillin/streptomycin, 5 % protein-free hybridoma medium (PFHM-II) (Gibco, Grand Island, NY), iron-saturated holotransferrin (200 µg/mL) (Sigma, St. Louis, MO), 15 % defined FBS (StemCell Technologies, Vancouver, BC, Canada), ascorbic acid (50 µg/ mL) and 0.055mM β-mercaptoethanol. In rescue experiments, recombinant mouse Vegf165 (2.5, 5, 10 ng/mL) (PreproTech, Rocky Hill, New Jersey) or recombinant mouse IHH (50, 100, 200 or 250 ng/.mL) (PreproTech) was added to EB cultures on day 6 of differentiation. In blocking experiments, recombinant mouse VEGF R2 (Flk-1) Fc chimera (5, 10, 20 ng/mL) (R & D Systems, Minneapolis, MN) or cylopamine (1, 5, 10, 20 µM) (dissolved in DMSO) (Sigma) was added to EB cultures on day 6. Cells were incubated at 37°C in 5% CO2.
Definitive Hematopoietic Progenitor Assay
EBs were digested into single cell suspension with Tryple Express (Gibco). Cells were counted using trypan blue and plated at 50,000 or 100,000 cells/mL (day 8, 9 and 10 EBs) with SCF (100 ng/mL), IL-3 (100 Units/mL) and EPO (5 Units/mL), granulocyte-macrophage colony stimulating factor (GM-CSF; 10ng/ mL) and macrophage colony stimulating factor (M-CSF; 5ng/mL) for definitive progenitor assays. Colonies were scored after 7 days incubation at 37°C in 5% CO2.
Reverse Transcriptase Polymerase Chain Reaction
EBs were collected after either 0 (undifferentiated ES cells), 2, 4, 6 or 8 days of differentiation and Trizol Reagent (Invitrogen; Carlsbad, CA) followed by a series of chloroform, isopropanol, and ethanol isolation phases was used to isolate total RNA. Total cellular RNA was quantified by UV spectroscopy and first-strand synthesis was performed using 2 µg total cellular RNA with oligo (dT) primers and reverse transcriptase (RT; SuperScript, Invitrogen). The expression of Gata-4 and Gata-6, was detected in EBs by RT-PCR. Total cellular RNA was isolated from DBA positive cells from day 5 and day 8 EBs using the RNeasy micro kit (Qiagen), and cDNA was synthesized using 0.8 µg of RNA. Endoderm gene expression patterns were analyzed by RT-PCR using primers for hepatic nuclear factor-4 alpha (Hnf-4α), Indian hedgehog (Ihh), hepatic nuclear factor-3 beta (Hnf-3β), vascular endothelial growth factor (Vegf), syndecan 4 (Sdc4). Additionally, total cellular RNA was isolated from c-Kit+, c-Kit+/Flk-1+, Flk-1+ cell populations and reverse transcription was performed using 0.8 µg as template. Gata-4 and Gata-6 genes were amplified by RT-PCR. β-actin served as the control in all experiments. All PCR products were amplified with 28 cycles of PCR and analyzed on a 2% agarose gel stained with ethidium bromide for visualization. See Table 1 for all primer sequences.
Table 1.
List of primer sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin (207 bp) | CGAGCACAGCTTCTTTGCAG | TTCTGACCCATTCCCACCAT |
| Gata-4 (240bp) | CTGGAAGACACCCCAATCTC | TGGTGGTAGTCTGGCAGTTG |
| Gata-6 (206bp) | CGGTGCTCCACAGCTTACAG | CCTGCTGAGGCCATTCATCT |
| Hnf-3β (367 bp) | CCGACTGGAGCAGCTACTATG | GGGCTCATGGAGTTCATGTT |
| Hnf-4α (356 bp) | AGCGTGAGGAAGAACCACAT | ATGTACTTGGCCCACTCGAC |
| Ihh (193 bp) | ACGTGCATTGCTCTGTCAA | CGGTCCAGGAAAATAAGC AC |
| Sdc4 (370 bp) | ACTTTGAGCTCTCGGGTTCT | TCGTAACTGCCTTCGTCCT T |
| Vegf (189bp) | ATTGAGACCCTGGTGGACAT | TATGTGCTGGCTTTGGTGAG |
Real-time Polymerase Chain Reaction
Total RNA was isolated from day 6 EBs using the RNeasy micro kit (Qiagen). Total cellular RNA was quantified by UV spectroscopy, and first-strand synthesis was performed using 0.8 µg total cellular RNA with RT primer mix and reverse transcriptase (QuantiTect Reverse Transcription; Qiagen). Using SYBR green PCR master mix (Applied Biosystems) and primers for Vegf and β-actin (see Table 1), the relative quantification of Vegf was determined in EBs and DBA sorted EB cells with the ABI 7500 using the comparative Δct method.
Enzyme-Linked ImmunoSorbent Assay
Day 8 EB culture media was centrifuged at 1200 rpm, and the concentration of VEGF in the supernatant was detected using the mouse VEGF ELISA Kit (Calbiochem, Darmstadt, Germany). Differentiation medium was used as the blank, and the absorbance of the standard (supplied in the kit) and supernatant collected from day 8 EBs was read at 450/540 nm. A standard curve was generated using the standard VEGF, and the concentration of VEGF secreted from the samples was extracted from the curve.
Flourescence Activated Cell Sorter (FACS) Analysis
EBs were collected and washed 3 times with 1X PBS. EBs were then digested with Hanks based enzyme free cell dissociation buffer (Gibco, Grand Island, NY) into a single cell suspension and cells were stained with PE-conjugated c-kit antibody and APC-conjugated Flk-1 antibody or FITC-conjugated Dolichos biflorus agglutinin (DBA) lectin for 30 minutes on ice. Additionally, a separate aliquot of cells was stained with IgG2b isotype control. Following antibody or lectin incubation, cells were washed with 1X PBS. Stained cells were analyzed by fluorescence activated cell sorter (FACS) for the detection of c-Kit/Flk-1 or DBA positive EB cells.
Results
Gata-4 and Gata-6 Gene Expression in EBs
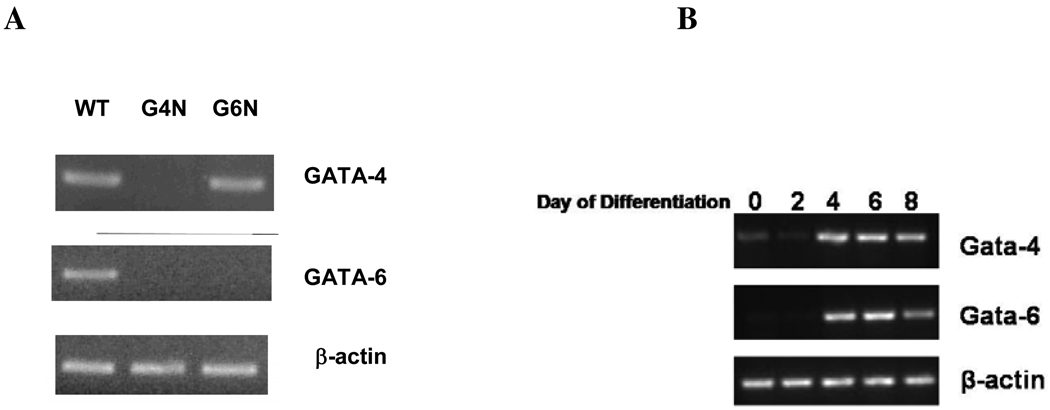
The expression of Gata-4 and Gata-6 genes was assessed in WT (wild-type), G4N (Gata-4 null) and G6N (Gata-6 null) EBs after 8 days of differentiation; the stage at which EB production of definitive progenitor cells is maximum [12]. Both Gata-4 and Gata-6 transcripts were detected in WT EBs. Consistent with previous reports [19, 20], G6N EBs did not express the Gata-6 gene but a Gata-4 transcript was detected in G6N EBs. However neither Gata-4 nor Gata-6 transcripts were detected G4N EBs (Fig. 1A). We also observed the temporal expression of Gata-4 and Gata-6 genes in WT EBs at days 0 (undifferentiated ES cells), 2, 4, 6 and 8 days of differentiation (Fig. 1B). Faint detection of Gata-4 and Gata-6 transcripts was observed in undifferentiated WT ES cells, consistent with previous reports, and the expression increased through day 8 of differentiation (Fig. 1.B) [22, 23].
