Abstract
Objective
The therapeutics for Bipolar Disorders (BD) is still far from adequate and new options with improved effectiveness, safety and tolerability in a wide range of patients are necessary. Preliminary data have suggested a role for dysfunctions targeting at the purinergic system in mood disorders. This study aimed to evaluate the efficacy and tolerability of the purinergic agents allopurinol and dipyridamole combined with lithium in bipolar mania.
Method
A randomized, placebo-controlled, double-blind study was performed in adult inpatients (n=180) with a DSM-IV-TR diagnosis of bipolar disorder, current episode manic with or without psychotic features (rapid cyclers and mixed episodes were not included). No antipsychotic agent was used during the study. Subjects were given fixed oral doses of either allopurinol 600 mg/day (n=60), dipyridamole 200 mg/day (n=60), or placebo (n=60) added to lithium for 4 weeks. Subjects were rated at baseline and days 7, 14, 21 and 28 using the Young Mania Rating Scale (YMRS) as the primary efficacy measure. The study was conducted between September 2003 and September 2006.
Results
Allopurinol resulted in greater mean reductions in YMRS scores from baseline to day 21 (p<.001) and day 28 (p=.003) compared with placebo using a linear model analysis (d=0.32, 95% C.I. = 0.07 to 0.57). Remission rates were significantly higher for allopurinol compared to dipyridamole and placebo (p=0.008). Lithium showed a significant antimanic efficacy even in the placebo group. Decrease in plasma uric acid levels showed a significant positive association with antimanic effects in the allopurinol group (p<0.001).
Conclusion
Allopurinol is clinically effective and well-tolerated adjunctively with lithium in manic episodes and may represent an alternative approach in the treatment of acute mania, especially for those presenting tolerability and safety issues with antipsychotics. The present results strongly support the involvement of the purinergic system in the pathophysiology and therapeutics of Bipolar Disorder. Further placebo-controlled studies with allopurinol compared with standard mood-stabilizers in mania and maintenance are warranted.
Keywords: adenosine, allopurinol, purinergic, mania, bipolar disorder, lithium, treatment, trial, purines, depression, uric acid
Introduction
The therapeutics of bipolar mania remain an important challenge in psychiatry. A significant number of patients either fail to respond to or are intolerant of the current therapeutic options1,2. Importantly, despite the wide use of antipsychotic agents in combination with lithium in bipolar mania, concerns about neurological adverse effects, arousal of depressive symptoms, long-term use and lack of adherence have limited their clinical utility, which has pointed out the need for new effective and well-tolerated approaches for this critical condition.
The involvement of purines and uric acid in mania has long been proposed3,4. Kraepelin was the first to describe an association between manic symptoms, uric acid excretion, hyperuricemia, and gout3. Cade also suggested a role for urate in manic behavior and used lithium to keep uric acid more soluble, before discovering its antimanic properties4. It was subsequently noted that remission from manic episodes temporarily coincided with an increased excretion of uric acid5. Similarly, an enhanced purinergic turnover was shown and proposed as a putative causative factor in the pathophysiology of mania4,6,7. Also, genetic data have highlighted a potential role for purinergic dysfunction in the pathophysiology of bipolar disorder and recurrent major depression8,9.
Uric acid is the end-product of purine metabolism. Purines are essential in energy metabolism both intracellularly with ATP as the energetic currency, and in the extracellular space with adenosine and ATP as key regulators of neurotransmission10. Adenosine is a widespread neuromodulator that acts mostly through Adenosine-1 and -2A receptors, and caffeine exerts its stimulating effects by blocking these receptors. According to both treatment guidelines11 and clinical expertise12, caffeine should be used sparingly if at all in bipolar disorder due to its well-known neurovegetative and stimulating effects. In contrast, animal studies show that adenosine agonists exert sedative, anticonvulsant, anti-aggressive, and antipsychotic-like effects13. Despite the paucity of pharmacological agents that clearly target the brain purinergic system (e.g. adenosine receptor agonists), two clinically available drugs, allopurinol (1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) and dipyridamole, have been described to regulate the brain purinergic metabolism. Allopurinol and its active metabolite oxypurinol are inhibitors of xanthine oxidase and have been proposed to present therapeutic effects in many pathological states by decreasing the production of uric acid, superoxide, and hydrogen peroxide14. Dipyridamole is a potent inhibitor of nucleoside uptake by cells, and it increases the extracellular concentrations of both endogenous and exogenous adenosine15,16.
Allopurinol has been shown to be effective as an add-on to different antipsychotic drugs in refractory schizophrenia, mania and aggressive behavior7,17,18,19,20. Previous studies in mania using allopurinol presented a small sample size, permitted other medications, and had a heterogeneous sample, which may limit the interpretation of the findings. Furthermore, allopurinol has been widely used for the treatment of mitochondrial disorders associated with increased oxidative stress levels14,21.
Interestingly, a number of findings suggest the presence of mitochondrial dysfunctions and concomitant changes on oxidative stress parameters has been reported in bipolar disorder22,23, which may indirectly indicate a potential role for allopurinol in bipolar disorder. We hypothesized that these two adenosinergic agents would have significant antimanic effects compared with placebo.
