Abstract
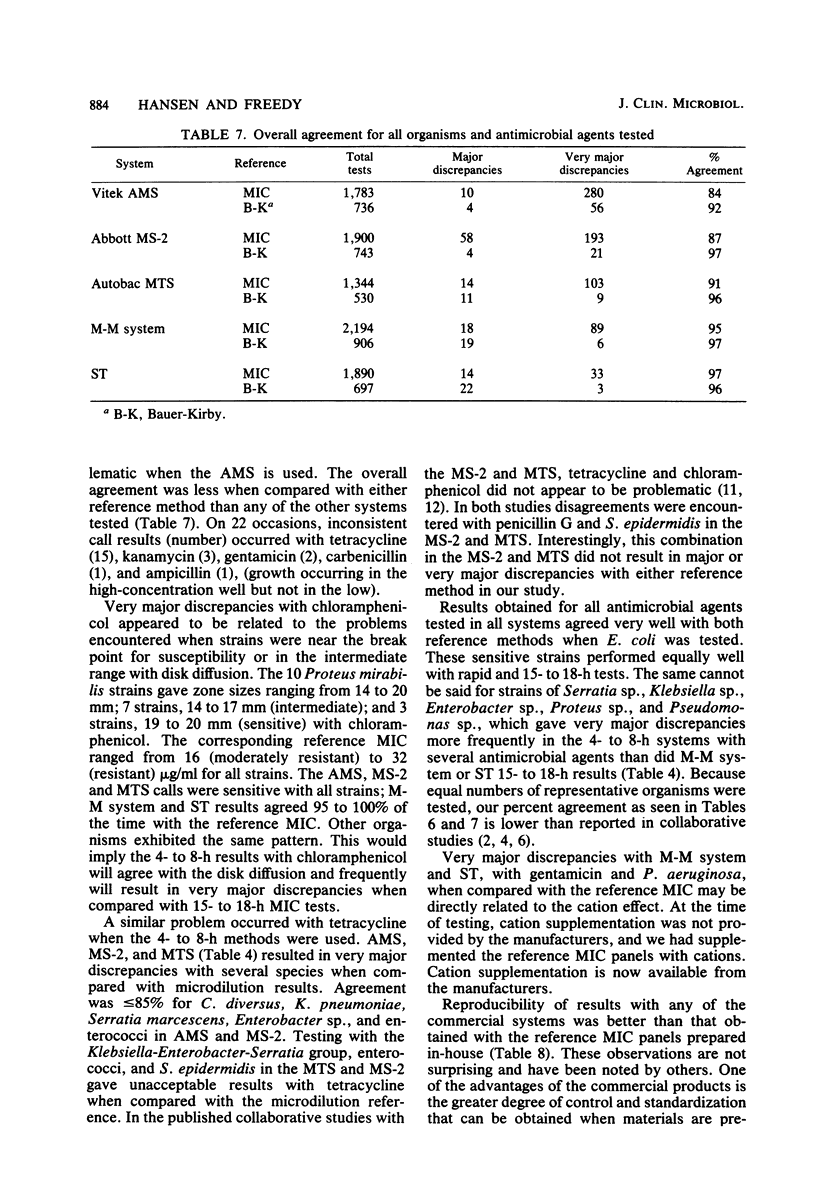
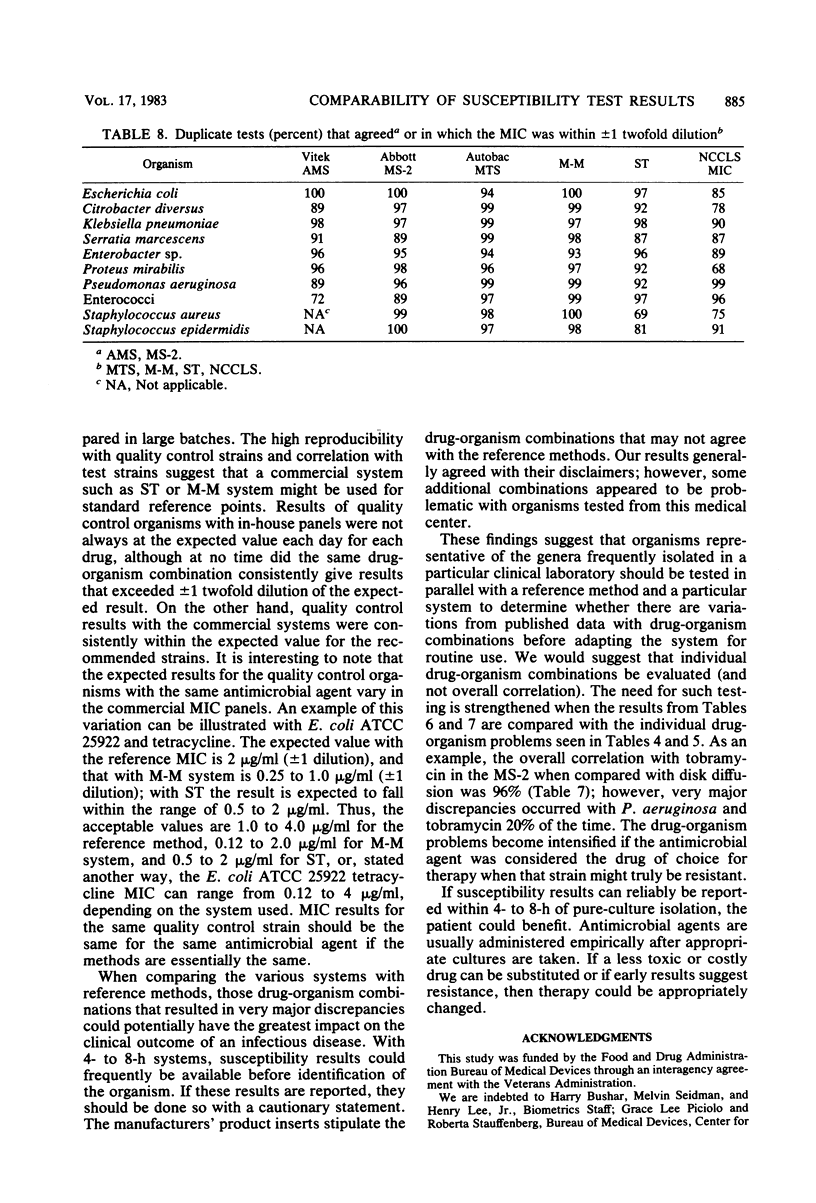
Susceptibility test results from 100 clinical isolates, using the AMS, MS-2, Autobac MTS, Micro-Media system, and Sensititre, were compared with results from the proposed National Committee for Clinical Laboratory Standards reference microdilution method for minimum inhibitory concentrations and with Bauer-Kirby results. Isolates were tested concurrently by each method on consecutive days to obtain duplicate results. The data were computer analyzed, using National Committee for Clinical Laboratory Standards guidelines for break point interpretation. Analysis was centered on drug-organism combinations and not on overall percent correlation. Data were analyzed for comparability to the reference methods and for reproducibility within each system. Commercial system results were very reproducible. Results from 4- to 8-h tests (AMS, MS-2, MTS) gave more very major discrepancies when compared with either reference method than did results from 15- to 18-h systems (Micro-Media, Sensititre).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. G., Green L. R., Talley R. L. Clinical evaluation of automated antibiotic susceptibility testing with the MS-2 system. J Clin Microbiol. 1980 Oct;12(4):527–532. doi: 10.1128/jcm.12.4.527-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Gavan T. L. Evaluation of the micro-media system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1978 Jan;13(1):61–69. doi: 10.1128/aac.