Figure 1. GATA-4 and GATA-6 transcripts were detected in WT EBs.
Total cellular RNA was isolated from WT, G4N and G6N day 8 EBs (A) and day 0, 2, 4, 6 and 8 WT EBs (B). Reverse transcription was performed using 0.8 µg as template. GATA-4, GATA-6 and β-actin were amplified with 28 cycles of PCR. Products were visualized on agarose gel stained with ethidium bromide.
Diminished Visceral Endoderm Formation and Gene Expression in G4N and G6N EBs
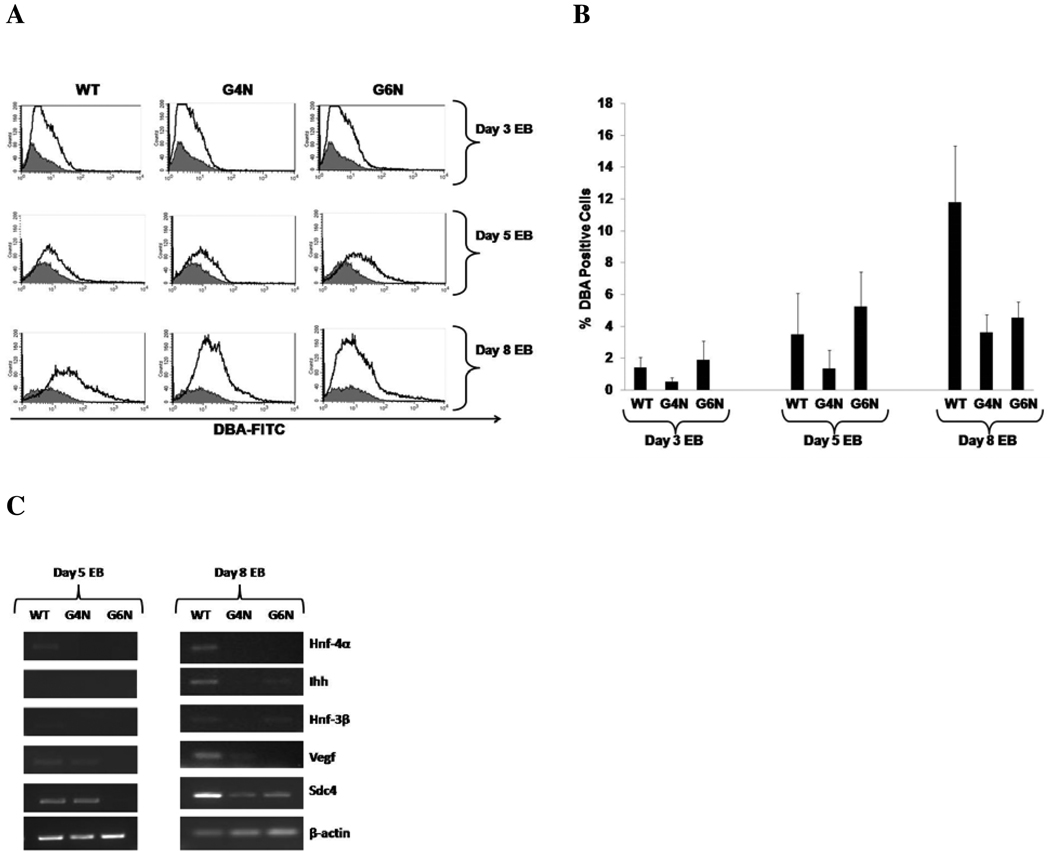
G4N and G6N EBs reportedly to lack a VE covering and display attenuated or failed expression of endoderm genes [19, 20]. We attempted to confirm the disruption in VE formation in EBs lacking Gata-4 or Gata-6 expression. To this end, we analyzed the binding of EB derived cells to the lectin DBA (Dolichos biflorus agglutinin); a characteristic previously attributed to VE cells in the mouse yolk sac [24, 25]. Day 3, 5 and 8 EB cells were stained with FITC-conjugated DBA and analyzed using fluorescence activated cell sorter (FACS) for the detection of DBA positively bound cells. We observed low levels of DBA bound cells in WT, G4N and G6N day 3 EBs (1.41%, 0.5% and 1.88%) and day 5 (3.57%, 1.33% and 5.22%), respectively (Fig. 2A, B). However, on day 8 the DBA positive cell population in WT EBs increased to 11.77% while G4N and G6N EBs contained only 3.61% and 4.54% DBA positively bound cells, respectively (Fig. 2A, B). The expression of hepatic nuclear factor-4 alpha (Hnf-4α), Indian hedgehog (Ihh), hepatic nuclear factor-3 beta (Hnf-3β), vascular endothelial growth factor (Vegf) and syndecan 4 (Sdc4), genes with known expression in VE cells, was examined in the DBA positive cell population of EBs at differentiation days 5 and 8 [26–29] (Fig. 2C). At day 5 of differentiation, we faintly detected the expression of Hnf-4α in WT EBs. Sdc4 and low levels of Vegf transcripts were detected in both WT and G4N day 5 EBs (Fig. 2C). By day 8 of differentiation, we clearly detected Hnf-4α, Ihh, Vegf and Sdc4 expression in WT EBs while Hnf-3β expression was faintly observed (Fig. 2C). Although Sdc4 expression was noted in both G4N and G6N day 8 EBs, G4N EBs had no observable detection of transcripts for Hnf-4α, Ihh and Hnf-3β and faint detection of Vegf expression (Fig. 2C). Transcripts for Ihh and Hnf-3β were observed in day 8 G6N EBs while Hnf-4α and Vegf were not detected (Fig. 2C). Together, the observed reduction in DBA positively bound cells and reduced expression of VE enriched genes within the DBA positive cell population confirm previous reports of disrupted VE formation in G4N and G6N EBs.
Figure 2. Diminished Visceral Endoderm Formation and Gene Expression in G4N and G6N EBs.
Cells from day 3, day 5 and day 6 EBs were stained with FITC-conjugated DBA and positive bound cells were analyzed by FACS analysis. (Representative experiment) (A). The average of four independent experiments are displayed (B). DBA positively bound cells were sorted from day 5 and day8 WT, G4N and G6N EBs. Total cellular RNA was isolated and reverse transcription was performed using 2.0 µg as template. Hnf-4α, Ihh, Hnf-3β, Vegf, Sdc4 and β-actin were amplified with 28 cycles of PCR. Products were visualized on agarose gel stained with ethidium bromide (C).