Method
Patient Selection
Men and women, aged 18 to 65 years, who were inpatients with a current diagnosis of bipolar I disorder, current episode manic with or without psychotic features as diagnosed by means of the Structured Clinical Interview for Axis I DSM-IV-TR Disorders, Research Version, Patient Version24 were eligible to participate. All subjects were studied between September 2003 and September 2006 in Porto Alegre and Sta Maria, Brazil. Subjects were required to have a score of greater than or equal to 22 on the Young Mania Rating Scale (YMRS) 25 at screening and at randomization (baseline). All subjects were in good physical health as determined by medical history, physical examination, blood laboratory values, electrocardiogram, chest x-ray, urinalysis and toxicology. Subjects were free of comorbid substance abuse or dependence for at least 5 weeks prior to screen and judged clinically not to be a serious suicide risk. Patients had not taken any psychopharmacologic treatment for at least 4 weeks before admission and had no detectable blood lithium levels when enrolled. Other exclusion criteria included rapid cycling in the past 12 months, current mixed episode, previous history of refractoriness to lithium, current Axis I psychiatric disorder other than bipolar disorder, or current clinically significant abnormal laboratory tests.
The study was approved by the local institutional review board at each study site. All subjects provided written informed consent before entry into the study.
Study Design
This 4-week, randomized, double-blind, placebo-controlled, parallel-group inpatient study compared allopurinol, dipyridamole, and placebo added to lithium in acute mania. One hundred and eighty patients were enrolled at two centers (Espirita Hospital of Porto Alegre [N=170]; and Federal University of Sta Maria, RGS, [n=10]). Before randomization, patients underwent a 2- to 7- day screening period. Subjects who met enrollment criteria were randomly assigned to allopurinol, dipyridamole, or placebo in a 1:1:1 ratio. All subjects and research staff were blinded to randomization codes. Study medications were supplied in identical capsules containing either 600 mg of allopurinol, 200 mg of dipyridamole, or placebo. Allopurinol and dipyridamole were purchased from Glaxo-Smithkline and Boehringer Ingelhein, Brazil.
Patients received fixed dosing of either allopurinol (600 mg/day), dipyridamole (200 mg/day) or placebo as add-on treatment to lithium. A dose of 600 mg/day of allopurinol and 200mg of dipyridamole was chosen based on the safety and tolerability evaluation from our pilot study26. Subjects unable to tolerate the fixed oral dose of allopurinol or dipyridamole were discontinued as no dose reduction was permitted. On the same day that the study medication was started, patients were also started on lithium 600 mg/day, and after 7 days, the dose of lithium was increased to 900 mg/day, and then a flexible dose was applied according to plasma levels. Plasma lithium levels were obtained at days 7, 14, and 21, and lithium doses were adjusted accordingly to achieve a level of 0.6- 1.2 mmol/L. Concomitant use of diazepam (maximum 20mg/day) on an as needed basis for agitation was allowed during the 4-week study period except for the 12 hour period before rating scales.
Assessment and Outcome Measures
Subjects were rated on a weekly basis at baseline and days 7, 14, 21 and 28. The YMRS was the primary outcome measure and the secondary outcome measure was the Clinical Global Impressions-Severity of Illness (CGI-S) scale. The primary efficacy variable was the reduction of the YMRS total score from baseline to endpoint (week 4). Raters were all board certified psychiatrists who displayed an inter-rater reliability of 0.90 for the YMRS. Clinical response was defined as a 50% or greater decrease in the YMRS rating scale from baseline. Remitters were those who had an end of study YMRS of 12 or less. An additional analysis examined remitters with YMRS score of 7 or less at endpoint given that some other studies have used this more stringent criterion. All adverse events were recorded. Switch into depression was evaluated according to DSM-IV-TR criteria for major depressive episode.
Statistical Analyses
The central hypothesis was that allopurinol and dipyridamole would reduce manic symptoms compared to placebo. All analysis was performed with the intent-to-treat sample in which all patients with at least one post-treatment measure were included. The difference between treatment groups was examined with a full factorial linear mixed model with restricted maximum likelihood estimation in which drug and time were fixed effects. Akaike's Information Criterion and Schwarz's Bayesian Criterion were used to determine the best fitting covariance structure. Thus, a first order autoregressive covariance structure was used and the intercept was included in the model. Least significant difference post hoc tests were carried out to examine significant omnibus effects, but Bonferroni corrections were applied to adjust for the number of weeks of active drug by the number of treatments studied. The raw p values are reported and results following correction are noted. For significant interactions, the drugs were compared at each time point to understand the timing of significant differences. Cohen's d with corresponding 95% confidence intervals are reported to show the size of differences at key comparison points. A secondary analysis was conducted with the linear mixed model including diazepam dose as a time dependent covariate in an attempt to determine whether additional medication influenced the drug comparisons. Additionally, psychosis was examined as a factor potentially contributing to response. Chi-square tests were used to compare the proportion of responders and remitters at the end of the study for each of the treatment groups. Number needed to treat (NNT) was calculated for these categorical outcomes using the intent-to-treat values. Demographic factors were examined across groups using analysis of variance for continuous measures and χ2 for categorical measures. Significance was evaluated at p<.05, two-tailed. Means and associated standard deviations are reported. SPSS 15.0.1 (SPSS Inc., Chicago, Ill) was used for this analysis.
Results
Patients
Two-hundred and forty-three subjects were screened yielding 180 subjects for randomization who met DSM-IV TR criteria for bipolar I disorder, current episode manic with or without psychotic features. Sixty-three subjects were excluded because of not meeting inclusion criteria (n=38), patient decision (n=15), unstable medical illness (n=6), or other reason (n=4) (Figure 1). A total of 141 subjects were examined in the primary analysis, which included add-on allopurinol (n=45), dipyridamole (n=50) or placebo (n=46). Subject's demographic and clinical characteristics are summarized in Table 1.
