Abstract
Immune activity is variable in many wild animals, despite presumed strong selection against immune incompetence. Much variation may be due to changes in prevalence and abundance of pathogens (and/or their vectors) in time and space, but the costs of immune defenses themselves may also be important. Induction of immune activity often increases energy and protein expenditure, sometimes to the point of compromising fitness. Whether immune defenses are expensive to maintain once they are generated, however, is less well appreciated. If so, organisms would face persistent challenges of allocating resources between immunity and other expensive physiological processes, which would mandate trade-offs. Mild food restriction (70% ad lib. diet) reduces secondary antibody responses in deer mice (Peromyscus maniculatus), functionally representing a cost of immune memory. In this study, we asked whether compromised immune memory was mediated by a decrease in size of the B cell population responsible for producing antibodies (i.e., spleen-derived B lymphocytes producing immunoglobulin G [IgG]). Two weeks of food restriction reduced total splenocytes, total splenic B lymphocytes (B220+ cells), and splenic B lymphocytes producing IgG (B220+/IgG+ cells) but did not affect body mass or two circulating antibody subclasses (IgG1 and IgG2a) in deer mice. These results further indicate that maintenance of immune memory is expensive and may be subject to trade-offs with other physiological processes.
Introduction
The immune system protects organisms against infection, but this protection comes at a cost (Lochmiller and Deerenberg 2000). Activation of immune defenses, particularly induction of fever, increases calorie and protein turnover (Martin et al. 2003; Klasing 2004; Lee 2006). Such immune costs are often sufficiently large to affect fitness (Martin et al. 2008), including reductions in sexual ornament elaboration (Verhulst et al. 1999), rates of reproductive maturation (Fair and Myers 2002; Prendergast et al. 2004), and reproductive success (Ilmonen et al. 2000; Bonneaud et al. 2003). Whether maintenance of immune defenses imparts ongoing costs has received little attention. If it does, the costs of immune protection would be persistent, not just transient, and thus would impose larger constraints on other physiological processes (i.e., trade-offs) than is conventionally appreciated.
Until recently, the costs of maintaining immune defenses were thought to be modest (Klasing and Calvert 1999; Klasing 2004). For example, pharmacological reduction of circulating leukocyte numbers did not decrease whole-body energy turnover in white-footed mice (Peromyscus leucopus) as expected (Derting and Compton 2003). Similarly, metabolic rates of domesticated mice genetically engineered to lack T and B cells were higher, not lower, than those of outbred conspecifics (Råberg et al. 2002). Contrary to these studies, maintenance of antibody-mediated immune memory was found to be expensive in deer mice (Martin et al. 2007). Two weeks of mild food restriction (FR; 70% of ad lib. [AL] consumption) prevented individuals from mounting secondary antibody responses, even though they were returned to AL food just before induction of secondary antibody responses. Restraint stress did not emulate or amplify FR effects on secondary antibody responses, indicating that FR effects were not mediated by increases in (potentially immunosuppressive) glucocorticoids (Martin et al. 2006). Functionally, compromised secondary antibody responses post-FR represent a cost of immune maintenance because the benefits of expending resources to generate immune memory (i.e., more rapid and targeted control of previously experienced infectious organisms) would not be realized in individuals experiencing food limitation.
Although a cost of maintaining the immune system was revealed in deer mice, the mechanism that produced this outcome was not identified. One viable possibility involves a post-FR decrease in the size of the cell population responsible for producing antibodies, specifically, spleen-derived B lymphocytes producing immunoglobulin G (IgG). To determine whether a change in B lymphocyte number post-FR was indeed the mechanism underlying the previous results, we generated immune memory in deer mice by exposing them to the same large immunogenic protein (keyhole limpet hemocyanin [KLH]) and FR paradigm as in the previous experiment and comparing antibody levels and number of splenic IgG progenitor cells among groups of deer mice. We predicted that FR would reduce both circulating antibody levels and splenic B cell numbers. Because some antibody isotypes (IgG2a) are more effective than others at promoting memory B cell proliferation, multiple antibody isotypes were measured (Klaus 1979). Also, because previous studies with deer mice indicated that day length (photoperiod) can affect immune activity, thus including IgG production (Demas and Nelson 1996, Demas and Nelson 1998a, 1998b; Martin et al. 2007), in addition to FR, deer mice experienced either long or short photoperiods throughout the experiment.