Definitive Hematopoiesis
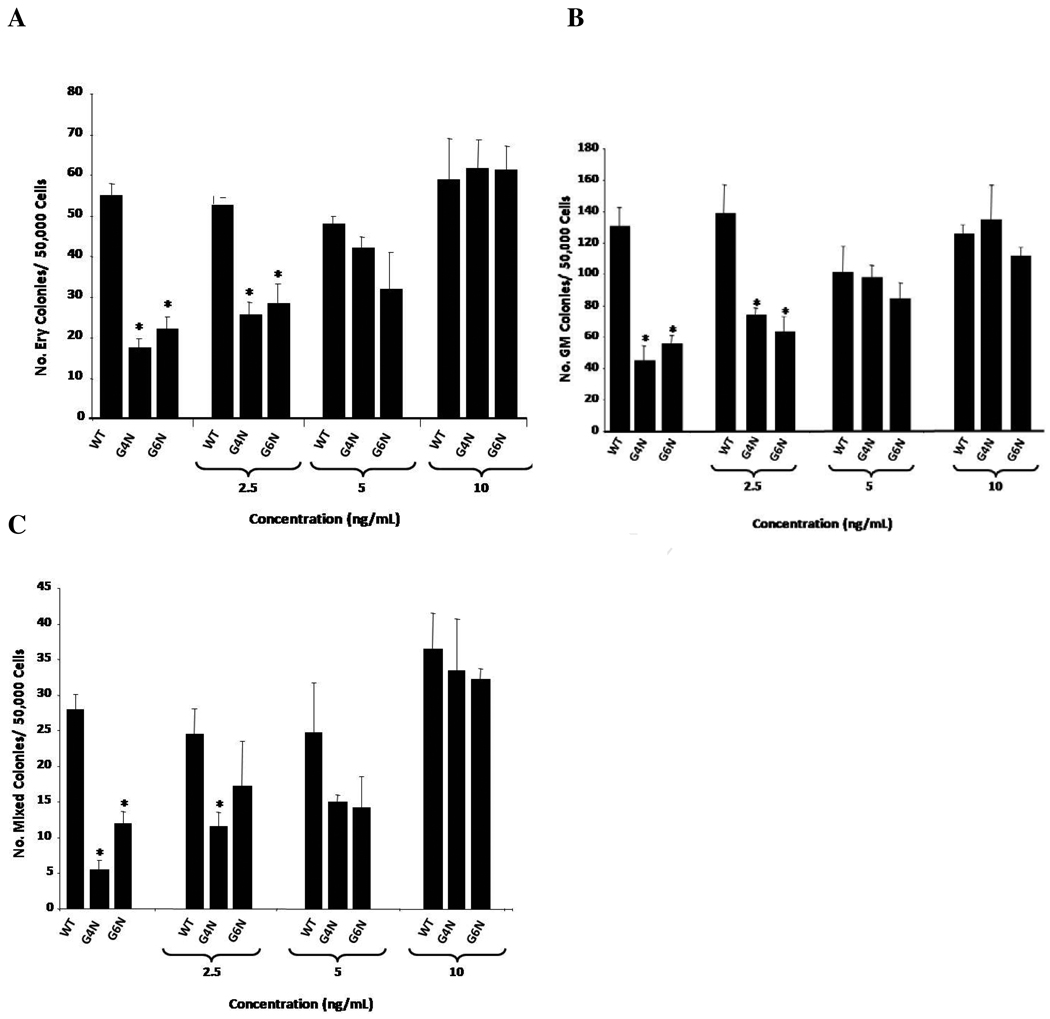
Initially, we compared the ability of WT, G4N and G6N EBs to commit to blast- colony forming cells (BL-CFC) and primitive erythroid progenitors using colony forming assays. In both cases, we observed no differences in BL-CFC or primitive erythroid progenitor colony forming ability between cell lines (data not shown). The ability of WT, G4N and G6N EBs derived cells to commit to definitive hematopoietic progenitors was tested using EBs plated into definitive progenitor colony forming assays at day 8, 9 and 10 of differentiation following dissociation into single cells. After 7 days of incubation, colonies were scored as either definitive erythroid (Ery), granulocyte-macrophage (GM) or mixed lineage (colonies containing both granulocyte-macrophage and definitive erythroid cells). Day 8 G4N and G6N EBs differentiated into significantly fewer definitive Ery, GM and mixed lineage progenitor colonies (Fig. 3) as compared to WT (p-values <0.001). By EB differentiation days 9 and 10, the number of definitive colonies was dramatically reduced to < 25% of day 8 EB content in all cell lines and was comparable between plated WT, G4N and G6N EBs (Fig. 3). Together, these results show that G4N and G6N EBs have an observable diminishment in definitive hematopoiesis at their peak day of detection.
Figure 3. Definitive hematopoiesis is diminished in G4N and G6N day 8 EBs.
Cells from day 8, 9 and 10 EBs were plated as single cells into definitive progenitor assays, and definitive erythroid (Ery), granulocyte-macrophage (GM) and mixed (Mixed) lineage colonies were scored after 7 days. (N = 5 independent experiments). Error bars represent s.e.m. *p<0.05.
VEGF Expression and Protein Secretion in EBs
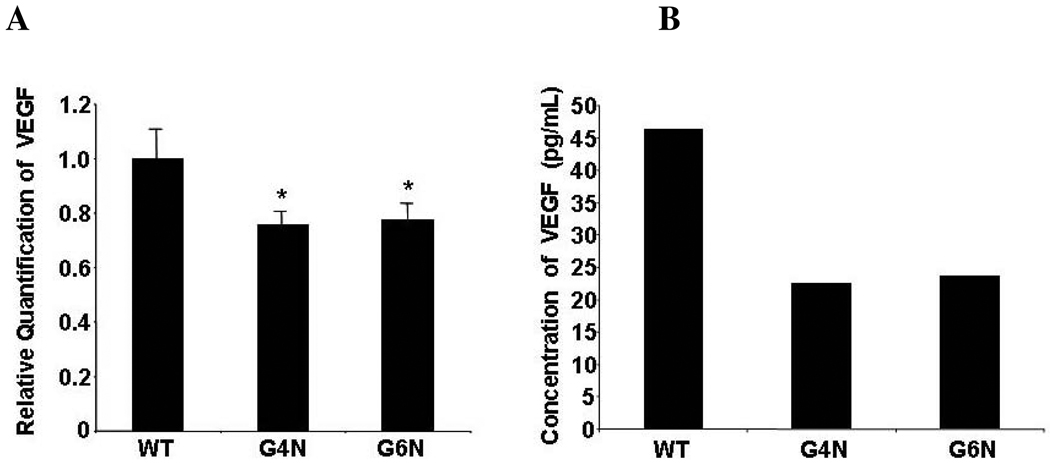
Among the VE enriched genes previous examined, we quantitated the expression level of Vegf in WT, G4N and G6N EBs using real-time PCR in light of previous reports concerning the essential role of Vegf in yolk sac blood island formation and primitive hematopoiesis [26, 27, 30]. We observed a significant reduction in Vegf expression in both G4N and G6N EBs in comparison to WT EBs (Fig. 4A). Using enzyme-linked immunosorbent assay (ELISA), we confirmed that the concentration of VEGF protein in the supernatant of G4N and G6N day 8 EBs was substantially reduced compared to the concentration of VEGF in supernatant of day 8 WT EBs (Fig. 4B).
Figure 4. VEGF mRNA levels and protein secretion are reduced in G4N and G6N EBs.
Total RNA was isolated from day 6 EBs. RNA was reverse transcribed and real-time PCR analysis was performed using the ABI 7500 for Vegf relative quantification. Error bars represent s.e.m. *p<0.05 (A). Supernatant from day 8 EBs was collected, and the concentration of VEGF protein was determined by ELISA (representative experiment) (B).