Figure 1. Patient flowchart.
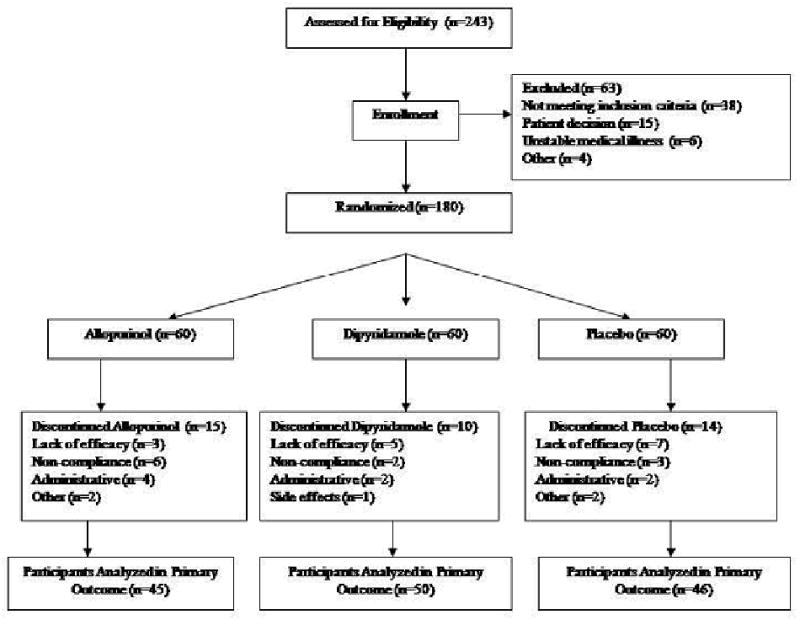
Table 1.
Characteristics of the sample
| Lithium + | ||||
|---|---|---|---|---|
| Characteristic | Allopurinol (N=45) |
Dipyridamole (N=50) |
Placebo (N=46) |
F, p |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age | 26.9 (8.3) | 29.3 (9.8) | 29.3 (8.5) | 1.12, .33 |
| Gender (Female) | 24 (53) | 28 (56) | 29 (63) | 0.94, .62 |
| Duration of Illness (years) | 6.1 (5.3) | 7.9 (8.2) | 7.9 (7.5) | 1.00, .37 |
| All mood episodes | 5.5 (4.2) | 4.7 (3.6) | 4.7 (3.3) | 0.70, .50 |
| Previous manic episodes (lifetime) | 4.0 (3.0) | 3.0 (2.6) | 3.1 (2.5) | 2.15, .12 |
| Hospitalizations | 3.6 (3.6) | 3.2 (3.1) | 3.1 (2.9) | 0.27, .76 |
| N (%) | N (%) | N (%) | χ2, p | |
| Family History of Mood Disorder (1st Degree Relatives) |
19 (42) | 27 (54) | 24 (52) | 1.49, .48 |
| Psychosis | 21 (48) | 22 (45) | 21 (46) | 0.08, .96 |
There were no statistically significant differences in any demographic or illness characteristics between treatment groups. Mean serum lithium levels at the end of the study were similar for the three groups (Table 2).
Table 2.
Side Effects
| Lithium + | |||||
|---|---|---|---|---|---|
| Characteristic | Allopurinol (N=45) | Dipyridamole (N=50) | Placebo (N=46) | Total (N=141) | χ2, p |
| N (%) | N (%) | N (%) | N (%) | ||
| Dizziness | 9 (20) | 15 (31) | 11 (24) | 35 (25) | 1.45, .48 |
| Diarrhea | 11 (24) | 10 (20) | 12 (26) | 33 (23) | 0.45, .80 |
| Nausea | 6 (13) | 12 (25) | 9 (20) | 27 (19) | 1.88, .39 |
| Somnolence | 7 (16) | 9 (18) | 3 (7) | 23 (16) | 3.06, .22 |
| Headache | 2 (4) | 9 (18) | 8 (17) | 19 (13) | 4.73, .09 |
| Dry skin/irritation | 4 (9) | 7 (14) | 5 (11) | 16 (11) | 0.70, .71 |
| Weight Loss | 4 (9) | 5 (10) | 4 (9) | 13 (9) | 0.08, .96 |
| Polyuria | 3 (7) | 6 (12) | 2 (4) | 11 (8) | 2.17, .34 |
| Vomiting | 6 (13) | 3 (6) | 1 (2) | 10 (7) | 4.39, .11 |
| Tremors | 2 (4) | 3 (6) | 5 (11) | 10 (7) | 1.53, .46 |
| Asthenia | 3 (7) | 1 (2) | 4 (9) | 8 (6) | 2.06, .36 |
| Myalgia | 3 (7) | 0 (0) | 4 (9) | 7 (5) | 4.17, .13 |
| Insomnia | 2 (4) | 2 (4) | 2 (4) | 6 (4) | 0.01, .99 |
| Libido Loss | 2 (4) | 3 (6) | 0 (0) | 5 (4) | 2.73, .26 |
| Ataxia | 2 (4) | 1 (2) | 2 (4) | 5 (4) | 0.51, .77 |
| Diplopia | 2 (4) | 1 (2) | 1 (2) | 4 (3) | 0.60, .74 |
| Minor Skin Reaction | 2 (4) | 1 (2) | 1 (2) | 4 (3) | 0.62, .73 |
| Irritability | 2 (4) | 1 (2) | 0 (0) | 3 (2) | 2.15, .34 |
| Sexual Dysfunction | 1 (2) | 1 (2) | 0 (0) | 2 (1) | 1.00, .61 |
| Appetite Increase | 0 (0) | 2 (4) | 0 (0) | 2 (1) | 3.77, .15 |
| Weight Gain | 0 (0) | 1 (2) | 1 (2) | 2 (1) | 0.96, .62 |
| Anxiety | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 2.15, .34 |
| Weakness | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 2.15, .34 |
| Arthritis Pain | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 2.15, .34 |
| Hypertension | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 2.15, .34 |
| Dystonia | 0 (0) | 1 (2) | 0 (0) | 1 (1) | 1.87, .39 |
| Galactorrhea | 0 (0) | 1 (2) | 0 (0) | 1 (1) | 1.87, .39 |
| Muscular Pain | 0 (0) | 1 (2) | 0 (0) | 1 (1) | 1.83, .40 |
| Severe Skin Rash | 0 (0) | 1 (2) | 0 (0) | 1 (1) | 1.83, .40 |
Completion rates for the drug groups were not significantly different from placebo (allopurinol: 38 of 45 [84%]; dipyridamole: 41 of 50 [82%]; and placebo: 31 of 46 [67%]) (χ2=4.57, df=2, p = .10).
