Abstract
Background
A high rate of comorbidity exists between mood disorders and alcohol dependence. Furthermore, both ketamine, a dissociative anesthetic with a recently described rapid-onset antidepressant effect, and ethanol are N-methyl-D-aspartate (NMDA) receptor antagonists. Previous investigations of healthy individuals with a family history of alcohol-dependence have found that these individuals have an attenuated response to ketamine's perceptual disturbance and dysphoric effects similar to that found in individuals with a self-reported history of alcohol dependence. This study investigated whether a family history of alcohol dependence influences ketamine's initial antidepressant effect.
Methods
Twenty-six subjects with DSM-IV treatment-resistant major depression were given an open-label intravenous infusion of ketamine hydrochloride (0.5 mg/kg) and rated using various depression scales at baseline, 40, 80, 120, and 230 minutes post-infusion. The primary outcome measure was Montgomery-Asberg Depression Rating Scale (MADRS) scores.
Results
Subjects with a family history of alcohol dependence showed significantly greater improvement in MADRS scores compared with subjects who had no family history of alcohol dependence.
Conclusions
A family history of alcohol dependence appears to predict a rapid initial antidepressant response to an NMDA receptor antagonist.
Keywords: alcohol dependence, depression, family history, glutamate, ketamine, NMDA receptor
Introduction
The lifetime prevalence of substance abuse disorders in persons suffering from major depression is estimated to range between 27 and 40% (1). Delayed recovery, persistent inter-episode symptomatology, and increased disability and mortality result from the combined impact of these diseases, and warrants a thorough investigation of their biological connection (2, 3).
In recent years, investigations of the pathophysiology of mood disorders and risk for alcoholism have focused on the glutamate system, the primary excitatory neurotransmitter in the mammalian brain (4). Of particular interest, the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors has emerged as a key biological target for therapeutics (4). In animal models, treatment with ketamine, an NMDA antagonist, results in behavioral effects similar to those seen after chronic antidepressant administration (5). Furthermore, in clinical studies of individuals with treatment-resistant major depression, administration of ketamine produced rapid and relatively sustained antidepressant effects (6, 7).
Studies of NMDA antagonists also highlight the role of the glutamate system in the signal for ethanol intoxication (8, 9). Ethanol binds with high affinity to the NMDA receptor, and administration of NMDA receptor antagonists mimics the subjective effects of ethanol intoxication (9, 10). Ethanol-dependent individuals show marked reductions to the subjective intoxicating effects of ketamine compared with healthy controls (9). More compellingly, in family history studies where healthy individuals with a positive family history of alcohol dependence were administered ketamine, these individuals displayed fewer perceptual alterations and lower dysphoric mood than those without a similar family history (11).
Thus, family history of ethanol-dependence predicts a different response to ketamine's perceptual disturbance and dysphoric effects in healthy individuals; however, the link between family history of alcohol-dependence and ketamine's antidepressant properties has not yet been examined. This study sought to determine whether antidepressant response to ketamine is altered in individuals with major depression with a positive family history of alcohol-dependence (FHP).
Methods and Materials
Twenty-six patients aged 18 to 65 years old participated in this study between October 2006 and May 2008. Participants fulfilled DSM-IV criteria for major depressive disorder and had no diagnosis of alcohol or substance abuse or dependence in the past 90 days, as determined by the Structured Clinical Interview for Diagnosis, DSM-IV (SCID). All patients were drug-free, in good health, and unmedicated for at least two weeks prior to the ketamine infusion, as determined by medical history, physical examination, routine blood labs, electrocardiogram, urinalysis, and urine toxicology. Patients received a complete description of the study, and written informed consent was subsequently obtained. The study was approved by the Central Nervous System Institutional Review Board (CNS-IRB) of the National Institutes of Health (NIH).