VEGF Rescues Definitive Hematopoiesis in G4N and G6N EBs
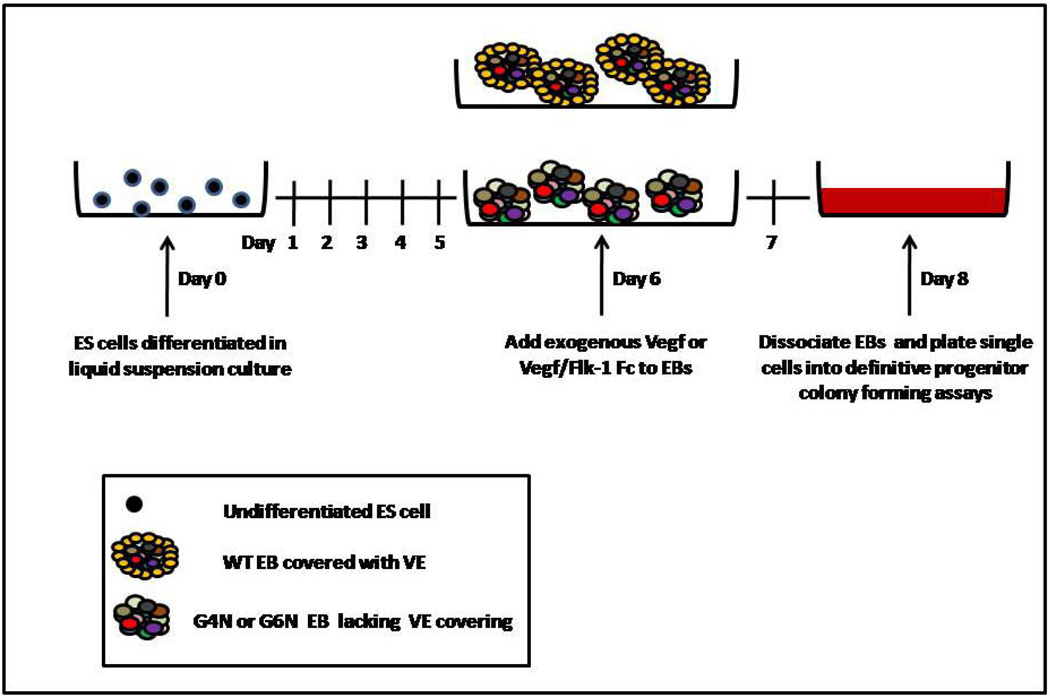
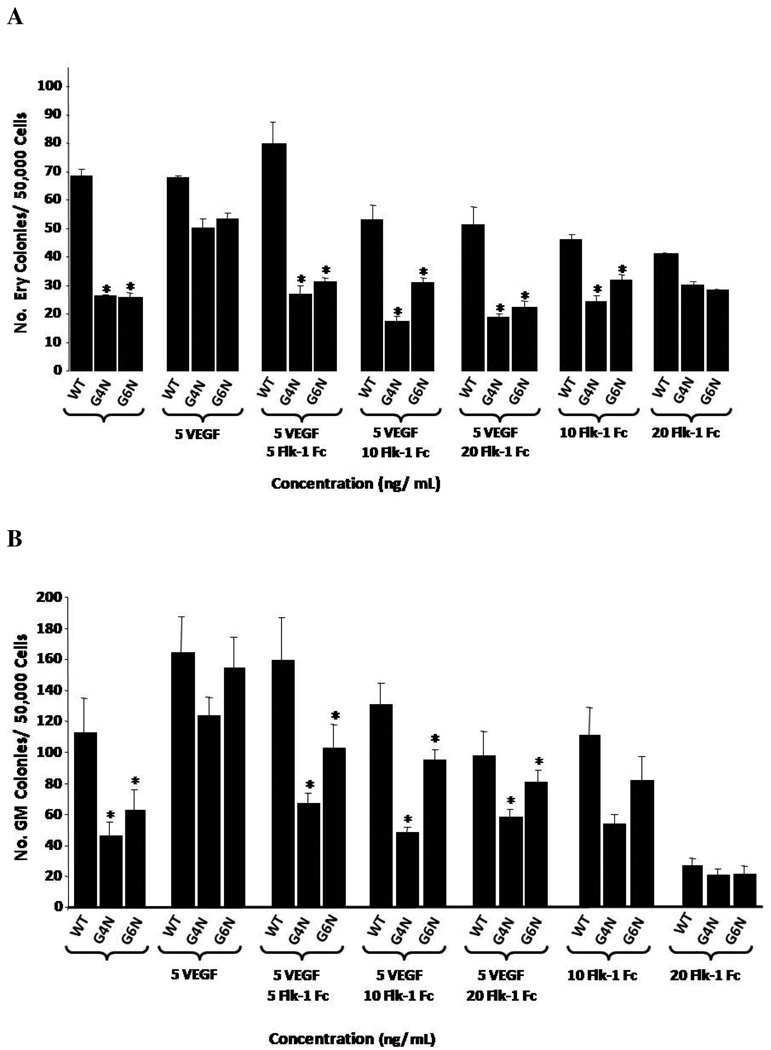
After observing a reduction in VEGF expression and protein secretion, we decided to test if the addition of exogenous VEGF to EB cultures could rescue definitive hematopoiesis in G4N and/or G6N EBs. EBs were either untreated or given recombinant mouse VEGF165 (2.5, 5, 10 ng/mL) after 6 days of culture. Following 2 additional days of culture, EBs were dissociated into single cells and plated into definitive progenitor colony assays (Fig. 5). We observed a dose-dependent rescue of definitive progenitor colony numbers with increasing concentrations of VEGF to EB cultures. The addition of 5 and 10 ng/mL of VEGF165 to EB cultures was sufficient to increase the number of definitive hematopoietic progenitor colonies in both G4N and G6N EBs to WT levels. (Fig. 6 A, B, C)
Figure 5. Schematic diagram of VEGF and Flk-1 Fc treatment of EB cultures.
In rescue experiments, EBs were treated with VEGF (2.5, 5 and 10 ng/mL) on EB differentiation day 6 and allowed to differentiate for 2 additional days. In blocking experiments, 5 ng/mL of VEGF and soluble Flk-1 Fc (5, 10, 20 ng/mL) were added to the culture medium on EB differentiation day 6 and EBs were differentiated for 2 additional days. In both cases, EBs were dissociated into single cells on day 8 and plated into definitive hematopoietic progenitor assays.
Figure 6. VEGF rescues definitive hematopoiesis in G4N and G6N EBs.
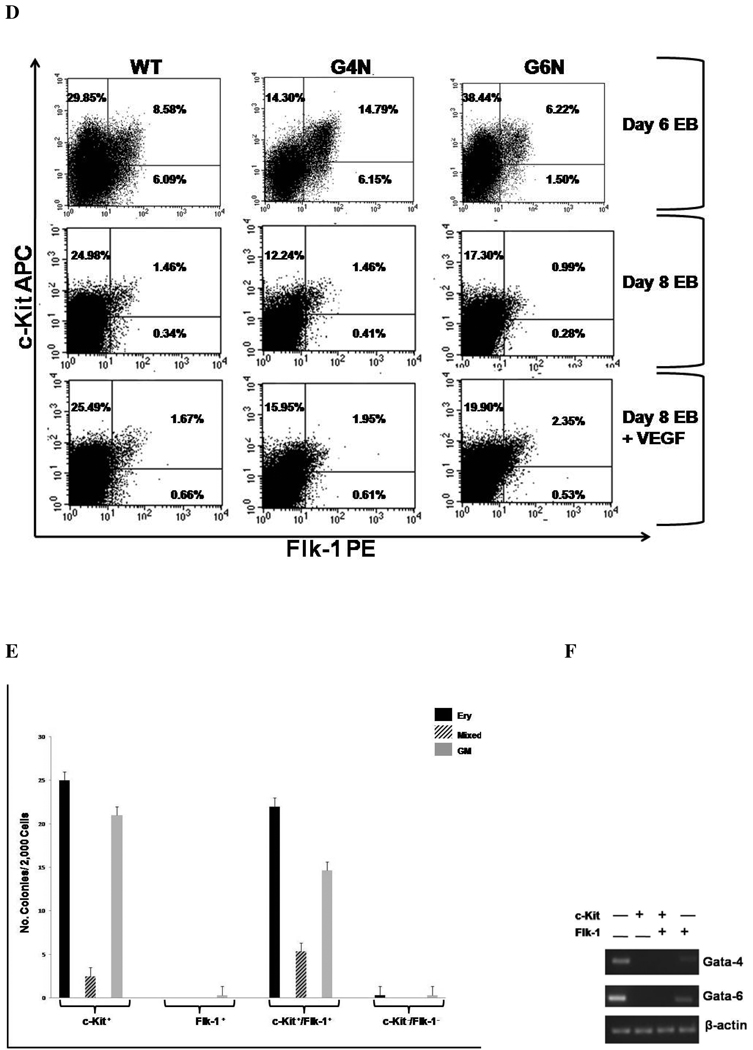
EBs were cultured for 6 days and VEGF (2.5, 5, 10 ng/ mL) was added to the culture medium. After 2 additional days, day 8 EBs were dissociated into single cells and plated into definitive hematopoietic progenitor colony formation assays. Definitive Erythroid (A), GM (B), and mixed (C) colonies were scored after 7 days. (N= 3 independent experiments). Error bars represent s.e.m. *p<0.05. EBs were dissociated into single cells on day 6 or on day 8 after being either treated or untreated with Vegf (5 ng/mL) on day 6. Cells were then stained with PE-conjugated c-Kit and APC-conjugated antibodies and analyzed by FACS analysis (representative experiment of 3 independent experiments) (D). Sorted cell populations were plated into definitive hematopoietic progenitor colony formation assays (representative experiment) (E.) Total cellular RNA was isolated from sorted cell populations and reverse transcription was performed using 0.8 µg as template. Gata-4 and Gata-6 expression were analyzed by RT-PCR (F). β-actin served as the positive control. Products were amplified with 28 cycles of PCR and visualized on agarose gel stained with ethidium bromide.