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M., White R. L., Bonner M. C., Lockwood W. R. Reliability of the MS-2 system in detecting methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1982 Feb;15(2):220–225. doi: 10.1128/jcm.15.2.220-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L. Evaluation of the Sensititre system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1980 Mar;17(3):464–469. doi: 10.1128/aac.17.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Gavan T. L., Barry A. L. Evaluation of the sensititre microdilution antibiotic susceptibility system against recent clinical isolates: three-laboratory collaborative study. J Clin Microbiol. 1980 Apr;11(4):426–429. doi: 10.1128/jcm.11.4.426-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C., Barry A. L., Gavan T. L. Evaluation of the Sceptor microdilution antibiotic susceptibility testing system: a collaborative investigation. J Clin Microbiol. 1981 Jan;13(1):184–194. doi: 10.1128/jcm.13.1.184-194.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenknecht F. D., Washington J. A., 2nd, Gavan T. L., Thornsberry C. Rapid determination of minimum inhibitory concentrations of antimicrobial agents by the Autobac method: a collaborative study. Antimicrob Agents Chemother. 1980 May;17(5):824–833. doi: 10.1128/aac.17.5.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Anhalt J. P., Washington J. A., 2nd, McCarthy L. R., Schoenknecht F. D., Sherris J. C., Spencer H. J. Clinical laboratory evaluation of the Abbott MS-2 automated antimicrobial susceptibility testing system: report of a collaborative study. J Clin Microbiol. 1980 Sep;12(3):375–390. doi: 10.1128/jcm.12.3.375-390.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