Patients underwent a single infusion of ketamine hydrochloride (0.5 mg/kg) infused over 40 minutes followed by a double-blind randomization to riluzole or placebo six hours post-infusion. Here we report only the results of the open-label ketamine phase (up to 230 minutes post-infusion). In a previous study (7), 88% of all responders to ketamine reached response criteria by 230 minutes. Ratings included the Montgomery-Asberg Depression Rating Scale (MADRS; (12)), 17-item Hamilton Depression Rating Scale (HDRS; (13)), the Beck Depression Inventory (BDI; (14)), the Clinician Administered Dissociative States Scale (CADSS; (15)) and the Brief Psychiatric Rating Scale (BPRS;); the latter scale was used to capture psychosis, dysphoria, and negative symptoms (11). Ratings were obtained at baseline (60 minutes prior to the infusion) and 40, 80, 120, and 230 minutes post-infusion. All items on each scale were used.
Diagnostic information about participants' relatives was ascertained prior to the infusion of ketamine by a clinician-administered interview, the Family Interview for Genetic Studies (FIGS; (16)) to assess family history of alcohol dependence and mood disorders. A FHP was defined here as the presence of at least one affected first-degree relative, or at least two second-degree relatives. Individual histories of alcohol abuse or dependence were determined by SCID.
Linear mixed models were used to examine the effect of family history of alcohol dependence on the course of response to the first day of ketamine infusion. The primary outcome measure was the MADRS. The model included fixed effects for group (family history of alcohol-dependence vs. none), a repeated measures time factor, as well as a group by time interaction. Fixed and random intercepts were included in the model along with a random effect for participant. Schwarz's Bayesian criterion was used to determine the best fitting covariance structure, which was first order autoregressive. Further analysis included additional covariates to determine whether they influenced the group difference. Similar group comparisons were performed using self-reported history of alcohol dependence, family history of depression, and childhood physical or sexual abuse as the grouping variables to verify that the initial grouping variable was the active factor. Bonferroni corrected post hoc tests were used following significant interactions. Results for the secondary analysis are reported prior to multiplicity correction, but significant results following correction are noted. Cohen's d is reported with 95% confidence interval for the 230 minute time point after Bonferroni correction. For all analyses, significance was evaluated at p≤0.05, two-tailed.
Results
Twenty-three of 26 patients had data on family history of alcohol abuse or dependence; 12 (52%) of 23 had FHP. FHP and negative family history (FHN) groups did not differ in their basic demographic and course of illness characteristics (Table 1). Plasma ketamine and norketamine levels were examined at 40 and 80 minutes, but there were no group differences (ketamine: F=0.74, df=1,22, p=.04; norketamine: F=0.15, df=1,21, p=.70) or group by time interactions (ketamine: F=0.05,df=1,21, p=.83; norketamine: F=3.15, df=1,20, p=.09). However, the FHP group had lower baseline scores on the MADRS, HDRS, and BPRS. Thus, baseline clinical measures were covaried out of all mixed models.
Table 1.