Next, we wanted to determine if EBs contain cells with surface expression of a VEGF receptor to permit responsiveness to VEGF and also display hematopoietic progenitor activity. We analyzed the level of c-Kit, a receptor expressed on virtually all hematopoietic progenitors [31], and Flk-1 (VEGF Receptor 2) expression on cells from day 6 and day 8 EBs that were either untreated or treated with 5 ng/mL of VEGF165 on day 6 of differentiation. Day 6 EBs, a stage containing mostly primitive progenitors, had 24.30% to 38.44% c-Kit+ cells, 6.22% to 14.79% c-Kit+/Flk-1+ cells and 1.5% to 6.15% Flk-1+ cells (Fig. 6D). In all cell lines, the c-Kit+, c-Kit+/Flk-1+ and FlK-1+ cell populations decreased from day 6 to day 8 of differentiation (Fig. 6D). Analysis of day 8 EBs revealed a decrease in the c-Kit+ cell population of G4N and G6N EBs compared to WT EBs (Fig. 6D). G6N day 8 EBs also had a decreased level of c-Kit+/Flk-1+ cells compared to WT EBs (Fig 6D). WT day 8 EBs showed virtually no difference in the level of c-Kit+ cells in response to VEGF treatment, while G4N and G6N day 8 EBs displayed modest increases the c-Kit+ and c-Kit+/Flk-1+ cell populations in response to VEGF treatment (Fig. 6D). The Flk-1+ cell population was comparable between all cell lines and slightly increased upon treatment with VEGF (Fig 6D). When we plated the c-Kit+, c-Kit+/Flk-1+, Flk-1+ and double negative populations from WT EBs into definitive hematopoietic progenitor colony forming assays, both the c-Kit+ and c-Kit+/Flk-1+ cell populations formed definitive progenitor colonies while colony formation was nearly absent in the Flk-1+ and double negative cell populations (Fig. 6E). We also wanted to confirm that neither Gata-4 nor Gata-6 genes are expressed in definitive hematopoietic progenitors contained within c-Kit+ and c-Kit+/Flk-1+ cell populations. We sorted c-Kit+, c-Kit+/Flk-1+, Flk-1+ and double negative cell populations from WT EBs and analyzed the expression of Gata-4 and Gata-6 genes by RT-PCR. Gata-4 and Gata-6 transcripts were detected in the double negative cell population and also in the Flk-1+cell population, albeit at lower levels. However, transcripts for Gata-4 and Gata-6 genes were not detected in either c-Kit+ or c-Kit+/Flk-1+ cell populations (Fig. 6F). These results demonstrate that EBs contain c-Kit positive definitive hematopoietic progenitor cells that co-express a receptor for VEGF, thus permitting responsiveness to the ligand. The lack of Gata-4 and Gata-6 expression in WT hematopoietic progenitor cells also suggest that the diminished definitive hematopoiesis observed in G4N and G6N EBs is not due to a lack of expression of these transcription factors in hematopoietic progenitor cells.
Blocking VEGF Ablates the Rescue of Definitive Hematopoiesis
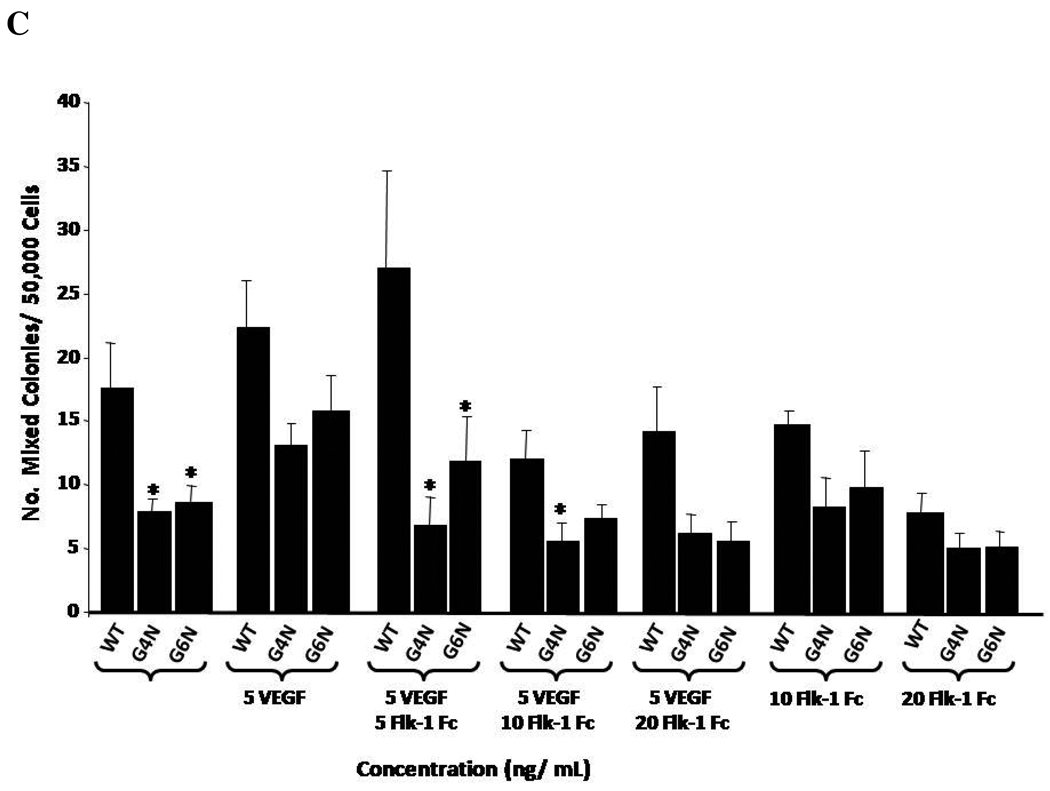
We decided to determine if the addition of soluble recombinant mouse VEGF Receptor 2 Fc chimera (Flk-1 Fc) to EB cultures on day 6 of differentiation blocks the rescue of definitive hematopoiesis in G4N and G6N EBs observed in response to the addition of VEGF (Figure 5). As in previous experiments, VEGF (5 ng/mL) rescued the impairment in definitive progenitor colony emergence in both G4N and G6N EBs. When we also supplemented EB cultures with soluble Flk-1 Fc, we noted a dose dependent decrease in colony numbers with increasing concentrations of Flk-1 Fc (Fig. 7 A, B, C). Although it was clear that the Flk-1 Fc blocks the VEGF induced rescue of definitive hematopoietic progenitors, no condition tested completely abolished definitive hematopoiesis, including Flk-1 Fc given alone (Fig. 7 A, B, C). These results demonstrate that the VEGF mediated rescue of definitive hematopoiesis can be specifically reversed.
Figure 7. VEGF mediated rescue of definitive hematopoiesis in day 8 EBs is abolished with soluble Flk-1/Fc.
EBs were cultured for 6 days and 5 ng/mL of VEGF and soluble Flk-1/ Fc (5, 10, 20 ng/mL) were added to the culture medium. After 2 additional days, day 8 EBs were dissociated into single cells and plated into definitive hematopoietic progenitor colony formation assays. Definitive Erythroid (A), GM (B), and mixed (C) colonies were scored after 7 days. (N= 3 independent experiments). Error bars represent s.e.m. *p<0.05.