Methods
Animals
Adult (~90-d-old) male Peromyscus maniculatus bairdii were obtained from the Peromyscus Genetic Stock Center at the University of South Carolina (Columbia), housed singly in polypropylene cages at room temperature and humidity (22.5°± 1°C and 50% ± 5% relative humidity), and exposed to either 16L: 8D (long day [LD]) or 8L: 16D (short day [SD]) each day (lights off at 1500 hours EST) for the entire experiment (half of the mice in each photoperiod condition). During an initial acclimation period, all mice were provided AL food (Harlan TekLad 8640; crude protein =22%, crude fat =5%, fiber =4.5%) and filtered chlorinated tap water. Two weeks before the first KLH injection, food intake was measured for four consecutive days for each mouse, and average daily food consumption was calculated. These data were used to establish (restricted) diets to be used later. All procedures were approved by the Ohio State University Institutional Animal Care and Use Committee and comply with current National Institutes of Health guidelines for animal research and Animal Behavior Society guidelines.
Experiment Progression
Immediately before initial KLH challenge, blood (~100 μL) was collected from the retro-orbital sinus of each mouse into heparanized microcapillary tubes while mice were under isoflurane anesthesia. Immediately after blood collection, each mouse was injected intraperitoneally (i.p.) with 150 μg KLH (CalBiochem, La Jolla, CA). After 7, 14, and 21 d, an additional blood sample was obtained to characterize primary antibody responses. After blood collection, samples were centrifuged at 7,000 rpm for 20 min, and plasma was removed and stored at −80°C until enzyme-linked immunosorbent assay (ELISA). Twenty-one days after initial KLH injection, mice were assigned randomly to AL or FR diets, which were administered for two consecutive weeks (Fig. 1). FR mice received 70% of food consumed under AL conditions; all other mice were maintained on AL food availability. Sample sizes in each group were as follows: LD, AL: 7; LD, FR: 9; SD, AL: 8; SD, FR: 8. Most mice in the FR group consumed all food provided; in the few cases when they did not, remaining food was removed and replaced with the appropriate amount of fresh food. We chose to maintain individuals that did not consume food on some occasions in the experiment in an effort to perform the most conservative analysis. Body mass was measured in all individuals before FR, every 3 d during the FR period, and at the time of the second KLH injection. After the FR period and for the remainder of the study, all mice were returned to AL access to food. One day after FR, a blood sample was taken (as above), and secondary antibody responses to KLH were induced (30 μg i.p.). Five days later, mice were decapitated while under deep isoflurane anesthesia, and trunk blood and spleens were collected for ELISA and flow cytometry, respectively. During all procedures and before injections or blood sampling, mice were handled and anesthetized comparably.
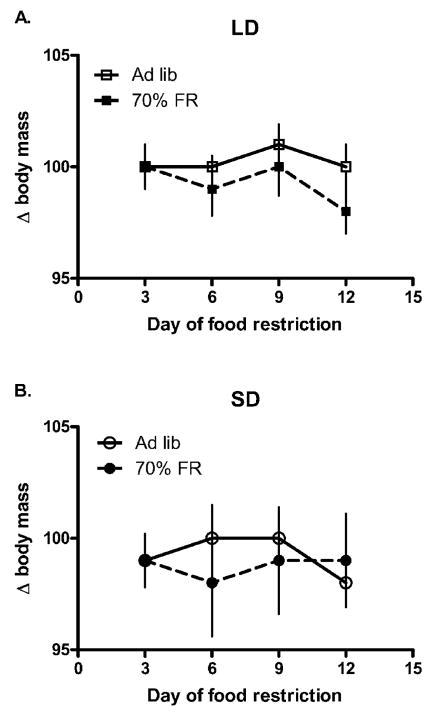
Figure 1.