Demographic and Course of Illness Characteristics
| Alcohol Dependence Family History | ||||
|---|---|---|---|---|
| Total | Positive | Negative | p | |
| (n=12) | (n=11) | |||
| N (%) | ||||
| Education (College Graduate) | 14 (64) | 9 (75) | 5 (50) | 0.22 |
| Sex (Male) | 14 (61) | 8 (67) | 6 (55) | 0.55 |
| MDD: Family History | 18 (78) | 9 (75) | 9 (82) | 0.69 |
| Alcohol Abuse/Dependence: Self-History | 8 (35) | 4 (33) | 4 (36) | 0.88 |
| Childhood Abuse | ||||
| • Physical | 9 (39) | 6 (50) | 3 (30) | 0.34 |
| • Sexual | 3 (13) | 2 (17) | 1 (10) | 0.65 |
| • Physical and/or Sexual | 10 (44) | 6 (50) | 4 (40) | 0.69 |
| Mean (SD) | ||||
| Age | 43.5 (14.1) | 47.4 (15.0) | 39.2 (12.2) | 0.17 |
| Age of Onset | 19.8 (9.9) | 20.9 (10.8) | 18.6 (9.3) | 0.59 |
| Duration of Illness (Years) | 23.7 (12.5) | 26.5 (14.3) | 20.6 (10.0) | 0.26 |
| Duration of Episode (Months) | 82.1 (103.5) | 88.3 (122.8) | 75.4 (83.0) | 0.77 |
| Clinical Measures | ||||
| • MADRS | 32.8 (4.3) | 30.8 (2.6) | 34.9 (4.8) | 0.02 |
| • HDRS | 20.2 (4.3) | 17.9 (2.7) | 22.6 (4.5) | 0.01 |
| • BDI | 26.8 (7.9) | 26.5 (8.5) | 27.1 (7.6) | 0.86 |
| • BPRS | 36.0 (6.1) | 33.6 (4.4) | 38.7 (6.8) | 0.04 |
| • BPRS Dysphoric | 19.6 (3.6) | 18.1 (3.1) | 21.2 (3.6) | 0.04 |
| • BPRS Negative | 6.7 (3.3) | 5.5 (2.0) | 8.0 (4.0) | 0.07 |
| • BPRS Positive | 4.1 (0.3) | 4.2 (0.4) | 4.1 (0.3) | 0.61 |
| • CADSS | 5.4 (9.7) | 3.7 (5.2) | 7.3 (13.0) | 0.39 |
| N (%) | ||||
| • Response (MADRS: 50% Decrease) | 10 (43) | 8 (67) | 2 (18) | 0.02 |
| • Remission (MADRS < 10) | 6 (26) | 5 (42) | 1 (9) | 0.08 |
Abbreviations: BDI: Beck Depression Inventory; BPRS: Brief Psychiatric Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; HDRS: Hamilton Depression Rating Scale, CADSS: Clinician Administered Dissociative States Scale.
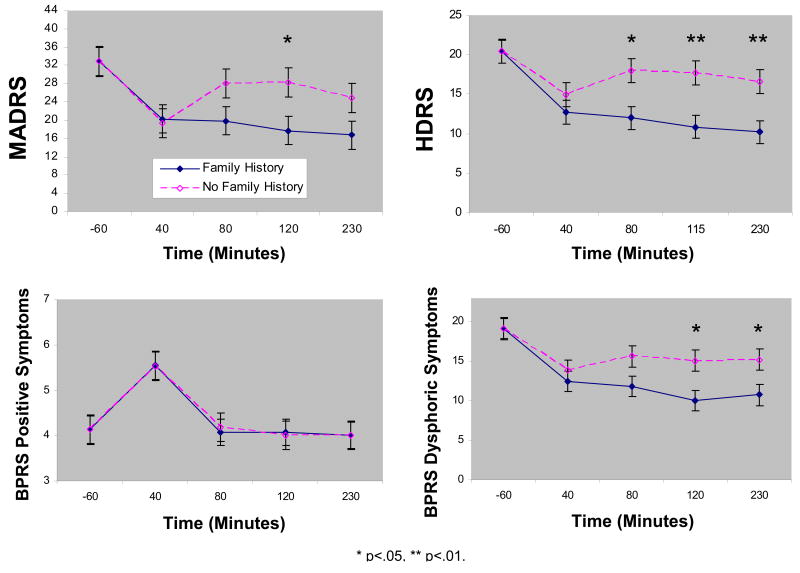
On the MADRS, the linear mixed model showed a significant group by time interaction (F=4.55, df=3,33, p=.009). After controlling for baseline, the FHP group had significantly lower MADRS scores at 120 minutes ketamine post-infusion (p=.03) and there were similar trends at 80 (p=.08) and 230 (p=.09) minutes post-infusion (230 minutes: d=0.68, 95% C.I.: -0.13-1.49) (Figure 1a). Additional analyses controlling for sex, age, duration of illness, and duration of current episode did not change these results. Further, an attempt to create propensity scores (17) using baseline demographic and clinical variables showed that only baseline depression was an independent predictor of FMP. Thus, a more general control for covariates was deemed unnecessary.