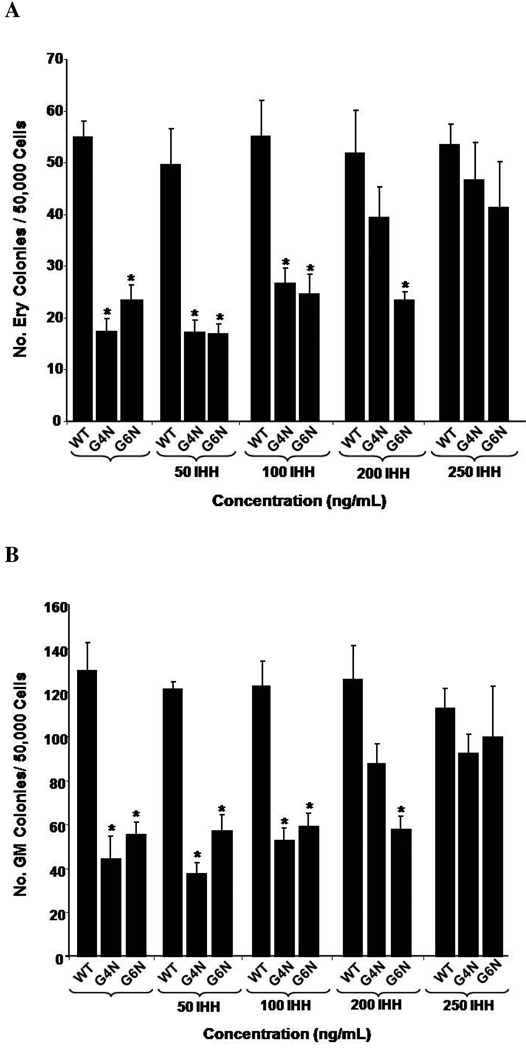
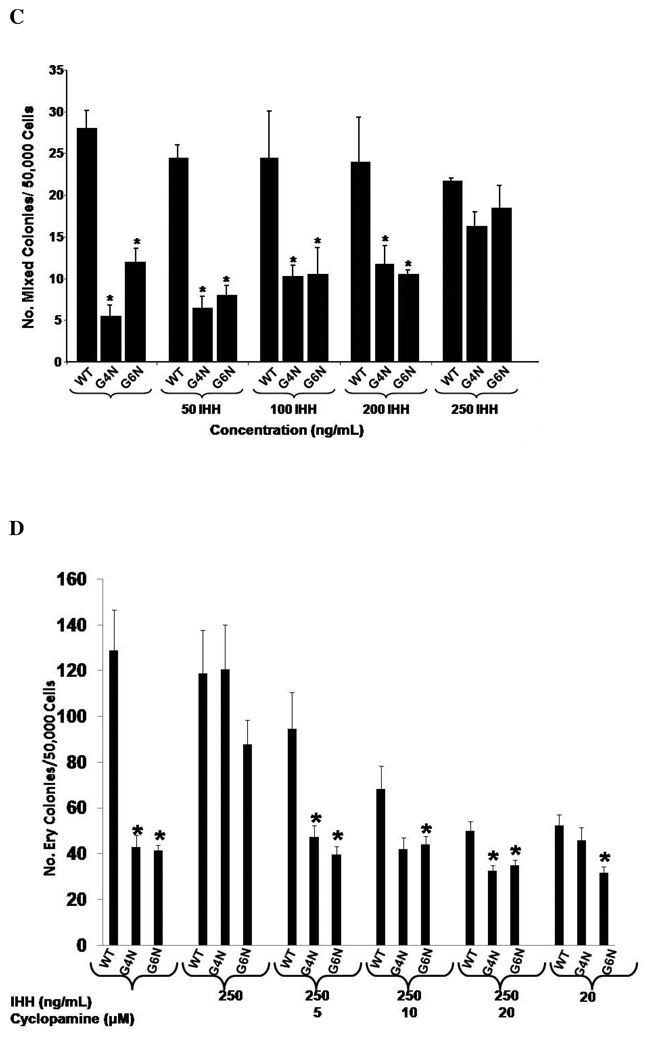
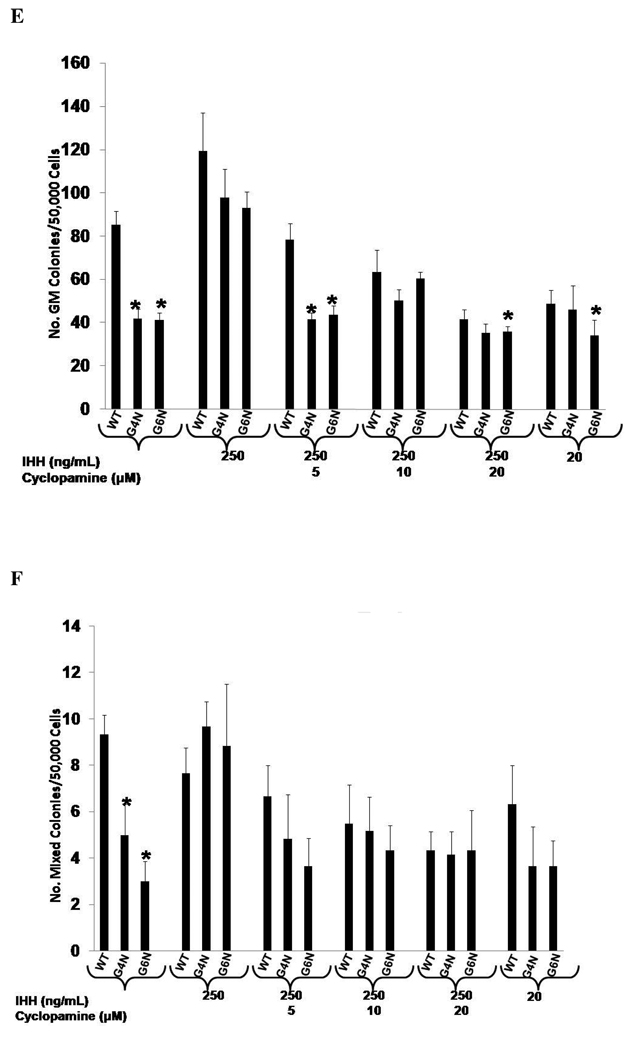
IHH Rescues Definitive Hematopoiesis in G4N and G6N EBs
Previous investigators have demonstrated that IHH, a VE derived factor, also serves as a key factor in blood island formation [9]. In our examination of VE enriched genes, we observed attenuated expression of Ihh in G4N and G6N EBs. Therefore we aimed to determine if exogenous IHH could also recover the reduced levels of definitive hematopoietic progenitors in G4N and G6N EBs. The addition of 250 ng/mL of IHH to EB cultures on differentiation day 6 was sufficient to rescue the impairment in definitive erythroid, granulocyte-macrophage and mixed lineage progenitor colony formation from day 8 G4N and G6N EBs to WT levels (Fig. 8 A, B, C). Next, we aimed to determine if adding cyclopamine, a well known antagonist to hedgehog signaling, could reverse the rescue of definitive hematopoiesis in G4N and G6N EBs noted with treatment of IHH to EB cultures. We noted a dose dependent decrease in definitive hematopoietic progenitor colony formation from WT, G4N and G6N EBs with increasing concentrations of cyclopamine added on differentiation day 6 (Fig. 8 D, E, F). The decrease in the number of definitive progenitor colonies occurred at lower concentrations of cyclopamine in G4N and G6N EBs than in WT EBs. Similar to the VEGF mediated rescue of definitive hematopoiesis in G4N and G6N EBs, these results demonstrate that the IHH mediated rescue of definitive hematopoiesis can be specifically reversed. Moreover, attempts to reverse the VEGF mediated rescue of definitive hematopoiesis with cyclopamine or the IHH mediated rescue of definitive hematopoiesis with Flk-1 Fc in G4N and G6N EBs proved unsuccessful (data not shown). We also observed no synergistic affects on definitive hematopoiesis when VEGF and IHH were added to EB cultures together (data not shown).