Efficacy
The linear mixed model for the YMRS showed a significant interaction between time and drug (F=2.78, df=8,471.9, p=.005) as well as a significant main effect for time (F=166.07, df=4,468.2, p<.0001). The drug main effect was not significant (F=2.20, df=2,148.3, p=.11). Figure 2 shows the estimated marginal means following restricted maximum likelihood estimation from the linear mixed model. At the end of the 4-week study, improvement in manic symptoms was significantly greater for allopurinol-treated patients. Post hoc tests show significantly lower YMRS total scores in the allopurinol group compared with the placebo group at week 3 (p<.001) and week 4 (p=.003) (d=0.32, 95% C.I. = 0.07 to 0.57), even after Bonferroni correction. The allopurinol group presented lower scores than the dipyridamole group at week 3 (p=.048) and week 4 (p=.009) (d=0.29, 95% C.I. = 0.02 to 0.59). The dipyridamole group showed no significant difference in YMRS scores from placebo at any time point.
Fig 2. Allopurinol significantly improved manic symptoms compared to placebo and dipyridamole groups at weeks 3 and 4.
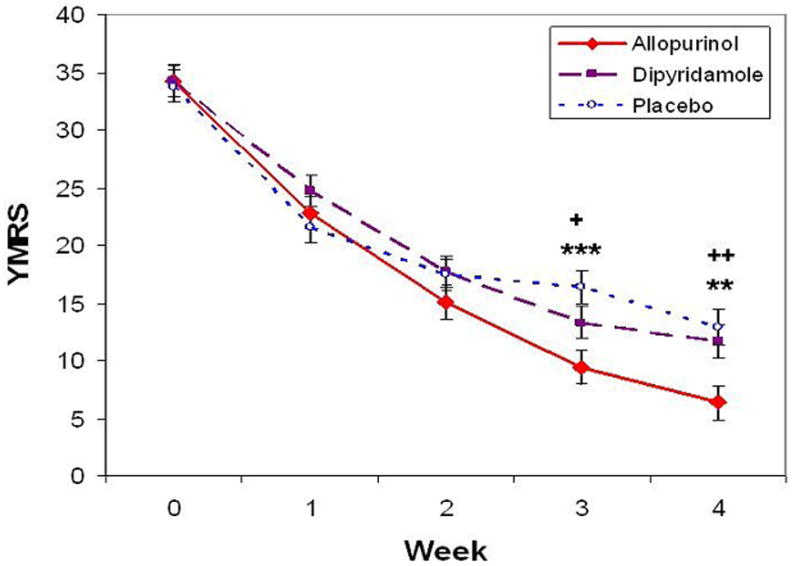
Allopurinol vs. Placebo:** p<.01, *** p<.001
Allopurinol vs. Dipyridamole:+ p<.05, ++ p<.01
A linear mixed model with CGI-S severity scores showed a significant interaction between drug and time (F=4.95, df=8,464.4, p<.001) and significant main effects for drug (F=5.76, df=2,158.9, p=.004) and time (F=184.73, df=4,464.4, p<.001). The group receiving allopurinol had significantly lower scores compared with the placebo group from weeks 2 through 4 (p<.001) (week 4: d=0.44, 95% C.I. = 0.23 to 0.64) and compared with the dipyridamole group for week 4 (p=.005) (d=0.29, 95% C.I. = 0.09 to 0.49). The dipyridamole group had lower scores than the placebo group at weeks 2 and 3 (p<.001), but not at week 4 (p=.13).