Figure 1.
Course of mood over 230 minutes in patients with treatment-resistant major depression and with or without family history of alcohol dependence who received ketamine (n=23). Values reflect group differences after controlling for baseline. Abbreviations: BPRS: Brief Psychiatric Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; HDRS: Hamilton Depression Rating Scale.
Overall, 43% of patients had at least a 50% decrease from baseline MADRS scores (Response) and 26% had a MADRS below 10 (Remission) at 230 minutes post-infusion. The FHP group had a significantly higher response rate (67%) than the FHN group (18%) (p=.02). The remission rate was higher in the FHP (42%) than FHN group (9%) and this approached significance (p=.08). This suggests a better rapid antidepressant response to ketamine in the FHP group.
A similar analysis using the HDRS and BDI confirmed these results, showing significant differences between the FHP and FHN groups from 80 to 230 minutes. Significant interactions between group and time were present for both measures (HDRS: F=2.92, df=3,37, p=.047 (Figure 1b); BDI: F=3.38, df=3,30, p=.03) and the effect sizes at 230 minutes were large (HDRS: d=1.06, 95% C.I.: 0.30-1.82; BDI: d=0.85, 95% C.I.: 0.05-1.64).
An examination of the CADSS indicated a significant interaction between group and time (F=2.85, df=3,43, p=.048). The FHP group had higher scores at 40 minutes only. A secondary analysis of the BPRS results showed a near significant interaction between group and time (F=2.70, df=3,40, p=.058). Covariates did not change this result. Positive and negative symptom subscales showed no group effects (Positive: F=0.00, df=1,13, p=.98 (Figure 1c); Negative: F=1.60, df=1,20, p=.22). However, there was a significant main effect of group for the dysphoric subscale (F=5.16, df=1,20, p=.03; Figure 1d). Patients with FHP had fewer dysphoric symptoms post-infusion than those with FHN.
Bonferroni corrections applied to all secondary analyses reflect continued significant effects for BDI, but effects for the HDRS, BPRS, BPRS subscales, and CADSS are no longer significant.
Additional analyses were performed to separately assess the putative role of personal history of alcohol abuse or dependence, family history of depression, and childhood physical or sexual abuse as grouping variables. These analyses showed no significant group differences prior to multiplicity correction.
Discussion
Twenty-six subjects with treatment resistant DSM-IV major depression received a ketamine infusion as part of a clinical research protocol. As assessed by MADRS, HDRS, and BDI scores, subjects with a FHP showed significantly greater improvement in depressive symptoms compared with subjects with FHN within 230 minutes after ketamine injection. Self-reported history of alcohol use disorders or family history of major depression did not predict response. FHP individuals also had attenuated dysphoric symptoms, replicating previous findings in the literature regarding the altered response to ketamine of individuals with a family history of alcohol dependence (11).
The potential clinical consequence of NMDA antagonists in the treatment of mood disorders necessitates an understanding of the factors that influence their efficacy in patient populations. The findings presented here suggest that family history of alcohol use disorders may serve as a useful marker for response to an NMDA antagonist.
One limitation of this study is its use of self-reporting to determine family history. Self-report of mood disorders and alcohol use disorders, however, is a well-established method used in family history studies, and is more sensitive when used in combination with diagnostic criteria such as the FIGs interview. Other limitations include the fact that the drug was open-label, the limited duration of the study, and the baseline group differences in depression. The open nature of the drug could bias the report of response, but it is unclear whether this would affect group differences. The limited duration of response examined suggests that interpretation of the findings needs to be limited to a very rapid clinical response. Future studies could examine whether family history of alcohol abuse modifies response over a longer time frame. Baseline differences could influence response, but controlling those differences did not change the results presented here.