Effects of 2-wk food restriction (FR; 70% ad lib. [AL] intake) on body mass in Peromyscus maniculatus housed in long-day (LD; A) and short-day (SD; B) photoperiods. Bars are means ±SEM. Sample sizes in each group were as follows: LD, AL: 7; LD, FR: 9; SD, AL: 8; SD, FR: 8.
KLH ELISA
To measure anti-KLH IgG, we employed a modified colorimetric ELISA developed for Peromyscus (Demas and Nelson 1996). Briefly, 96-well plates were coated with KLH, and then thawed plasma samples were diluted with phosphate-buffered saline–Tween (PBS-T; 1:200), vortexed, and added to plates in duplicate. Positive (plasma samples from Peromyscus already determined to have high anti-KLH IgG titers) and negative (plasma from KLH-naive mice) controls were also added to each plate in triplicate. Plates were then sealed, incubated at 37°C for 3 h, and washed with PBS-T, and then one of two secondary antibodies (both alkaline-phosphatase conjugated antimouse: IgG1 [ICN Biomedicals, Aurora, OH] and IgG2a [Serotec, Oxford]) was added to each well (dilutions: IgG1, 1:750; IgG2a, 1:2,000); both subclasses were measured in all samples but on different plates. Plates were again incubated (37°C for 1 h) and washed with PBS-T, and then each well was treated with p-nitrophenyl phosphate. Exactly 20 min later, optical density (OD) of each well was read (405-nm filter; BioRad Benchmark microplate reader, Richmond, CA), and mean OD of each sample was calculated. Data analysis was performed on sample OD readings, expressed as a percentage of the positive controls on each plate. All samples were measured blind to sample identity, and mean intra- and interplate variation were <10%.
Flow Cytometry
Spleens were excised and macerated in Hanks’s buffered saline solution by using a stomacher machine. The resultant cell suspension was washed via centrifugation (600 g for 10 min at 4°C), and red blood cells were lysed with buffer (0.16 M NH4Cl, 10 mM KHCO3, and 0.13 mM EDTA). After a second wash, cells were filtered through a 70-μm nylon mesh filter and then resuspended in CTLL RPMI 1640 (containing 0.075% sodium bicarbonate, 1.5 mM L-glutamine, and 0.00035% 2-mecrap-toethanol) + 10% heat-inactivated fetal bovine serum (FBS). Cells in the preparation were enumerated using a Z2 particle counter (Beckman Coulter, Fullerton, CA) and adjusted to a concentration of 5 × 106 cells mL−1. A total of 2.5 × 105 cells was then incubated at 4°C for 45 min with PE-conjugated anti-IgG (polyclonal; eBioscience, San Diego, CA) and PerCP-conjugated anti-B220 (clone RA3-6B2; BD Pharmingen, San Diego, CA) antibodies. Antibody labeling was performed by a standard lyse wash procedure using FACS lysing solution (BD Immunocytometry Systems, San Jose, CA) and supplemented PBS (Dulbecco’s PBS without calcium or magnesium, 2% FBS, 0.1% NaN3). A total of 10,000 cells from each sample was analyzed on a dual-laser flow cytometer (FACSCalibur, BD Immunocytometry Systems) using CellQuest Pro (ver. 4.0.2) and Attractors software (ver. 3.1.0). Cells were identified on the basis of forward- and side-scatter characteristics and antibody staining. Matched isotype controls were used for both antibodies to set negative staining criteria.
Data Analysis
Before analysis, data were tested to ensure that requirements of parametric statistics were met. To compare antibody responses, ANOVA or repeated-measures ANOVA was conducted on primary and secondary responses or individual time points, with FR, photoperiod treatment, and their interaction as factors. Body mass change during and before FR was analyzed similarly. Splenic leukocyte numbers were analyzed using univariate ANOVA with the above factors. All analyses were conducted with SPSS (ver. 15) with α=0.05.