Figure 8. IHH specifically rescues definitive hematopoiesis in G4N and G6N EBs.
EBs were cultured for 6 days and IHH (50, 100, 200, 250 ng/ mL) was added to the culture medium. After 2 additional days, day 8 EBs were dissociated into single cells and plated into definitive hematopoietic progenitor colony formation assays. Definitive Erythroid (A), GM (B), and mixed (C) colonies were scored after 7 days. EBs were cultured for 6 days and IHH (250 ng/ml) and cylopamine (5, 10, 20 µM) were added and EB cells were plated after two additional days of culture into definitive hematopoietic progenitor colony forming assays. Colonies were scored as definitive erythroid (D), GM (E), and mixed (F) after 7 days. (N= 3 independent experiments). Error bars represent s.e.m. *p<0.05
Discussion
To determine if the absence of Gata-4 or Gata-6 and the associated loss of a VE covering affect hematopoiesis in EBs, we compared the hematopoietic potential of G4N and G6N EB cells to WT EB cells using progenitor colony assays. We observed a significant diminishment in definitive hematopoiesis on the peak day of detection in G4N and G6N EBs that does not appear to be a cell-autonomous effect since neither Gata-4 nor Gata-6 expression was detected in WT definitive progenitor colonies or in progenitor enriched cell populations. Among several VE genes analyzed, VEGF expression and protein secretion was determined to be decreased in both G4N and G6N EBs. The relevancy of this decrease in VEGF expression and protein secretion in G4N and G6N EBs was demonstrated by the rescue of their diminished definitive hematopoiesis with the addition of exogenous VEGF to EB cultures prior to colony forming assays. FACS analysis of c-Kit+ and c-Kit+/Flk-1+ cell populations of day 8 EB cells confirmed a reduction in the hematopoietic progenitor population in G4N and G6N EBs. The c-Kit+ and c-Kit+/Flk-1+ cell populations increase upon treatment with VEGF and coincide with the observed rescue in the definitive progenitor colonies in G4N and G6N EBs in colony forming assays. We specifically blocked the VEGF mediated rescue of definitive hematopoietic progenitors using soluble Flk-1 Fc. Furthermore, we also observed that G4N and G6N EBs have attenuated Ihh expression. The addition of IHH to EB cultures also successfully rescued definitive hematopoiesis in G4N and G6N EBs; in a specifically reversible manner.
Gata-4−/− mice reportedly die between E7.0 and E10.5 because of the failure of splanchnic mesoderm to migrate to the ventral midline and form the pericardial cavity and heart tube [32, 33]. Gata-6−/− mice die between E6.5 and E7.5 as a result of a block in endoderm differentiation [20]. Cells phenotypically resembling VE have been recognized in both Gata-4 and Gata-6 deficient embryos, although Gata-6 deficient embryos have attenuated or no detectable expression of VE genes [20, 32, 33].
While Gata-4−/− and Gata-6−/− mice both develop yolk sac VE, the opposite has been observed during the differentiation of Gata-4−/− and Gata-6−/− ES cells into EBs [19, 20]. Neither Gata-4−/−nor Gata-6−/− ES cell derived EBs form a VE covering [19, 20]. In both cases, the surface of EBs contains relatively undifferentiated cells lacking phagocytic vacuoles, and microvilli that are characteristic of VE. In addition to a disruption in VE formation, Bielinska et al. [21] observed impaired or disorganized erythropoiesis in G4N EBs; evidenced by scarce and dispersed benzidine staining [21]. Bielinska et al. [21] showed a marked reduction in εy- globin expression in day 7 EBs. They observed no reduction in β-major in EBs from 6 to 7 days of differentiation but noted a modest reduction in β-major at 10 days of differentiation. From these findings, the authors suggested that VE deficiency reduces primitive erythropoiesis more than definitive erythropoiesis [21].
We observed a significant reduction in definitive but not primitive hematopoiesis. The differences in our results and those of Bielinska et al. [21] may be attributed to the different assays used to assess hematopoietic progenitor potential. Bielinska et al. [21] used globin gene expression and benzidine staining to determine hematopoietic progenitor potential, while we used hematopoietic progenitor colony forming assays. Furthermore, the difference in days of EB differentiation used in the analyses may also contribute to the conflicting findings of our studies and that of Bielinska et al. [21]. In kinetic analysis of lineage specification, Keller et al. [12] demonstrated that EryP cells were routinely detected from day 5 to a maximum level at day 7, and then EryP cells declined to undetectable levels by day 10. Definitive hematopoietic progenitor cells, detected as early as day 5 or 6, all reached maximum detection at day 8 followed by a steep decline [12]. We performed EryP colony forming assays using EBs differentiated for 4 to 7 days to span the minimum to maximum range of detection and noted no difference among cell lines tested (data not shown). However, we observed diminished definitive hematopoiesis in EBs at day 8 of differentiation (day of maximum detection of definitive hematopoietic progenitors). When we plated day 9 and day 10 EB cells into progenitor colony forming assays, we saw no difference in the colony forming ability between WT, G4N and G6N EBs. Thus, Bielinska et al. [21] may have underestimated the reduction in definitive hematopoiesis in G4N EBs because of the differentiation days of EB (days 6, 7 and 10) examined in their analysis.
Importantly, we believe that our observed reduction in definitive hematopoiesis is caused by a reduction in hematopoietic supportive factors VEGF and IHH; resulting from the failure of G4N and G6N EBs to form a VE covering. Defective visceral endoderm formation is evidenced by a decrease in DBA positively binding cells in G4N and G6N EBs as well as by a reduction in the expression of visceral endoderm enriched genes. Reduced expression of Hnf-3β and Hnf-4α in G4N and G6N EBs may be the result of GATA-4 and/or GATA-6 indirect regulation of these factors through the known intermediate Fetoprotein transcription factor (FTF); which can be directly regulated by GATA-4 or GATA-6 binding to GATA elements in the FTF promoter [34]. Similarly, reduced Vegf and Ihh expression in G4N and G6N EBs may reflect an indirect regulation of these factors by GATA-4 via a Fibroblast growth factor (Fgf) intermediate [35]. Additionally, the Vegfa gene promoter contains 3 putative Gata binding sites, and in vivo studies have demonstrated that Gata-4 can directly bind the Vegfa gene promoter and regulate cardiomyocyte angiogenesis via induction of Vegf expression [36].
While there have been numerous reports on a requirement of Vegf expression and secretion for proper yolk sac hematopoiesis (particularly in primitive hematopoiesis), its specific contribution to this process in embryos and EBs remains elusive. Vegf+/− mice are embryonic lethal ~E10.5–12 [27, 37]. Among other abnormalities, the blood supply of the yolk sac in Vegf+/− mouse E9.5–11.5 embryos was defective [27]. Vegf is expressed both in yolk sac mesoderm and VE [38]. However, experiments using hypomorphic Vegflo./lo or Vegflo/+ expressing tetraploid embryos (activity presumed to be 50% and 75% of wild-type, respectively) have demonstrated that Vegf expression in mesoderm does not sufficiently promote blood island formation, while a threshold of Vegf expression in VE is necessary and sufficient for this process to occur [26]. Additionally, a dose-dependent relationship between VEGF activity and the enhancement of the expansion and survival of primitive erythrocytes has also been previously demonstrated [30].