The precise reasons underlying the better response of the FHP group to ketamine is unknown. However, the NMDA receptor is known to be a target for ethanol's action, and emerging data suggests that genetically determined alterations in NMDA receptor subunits may be associated with alcohol dependence. Thus, for example, a recent study found evidence of an association of NR2A with alcohol dependence (18), and alcohol has been shown to regulate expression of NR2A in brain regions implicated in addiction-related neurobiological processes, including amygdala and hippocampus (19). Thus, albeit quite speculative, it remains a possibility that the genetic variations in NMDA receptor subunits that increase vulnerability to alcohol dependence alter the sensitivity of the NMDA complex. This altered sensitivity of the NMDA receptor complex, may thus impart a greater response to ketamine's antidepressant effects (ketamine acts partially as an NR2A antagonist (20).
In the future, candidate gene investigations may provide two-fold insight: clarifying the biological association between alcohol dependence and major depression, and providing the ability of heritable biomarkers to predict antidepressant response to an NMDA antagonist. The significant findings of this investigation warrant continued examination into the predictive value and biological implications of a family vulnerability to alcoholism in the study of mood disorders.
Acknowledgments
This study was supported by the Intramural Research Program at the National Institute of Mental Health, and the National Alliance for Research on Schizophrenia and Depression (CZ). Ioline Henter provided invaluable editorial assistance.
Footnotes
Study ID Numbers: 040222; 04-M-0222; Record first received: July 2004; ClinicalTrials.gov Identifier: NCT00088699
Financial Disclosures: We would like to acknowledge the support of the Intramural Research Program of the National Institute of Mental Health. The author(s) declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. A patent application for the use of ketamine in depression has been submitted listing Drs. Manji and Zarate among the inventors. Drs. Manji and Zarate have assigned their rights on the patent to the US government.
References
- 1.Davis LL, Frazier EC, Gaynes BN, Trivedi MH, Wisniewski SR, Fava M, et al. Are depressed outpatients with and without a family history of substance use disorder different? A baseline analysis of the STAR*D cohort. J Clin Psychiatry. 2007;68:1931–8. doi: 10.4088/jcp.v68n1214. [DOI] [PubMed] [Google Scholar]
- 2.Preisig M, Fenton BT, Stevens DE, Merikangas KR. Familial relationship between mood disorders and alcoholism. Compr Psychiatry. 2001;42:87–95. doi: 10.1053/comp.2001.21221. [DOI] [PubMed] [Google Scholar]
- 3.Davis LL, Frazier E, Husain MM, Warden D, Trivedi M, Fava M, et al. Substance use disorder comorbidity in major depressive disorder: a confirmatory analysis of the STAR*D cohort. Am J Addict. 2006;15:278–85. doi: 10.1080/10550490600754317. [DOI] [PubMed] [Google Scholar]
- 4.Sanacora G, Rothman DL, Mason G, Krystal JH. Clinical studies implementing glutamate neurotransmission in mood disorders. Ann N Y Acad Sci. 2003;1003:292–308. doi: 10.1196/annals.1300.018. [DOI] [PubMed] [Google Scholar]
- 5.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–40. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- 9.Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D'Souza DC. NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci. 2003;1003:176–84. doi: 10.1196/annals.1300.010. [DOI] [PubMed] [Google Scholar]
- 10.Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, et al. Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–9. doi: 10.1192/bjp.bp.105.015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–82. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–69. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 15.Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–36. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell ME. Family Interview for Genetic Studies (FIGS): Manuel for FIGS. Bethesda, MD: 1992. [Google Scholar]
- 17.Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med. 2002;137:693–5. doi: 10.7326/0003-4819-137-8-200210150-00015. [DOI] [PubMed] [Google Scholar]
- 18.Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, et al. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–38. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- 19.Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006;187:455–66. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- 20.Petrenko AB, Yamakura T, Fujiwara N, Askalany AR, Baba H, Sakimura K. Reduced sensitivity to ketamine and pentobarbital in mice lacking the N-methyl-D-aspartate receptor GluRepsilon1 subunit. Anesth Analg. 2004;99:1136–40. doi: 10.1213/01.ANE.0000131729.54986.30. [DOI] [PubMed] [Google Scholar]