Results
Effects of FR and Photoperiod on Body Mass
Photoperiod did not significantly affect body mass before FR (univariate F1, 29=0.58, P =0.45). Neither FR (repeated-measures F5, 140=1.0, P =0.43) nor photoperiod (repeated-measures F5, 140=0.48, P =0.79) affected body mass loss before the secondary antigen challenge (Fig. 1). Likewise, neither FR (univariate F1, 29=1.44, P =0.24) nor photoperiod (univariate F1, 29=0.65, P =0.43) affected body mass on the day of the second KLH challenge.
Effects of FR and Photoperiod on Circulating IgG
Mice mounted IgG1 (repeated-measures F3, 93=107.5, P <0.001) and IgG2a (repeated-measures F3, 93=52.6, P < 0.001) primary antibody responses against KLH because anti-KLH antibody titers increased significantly over time within individuals. However, photoperiod did not affect primary responses (repeated-measures IgG1: F3, 93=1.1, P =0.39; IgG2a: F3, 93 =0.33, P =0.80). After the primary antibody responses but before the second antigen challenge, IgG1 titer was not affected by FR (univariate F1, 29=0.03, P =0.85) or photoperiod (univariate F1, 29=0.08, P =0.85). The same was true for IgG2a; it was not affected by either FR (univariate F1, 29= 1.9, P =0.17) or photoperiod (univariate F1, 29=0.23, P =0.64).
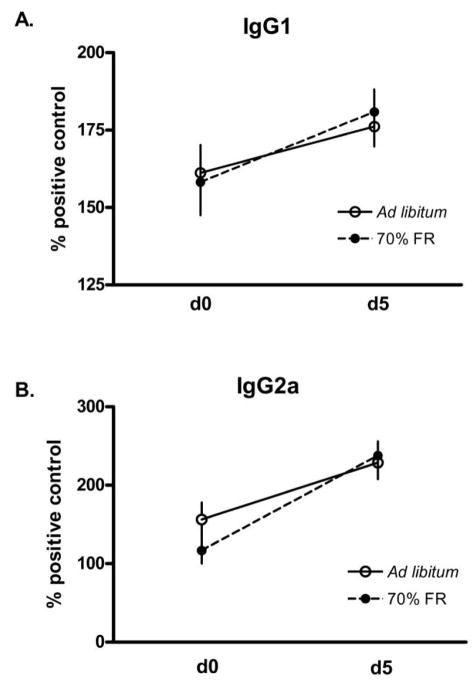
All mice mounted significant IgG1 (univariate F1, 29=5.7, P =0.02) and IgG2a (univariate F1, 29=67.5, P < 0.001) secondary responses against KLH, as indicated by elevated antibody titers 5 d after the second KLH challenge. To ascertain whether FR affected secondary antibody responses, we examined IgG1 and IgG2a titers 5 d after the secondary antigen challenge as well as the change in titer during this period. IgG1 titers 5 d after the second KLH injections were not influenced by FR (univariate F1, 29=0.37, P =0.55; Fig. 2A) or photoperiod (univariate F1, 29=0.04, P =0.84). IgG2a titers 5 d after the second KLH injection were also not influenced by FR (univariate F1, 29=0.09, P =0.77; Fig. 2B) or photoperiod (univariate F1, 29=0.09, P =0.77). Similarly, changes in IgG before the second KLH challenge and 5 d after the second challenge were not affected by treatments. IgG1 and IgG2a produced during the secondary antibody response were not affected by FR (repeated-measures IgG1: F1, 29=0.05, P =0.83; IgG2a: F1, 29=3.8, P =0.06) or photoperiod (repeated-measures IgG1: F1, 29 =0.02, P =0.89; IgG2a: F1, 29=0.49, P =0.49).
Figure 2.
Effects of 2-wk food restriction (70% ad lib. intake) on secondary antibody (IgG1 [A] and IgG2a [B]) production in Peromyscus maniculatus. Bars are means ± SEM.