We postulate that while there remains an appreciable amount of VEGF expression and protein secretion in both G4N and G6N EBs, the disruption of VE in G4N and G6N EBs is sufficient to significantly reduce the production of VEGF and definitive hematopoietic progenitors. Since neither Gata-4 nor Gata-6 transcripts are detected in hematopoietic progenitor colonies or enriched cell populations, we are able to rule out Gata-4 and Gata-6 transcription factors affecting definitive hematopoietic progenitor cells in a cell autonomous manner. Vegf, however, is known to be endogenously expressed in both endoderm and mesoderm cells and their derivatives (including hematopoietic and endothelial cells) [38]. Consistent with the regulatory mechanism of other hematopoietic related cytokines such as IL-6, IL-3 and GM-CSF [39–41], VEGF has been demonstrated to regulate the survival of hematopoietic stem (HSC) and progenitor cells (HPC) in vivo and in vitro by an internal autocrine loop mechanism [42]. Bone marrow derived Vegf deficient HPCs are unable to form hematopoietic colonies in in vitro progenitor colony forming assays, and HSC/HPC cells fail to repopulate lethally irradiated host mice in transplantation experiments [42]. Although not examined in this study, we presume that autocrine VEGF signaling in hematopoietic progenitors and/or the secretion of VEGF protein from other cells such as definitive endoderm or residual primitive endoderm likely contribute to the hematopoietic program of WT, G4N and G6N EBs.
The findings presented here also highlight the role of IHH in definitive hematopoiesis in EBs given its ability to rescue definitive hematopoietic progenitors in G4N and G6N EBs. The results of this study corroborate in vivo observations of Ihh null mice which appear to have paler yolk sacs than wild-type littermates; indicating fewer hematopoietic cells [43]. Similar to the observed inhibitory effect of cyclopamine on hematopoietic and endothelial gene expression during in vitro cultures of embryos, the addition of cyclopamine to EB cultures in this study not only completely ablated the IHH mediated rescue of definitive hematopoiesis in G4N and G6N EBs, but it also resulted in a decrease in definitive hematopoiesis in WT EBs [44]. Interestingly, hedgehog dependent induction of Vegf expression has been observed in coronary vascular development [35]. However, in the present study, administering cyclopamine in combination with exogenous VEGF or Flk-1 Fc in combination with exogenous IHH did not ablate their rescue of definitive hematopoiesis; suggesting independent functions in this system.
Our finding that a VEGF and IHH mediated signal results in diminished definitive hematopoiesis in VE deficient G4N and G6N EBs does however provide new insights into the overall understanding of the influence of VE on hematopoietic specification in EBs. This is particularly important because the ES/EB system has become a classically utilized in vitro model of embryonic hematopoietic process. Moreover, while many reports have highlighted a role for VE secreted factors in embryonic yolk sac primitive hematopoiesis, this study underscores the importance of VE derived factors VEGF and IHH in definitive hematopoiesis in EBs.
Acknowledgements
This research was supported by the Graduate Assistance in Areas of Need (GAAN), NIH R01 HL063169-05A1S1 and the Riley Children’s Foundation. We would like to thank Rebecca Chan MD/PhD and Yahaira Naaldijk for their technical support. We would also like to thank Tiffany Lewallen, Marilyn Wales and Janice Walls for their help in the preparation of the manuscript. No financial interest/relationships with financial interest relating to the topic of this article have been declared.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 2.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 3.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111:3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabin F. Studies on the origin of blood vessels and of red blood corpuscules as seen in the living blastoderm of chicks during the second day of incubation. Contributions to Embryology. Carnegie Inst. Pub. 1920:214–262. [Google Scholar]
- 5.Murray P. The develpment in vitro of blood of the early chick embryo. Proc Roy Soc. 1932;111:497–521. [Google Scholar]
- 6.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Bielinska M, Narita N, Wilson DB. Distinct roles for visceral endoderm during embryonic mouse development. Int J Dev Biol. 1999;43:183–205. [PubMed] [Google Scholar]
- 8.Palis J, McGrath KE, Kingsley PD. Initiation of hematopoiesis and vasculogenesis in murine yolk sac explants. Blood. 1995;86:156–163. [PubMed] [Google Scholar]
- 9.Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 10.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 11.Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 12.Keller G, Kennedy M, Papayannopoulou T, Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe K, Niwa H, Iwase K, et al. Endoderm-specific gene expression in embryonic stem cells differentiated to embryoid bodies. Exp Cell Res. 1996;229:27–34. doi: 10.1006/excr.1996.0340. [DOI] [PubMed] [Google Scholar]
- 15.Choi D, Lee HJ, Jee S, et al. In vitro differentiation of mouse embryonic stem cells: enrichment of endodermal cells in the embryoid body. Stem Cells. 2005;23:817–827. doi: 10.1634/stemcells.2004-0262. [DOI] [PubMed] [Google Scholar]
- 16.Arceci RJ, King AA, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrisey EE, Ip HS, Lu MM, Parmacek MS. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 18.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 19.Soudais C, Bielinska M, Heikinheimo M, et al. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- 20.Morrisey EE, Tang Z, Sigrist K, et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bielinska M, Narita N, Heikinheimo M, Porter SB, Wilson DB. Erythropoiesis and vasculogenesis in embryoid bodies lacking visceral yolk sac endoderm. Blood. 1996;88:3720–3730. [PubMed] [Google Scholar]
- 22.Fujikura J, Yamato E, Yonemura S, et al. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed] [Google Scholar]
- 24.Noguchi M, Noguchi T, Watanabe M, Muramatsu T. Localization of receptors for Dolichos biflorus agglutinin in early post implantation embryos in mice. J Embryol Exp Morphol. 1982;72:39–52. [PubMed] [Google Scholar]
- 25.Sato M, Muramatsu T. Reactivity of five N-acetylgalactosamine-recognizing lectins with preimplantation embryos, early postimplantation embryos, and teratocarcinoma cells of the mouse. Differentiation. 1985;29:29–38. doi: 10.1111/j.1432-0436.1985.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 26.Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development. 2002;129:1881–1892. doi: 10.1242/dev.129.8.1881. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 28.Farrington SM, Belaoussoff M, Baron MH. Winged-helix, Hedgehog and Bmp genes are differentially expressed in distinct cell layers of the murine yolk sac. Mech Dev. 1997;62:197–211. doi: 10.1016/s0925-4773(97)00664-3. [DOI] [PubMed] [Google Scholar]
- 29.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 30.Martin R, Lahlil R, Damert A, et al. SCL interacts with VEGF to suppress apoptosis at the onset of hematopoiesis. Development. 2004;131:693–702. doi: 10.1242/dev.00968. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa M, Matsuzaki Y, Nishikawa S, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo CT, Morrisey EE, Anandappa R, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 33.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 34.Pare JF, Roy S, Galarneau L, Belanger L. The mouse fetoprotein transcription factor (FTF) gene promoter is regulated by three GATA elements with tandem E box and Nkx motifs, and FTF in turn activates the Hnf3beta, Hnf4alpha, and Hnf1alpha gene promoters. J Biol Chem. 2001;276:13136–13144. doi: 10.1074/jbc.M010737200. [DOI] [PubMed] [Google Scholar]
- 35.Lavine KJ, White AC, Park C, et al. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes Dev. 2006;20:1651–1666. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heineke J, Auger-Messier M, Xu J, et al. Cardiomyocyte GATA4 functions as a stress-responsive regulator of angiogenesis in the murine heart. J Clin Invest. 2007;117:3198–3210. doi: 10.1172/JCI32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 38.Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- 39.Tosato G, Tanner J, Jones KD, Revel M, Pike SE. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990;64:3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 1999;96:12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pech N, Hermine O, Goldwasser E. Further study of internal autocrine regulation of multipotent hematopoietic cells. Blood. 1993;82:1502–1506. [PubMed] [Google Scholar]
- 42.Gerber HP, Malik AK, Solar GP, et al. VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature. 2002;417:954–958. doi: 10.1038/nature00821. [DOI] [PubMed] [Google Scholar]
- 43.Byrd N, Becker S, Maye P, et al. Hedgehog is required for murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- 44.Nagase M, Nagase T, Koshima I, Fujita T. Critical time window of hedgehog-dependent angiogenesis in murine yolk sac. Microvasc Res. 2006;71:85–90. doi: 10.1016/j.mvr.2005.11.006. [DOI] [PubMed] [Google Scholar]