Current psychosis was examined as an additional factor that might be contributing to changes over time. However, the models for the YMRS and CGI-S showed no significant interactions with psychosis (YMRS: psychosis × time, F=0.46, df=4,450.0, p=.77; psychosis × drug, F=0.09, df=2,142.7, p=.91, psychosis × time × drug, F=0.67, df=8,450.8, p=.72) (CGI: psychosis × time, F=0.10, df=4,446.2, p=.98; psychosis × drug, F=0.32, df=2,152.4, p=.73, psychosis × time × drug, F=1.07, df=8,446.4, p=.38) and no significant main effects of psychosis (YMRS: F=0.41, df=1,142.7, p=.52; CGI: F=0.64, df=1,152.4, p=.43). Thus, psychosis status did not influence the drug effects over time. The use of diazepam over the course of the study was examined with a linear mixed model. The dose decreased significantly over the course of the trial (F=20.76, df=3,381.4, p<.0001), but the increases did not differ by group (Drug: F=0.92, df=2,150.6, p=.40; Time × Drug: F=1.56, df=6,381.4, p=.16). Given the decreases in dose, diazepam was used as a covariate in a secondary analysis of the YMRS total score. This did not take away the significant interaction between drug and time (F=2.44, df=8,457.2, p=.01). Intention-to-treat remission rates for which remission is counted at the last observed timepoint were significantly different among the three groups after 28 days with a more liberal (YMRS score <12) (p=.008) or more conservative (YMRS score <7) (p=.03) definition of remission. The allopurinol group had a higher remission rate (YMRS score <12, p=.002; YMRS score <7, p=.008) than the other two groups, which were not different (YMRS score <12, p=.87; YMRS acore <7, p=.78). Intention-to-treat mania response rates were higher in the allopurinol group, but this was not quite significant after 28 days (p=.06) (see Table 3).
Table 3.
Efficacy Measures and Treatment Characteristics
| Lithium + | ||||
|---|---|---|---|---|
| Characteristic | Allopurinol (N=45) | Dipyridamole (N=50) | Placebo (N=46) | F, p |
| Mean (SE) | Mean (SE) | Mean (SE) | ||
| YMRS | ||||
| Baseline | 34.2 (1.4) | 34.2 (1.3) | 33.8 (1.4) | 0.03, .96 |
| 1 Week | 22.8 (1.4) | 24.7 (1.3) | 21.6 (1.4) | 1.38, .25 |
| 2 Weeks | 15.1 (1.4) | 17.7 (1.3) | 17.5 (1.4) | 1.09, .34 |
| 3 Weeks | 9.3 (1.4) | 13.3 (1.4) | 16.4 (1.4) | 6.04, .003 |
| 4 Weeks | 6.3 (1.5) | 11.7 (1.4) | 12.8 (1.6) | 5.39, .005 |
| CGI Severity | ||||
| Baseline | 5.4 (0.2) | 5.2 (0.2) | 5.1 (0.2) | 0.83, .84 |
| 1 Week | 4.2 (0.2) | 4.3 (0.2) | 4.2 (0.2) | 0.30, .74 |
| 2 Weeks | 2.9 (0.2) | 3.2 (0.2) | 3.8 (0.2) | 7.03, .001 |
| 3 Weeks | 1.8 (0.2) | 2.1 (0.2) | 3.0 (0.2) | 13.85, <.001 |
| 4 Weeks | 1.4 (0.2) | 2.0 (0.2) | 2.4 (0.2) | 9.06, <.001 |
| Diazepam | ||||
| 1 Week | 46.7 (4.8) | 50.8 (4.6) | 49.1 (4.8) | 0.20, .82 |
| 2 Weeks | 39.6 (4.8) | 44.8 (4.6) | 45.0 (4.8) | 0.42, .66 |
| 3 Weeks | 28.9 (4.9) | 41.2 (4.7) | 45.5 (4.8) | 3.12, .046 |
| 4 Weeks | 16.3 (5.1) | 28.0 (4.8) | 36.1 (5.0) | 3.92, .02 |
| Uric Acid | ||||
| Baseline | 4.8 (0.2) | 4.7 (0.2) | 4.7 (0.2) | 0.11, .89 |
| Week 4 | 3.3 (0.2) | 4.7 (0.2) | 4.4 (0.2) | 16.93, <.001 |
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Serum Lithium | 0.98 (0.20) | 0.99 (0.19) | 0.95 (0.20) | 0.50, .61 |
| N (%) | N (%) | N (%) | χ2, p | |
| Study Completion | 38 (84) | 41 (82) | 31 (67) | 4.57, .10 |
| Response | ||||
| 3 Weeks | 36 (90) | 30 (70) | 22 (55) | 12.14, .002 |
| 4 Weeks | 35 (92) | 30 (73) | 23 (74) | 5.33, .07 |
| End | 37 (82) | 31 (62) | 29 (63) | 5.56, .06 |
| Remission | ||||
| YMRS < 12 | ||||
| 3 Weeks | 32 (80) | 28 (65) | 15 (38) | 15.66, .0004 |
| 4 Weeks | 34 (90) | 27 (66) | 20 (65) | 7.52, .02 |
| End | 37 (82) | 28 (56) | 25 (54) | 9.71, .008 |
| YMRS < 7 | ||||
| 3 Weeks | 21 (53) | 14 (33) | 9 (23) | 8.13, .02 |
| 4 Weeks | 27 (71) | 21 (51) | 14 (45) | 5.36, .07 |
| End | 29 (64) | 21 (42) | 18 (39) | 7.04, .03 |
The NNT for the allopurinol group compared to the placebo group was 5.2 for response rate, 3.6 for the more liberal remission rate, and 4.0 for the more conservative remission rate. Compared with the dipyridamole group, the NNT for the allopurinol group was 4.9 for response rate, 3.8 for the more liberal remission rate, and 4.5 for the more conservative remission rate.
Additional models were run in the same manner as the total YMRS for the individual items to identify specific areas for which allopurinol may be helpful to patients. All symptoms were significantly improved over time (p<.001). Significant time by drug interactions were found for items 1 (Elevated Mood) (F=3.52, df=8,466.0, p<.001), 2 (Increased Motor Activity or Energy) (F=2.14, df=8,475.5, p=.03), 8 (Thought Content) (F=2.03, df=8,478.0, p=.04), and 11 (lack of Insight) (F=3.31, df=8,483.9, p=.001). At the end of the study, the allopurinol group had significantly lower scores on elevated mood compared to placebo and significantly lower scores on thought content and lack of insight compared with placebo and dipyridamole. A significant main effect for drug was present for symptom 7 (Language or Thought Disorder) (F=3.56, df=2,164.4, p=.03). This reflected generally higher scores for the dipyridamole group compared with the allopurinol group, but these differences were stronger toward the end of the study, not at baseline.
There was no statistically significant difference between treatment groups in the incidence rates of clinically relevant changes in vital signs and laboratory measures, except for uric acid levels. Uric acid levels were measured at baseline and after 3 weeks of treatment. The linear mixed model showed a significant interaction between treatment group and time (F=21.40, df=2,123.1, p<.001) as well as significant main effects for treatment (F=5.00, df=2,135.3, p=.008) and time (F=29.36, df=1,123.1, p<.001). Post hoc tests showed significantly lower levels in the allopurinol group at week 3 compared with the dipyridamole (p<.001) and placebo groups (p<.001). Only the allopurinol group had a significant decrease in uric acid levels compared with baseline (p<.001).
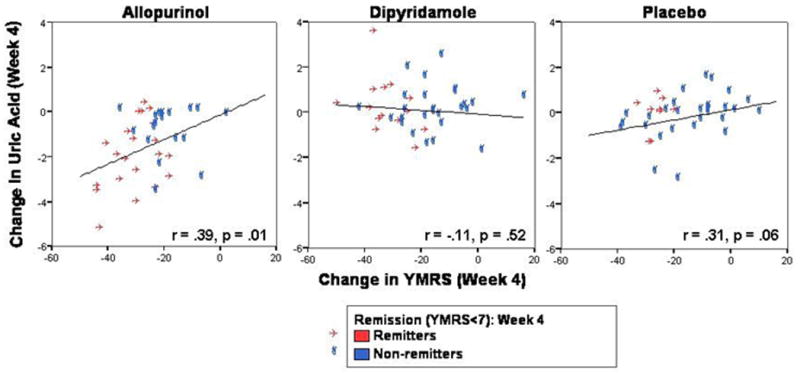
The correlation between the absolute changes in YMRS scores and uric acid levels from baseline to endpoint was significant for the entire sample (r=.25, p=.007). The correlation was significant for the allopurinol group (r=.39, p=.01), a trend for the placebo group (r=.31, p=.06), but non-significant for the dipyridamole group (r=-.11, p=.52) (Figure 3). Thus, improvement in mania was associated with decreasing uric acid levels for the lithium plus allopurinol or placebo groups. For the entire sample, the change in uric acid level was positively related to changes in several YMRS items: increased motor activity and energy (r=.21, p=.02), language or thought disorder (r=.24, p=.009), thought content (r=.22, p=.02), and lack of insight (r=.24, p=.01). Lastly, switch into depression was observed in 7.8% of subjects, with no difference between groups (p=.09) (Table 2).
Figure 3. A positive association between the antimanic efficacy of Allopurinol and plasma uric acid levels and a trend in the Placebo group was observed.
Predictors
Age, duration of illness, episodes, hospitalizations, prior medication usage, psychosis, sex, and baseline uric acid levels were correlated to the absolute change in YMRS score. Only sex and prior use of lithium were related to response. Women had better response rates than males (r=.31, p<.001) and patients with prior lithium usage responded better than others (r=.20, p=.01). For the allopurinol group alone, more hospitalizations (r=-.34, p=.04) and more episodes (r=-.38, p=.02) were related to better response. No predictors were related to dipyridamole response. For the lithium plus placebo group, women had better response rates than men (r=.42, p=.02).
Adverse Effects
Overall, lithium, allopurinol, and dipyridamole were well tolerated. The most common side effects were dizziness (25%) and diarrhea (23%). The treatment groups were not significantly different on any other side effects (Table 2). Only one patient (in the dipyridamole) presented a severe adverse effect (skin rash).
Discussion
This is the first double-blind controlled study showing therapeutic effects of allopurinol in bipolar mania combined with lithium monotherapy. Allopurinol was significantly more effective than dipyridamole and placebo in reducing manic symptoms and was also well- tolerated. Antimanic effects induced by allopurinol showed a significant association with uric acid levels, suggesting a role for purinergic system dysfunction in mania. These data confirm our preliminary results showing the efficacy of allopurinol combined with lithium monotherapy in 90 manic subjects26. Subsequently, the antimanic efficacy of allopurinol as adjunct therapy to haloperidol plus lithium was also shown19.
Double-blind studies have shown the superior efficacy of lithium compared with placebo as monotherapy for mania, but many patients persist with manic symptoms28. First-generation antipsychotics are still a widely used option as add-on treatment to mood stabilizers in mania29. Despite their efficacy, concern about adverse effects with these agents (particularly extrapyramidal symptoms and tardive dyskinesia) has limited their use with standard mood stabilizers in bipolar mania. Also, typical antipsychotics commonly induce or worsen depressive symptoms of bipolar disorder30. Likewise, despite a clear superiority and positive effects over typical antipsychotics, atypical antipsychotics have also been associated with weight gain and metabolic disturbances30. Different from second-generation antipsychotics, allopurinol showed no weight gain or metabolic dysfunction. Finally, allopurinol was clearly superior to placebo, but differently from some antipsychotics, separation occurred not earlier than week 2. Given the absence of sedative effects and good tolerability, a higher dose of allopurinol may be useful to accelerate its onset of action.
This study had important methodological strengths and limitations. This was a prospective, placebo-controlled randomized trial and enrolled a relatively large sample of subjects with manic episode. Also, none of the patients were treated with any typical or atypical antipsychotics during the follow-up period, which based on their potential antimanic effects, could produce a decreased effect size when evaluating the therapeutic effectiveness of allopurinol and dipyridamole.
Although we used benzodiazepines in this study which could have some effects in reducing manic symptoms, controlling for benzodiazepine use did not change our results. We excluded patients with mixed episodes, rapid cycling and comorbidity with substance abuse (potential factors predictive of non-response to lithium), therefore limiting the generalization of the present data. In contrast to most of the data available on lithium efficacy in mania (mostly using antipsychotic drugs in manic patients poorly responsive to lithium), this sample included patients who were potential responders to lithium and, thus, the high response and remission rates observed even in the placebo group clearly show a significant antimanic efficacy of lithium in the absence of rapid cycling/mixed episode and/or drug use.
An additional limitation of this study was not including depression rating scales. We decided not to include scales for measuring depression as we were specifically interested in the antimanic effects of purinergic agents. For that reason, we also excluded patients with mixed episodes; subjects with these features are at a greater risk of cycling into depression31. It could be argued that although allopurinol was effective in acute manic episodes, depression symptoms could have worsened. We did not find this to be the case; there was no difference in the number of subjects meeting DSM-IV criteria for major depressive episode at the end of the study. Future studies would need to include patients with these characteristics. Finally, our findings apply only to acute mania; additional trials would need to be conducted to examine the efficacy and safety of allopurinol during the continuation and maintenance phases of treatment of bipolar disorder.
The putative mechanisms involved in the therapeutic effects of allopurinol in bipolar mania may include activation of adenosinergic activity, mostly through A14. Adenosine inhibits the release of glutamate, dopamine and noradrenaline16. Xanthine and hypoxanthine, the precursors of uric acid, accumulate and can be salvaged by the enzyme hypoxanthine-guanine-phosphorybosil-transferase, producing the nucleosides adenosine, guanosine, and inosine, which have shown to possess neuromodulatory actions, most of them inhibitory4. Similar to diverse mood stabilizers, allopurinol has also presented anticonvulsant properties as add-on therapy in refractory epilepsy33,34. Allopurinol also has neuroprotective effects presumably by limiting the production of free radicals leading to a decreased activity of superoxide dismutase14, an enzyme for which levels have been shown to increase during manic episodes22.
Here we observed a positive association between the antimanic efficacy of allopurinol and plasma uric acid levels and a trend in the placebo group. Previous studies have described a direct association of elevated uric acid levels with increased locomotor activity and refractory mania7,33. In addition, Anumonye et al5 (1968) described a direct association between increased excretion of uric acid and remission of manic episodes. Also, lithium might exert antimanic effects targeting the uric acid metabolism per se, which was its main indication before used in BD. These data reinforce a role for purines in the pathophysiology and therapeutics of mania. Dipyridamole failed to show antimanic efficacy in the present study. One possibility for its lack of efficacy is that dipyridamole may not reliably cross the bloodbrain- barrier34. Overall, allopurinol was shown to be an efficacious and well-tolerated antimanic agent when combined with lithium. The present study confirmed our preliminary data and supports the purinergic dysfunction model in mania4. Further studies with allopurinol in acute mania and maintenance in bipolar disorder are warranted.
Acknowledgments
This study was supported by Stanley Medical Research Institute (Bethesda, M.D.)-Grant Award 03T-356- BD.
Footnotes
Presented in the Seventh International Conference on Bipolar Disorder, Jun.7-9, 2007, Pittsburgh, P.A.
Trial Registration: clinicaltrials.gov Identifier: NCT00560079
The authors report no other financial affiliations relevant to the subject of this article.
References
- 1.Keck PE., Jr The management of acute mania. Bmj. 2003;327(7422):1002–3. doi: 10.1136/bmj.327.7422.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gitlin M. Treatment-resistant bipolar disorder. Mol Psychiatry. 2006;11(3):227–40. doi: 10.1038/sj.mp.4001793. [DOI] [PubMed] [Google Scholar]
- 3.Kraepelin E. Manic-Depressive Insanity and Paranoia. Edinburgh, Scotland: E & S Livingstone; 1921. 1921. [Google Scholar]
- 4.Machado-Vieira R, Lara DR, et al. Purinergic dysfunction in mania: an integrative model. Med Hypotheses. 2002;58(4):297–304. doi: 10.1054/mehy.2001.1543. [DOI] [PubMed] [Google Scholar]
- 5.Anumonye A, Reading HW, et al. Uric-acid metabolism in manicdepressive illness and during lithium therapy. Lancet. 1968;1(7555):1290–3. doi: 10.1016/s0140-6736(68)92300-3. [DOI] [PubMed] [Google Scholar]
- 6.Brooks SC, Linn JJ, et al. Serotonin, folic acid, and uric acid metabolism in the diagnosis of neuropsychiatric disorders. Biol Psychiatry. 1978;13(6):671–84. [PubMed] [Google Scholar]
- 7.Machado-Vieira R, Lara DR, et al. Therapeutic efficacy of allopurinol in mania associated with hyperuricemia. J Clin Psychopharmacol. 2001;21(6):621–2. doi: 10.1097/00004714-200112000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Barden N, Harvey M, et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141(4):374–82. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- 9.Lucae S, Salyakina D, et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15(16):2438–45. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27(3):166–76. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Yatham LN, Kennedy SH, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: consensus and controversies. Bipolar Disord. 2005;7 Supp 3:5–69. doi: 10.1111/j.1399-5618.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 12.Kilzieh N, Akiskal HS. Rapid-cycling bipolar disorder. An overview of research and clinical experience. Psychiatr Clin North Am. 1999;22(3):585–607. doi: 10.1016/s0193-953x(05)70097-6. [DOI] [PubMed] [Google Scholar]
- 13.Lara DR, Dall'Igna OP, et al. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):617–29. doi: 10.1016/j.pnpbp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Pacher P, Nivorozhkin A, et al. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daly JW. Adenosine receptors: targets for future drugs. J Med Chem. 1982;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB, Chen JF, et al. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 17.Brunstein M, Ghisolfi ES, et al. A clinical trial of adjuvant allopurinol therapy for moderately refractory schizophrenia. J Clin Psychiatry. 2005;66(2):213–9. doi: 10.4088/jcp.v66n0209. [DOI] [PubMed] [Google Scholar]
- 18.Akhondzadeh S, Safarcherati A, et al. Beneficial antipsychotic effects of allopurinol as add-on therapy for schizophrenia: a double blind, randomized and placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):253–9. doi: 10.1016/j.pnpbp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Akhondzadeh S, Milajerdi, et al. Allopurinol as an adjunct to lithium and haloperidol for treatment of patients with acute mania: a double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2006;8:485–9. doi: 10.1111/j.1399-5618.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- 20.Lara DR, Belmonte-de-Abreu P, et al. Allopurinol for refractory aggression and self-inflicted behaviour. J Psychopharmacol. 2000;14(1):81–3. doi: 10.1177/026988110001400112. [DOI] [PubMed] [Google Scholar]
- 21.Sanganahalli BG, Joshi PG, et al. Xanthine oxidase, nitric oxide synthase and phospholipase A(2) produce reactive oxygen species via mitochondria. Brain Res. 2005;1037(12):200–3. doi: 10.1016/j.brainres.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Machado-Vieira R, Andreazza AC, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: A possible role for lithium antioxidant effects. Neurosci Lett. 2007;421(1):33–6. doi: 10.1016/j.neulet.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Kato T, Kato N. Mitochondrial dysfunction in bipolar disorder. Bipolar Disord. 2000;2(3 Pt 1):180–90. doi: 10.1034/j.1399-5618.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 25.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity, and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 26.Machado-Vieira R, Kapczinski F, et al. A double-blind controlled study of adjunctive allopurinol or dipyridamole to lithium in the treatment of bipolar mania: preliminary results. Bipolar Disorders. 2005;7(Suppl 2):71. [Google Scholar]
- 27.Bowden CL, Brugger AM, et al. Efficacy of divalproex vs lithium and placebo in the treatment of mania. The Depakote Mania Study Group. JAMA. 1994;271:918–924. [PubMed] [Google Scholar]; 27 Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health; 1976. pp. 218–222. [Google Scholar]
- 28.Letmaier M, Schreinzer D, et al. Drug therapy of acute manias. A retrospective data analysis of inpatients from 1997 to 1999. Nervenarzt. 2004;75(3):249–57. doi: 10.1007/s00115-003-1554-x. [DOI] [PubMed] [Google Scholar]
- 29.Zarate CA, Tohen M. Double blind comparison of the continued use fo antipsychotic treatment versus its discontinuation in remitted manic patients. Am J Psychiatry. 2004;161:169–171. doi: 10.1176/appi.ajp.161.1.169. [DOI] [PubMed] [Google Scholar]
- 30.Newcomer JW. Metabolic considerations in the use of antipsychotic medications: a review of recent evidence. J Clin Psychiatry. 2007;68 Supp 1:20–7. [PubMed] [Google Scholar]
- 31.Zarate CA, Tohen M, et al. Cycling into depression from a first episode of mania: a case-comparison study. Am J Psychiatry. 2001;158:1524–6. doi: 10.1176/appi.ajp.158.9.1524. [DOI] [PubMed] [Google Scholar]
- 32.Zagnoni PG, Bianchi A, et al. Allopurinol as add-on therapy in refractory epilepsy: a double-blind placebo-controlled randomized study. Epilepsia. 1994;35(1):107–12. doi: 10.1111/j.1528-1157.1994.tb02919.x. [DOI] [PubMed] [Google Scholar]
- 33.Togha M, Akhondzadeh S, et al. Allopurinol as adjunctive therapy in intractable epilepsy: a double-blind and placebo-controlled trial. Arch Med Res. 2007;38(3):313–6. doi: 10.1016/j.arcmed.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Barrera CM, Hunter RE, et al. Hyperuricemia and locomotor activity in developing rats. Pharmacol Biochem Behav. 1989;33(2):367–9. doi: 10.1016/0091-3057(89)90515-7. [DOI] [PubMed] [Google Scholar]