Effects of FR and Photoperiod on Splenic B Lymphocytes
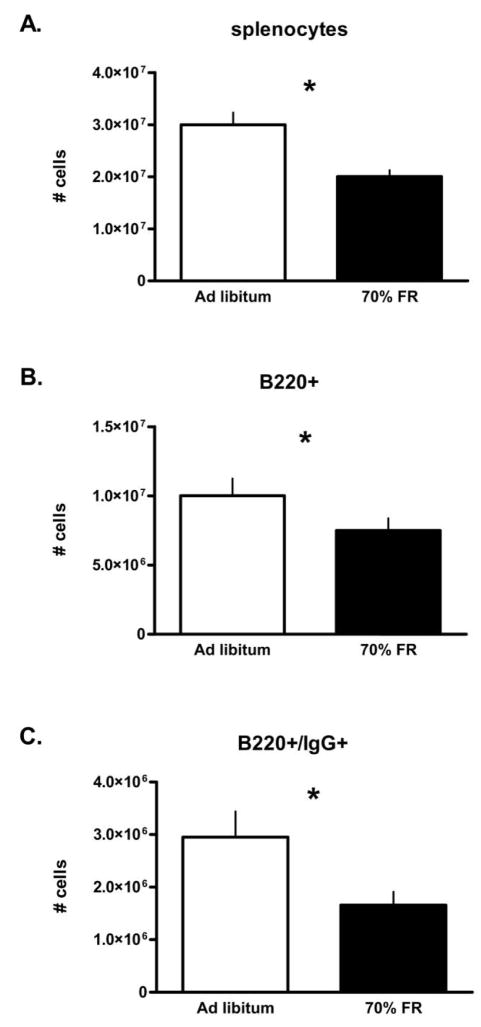
Mice that underwent 2 wk of FR had significantly fewer splenocytes (univariate F1, 29=5.0, P =0.03; Fig. 3A), significantly fewer B220+ cells (univariate F1, 29=7.6, P =0.01; Fig. 3B), and significantly fewer B220+/IgG+ cells (univariate F1, 29= 4.35, P =0.05; Fig. 3C) compared to AL mice. Photoperiod did not significantly affect any of these cell numbers, however (total splenocytes: univariate F1, 29=0.28, P =0.60; B220+ cells: F1, 29 =0.21, P =0.65; B220+/IgG+ cells: F1, 29=0.01, P =0.90).
Figure 3.
Effects of 2-wk food restriction (FR; 70% ad lib. intake) on splenic IgG-producing cells in Peromyscus maniculatus. A, Total splenocytes; B, B lymphocytes (B220+); and C, IgG-producing B lymphocytes (B220+/IgG+). Bars are means + SEM; asterisks indicate significant effect of FR (P < 0.05).
Discussion
Immunological memory results when antigen-specific B cells differentiate into long-lived, quiescent memory B lymphocytes. These memory B cells possess high-affinity receptors for specific antigens and increased IgG expression on cell membranes (relative to other isotypes). As a result, memory B cells produce more antibodies more rapidly during second antigen exposures (Abbas et al. 2000). Immunological memory is maintained through ongoing, low-intensity cell proliferation. If maintenance of this immune memory is costly, then the abundance of memory B cells should decrease, and subsequently, secondary antibody responses should be diminished when animals are food restricted. Previously, we demonstrated the latter (Martin et al. 2007); in this study, we document the former. Combined, significant reductions in numbers of splenocytes, splenic B lymphocytes, and splenic B lymphocytes expressing IgG and a diminution in capacity to generate IgG post-FR functionally (but indirectly) represent a cost of immunological memory in deer mice.
The above data document an effect of FR on splenic B cell populations, but the specific mechanisms producing such differences remain unidentified. One possibility involves energy availability for proliferative processes to the memory B cells themselves and/or to antigen-specific T helper cells that facilitate B cell responses. Cell proliferation in general (Epel 1963) and humoral immune responses in particular (Demas et al. 2003; Bourgeon et al. 2006) are expensive, so lack of calories, fat, or protein may directly compromise memory B cell proliferation. An alternative possibility is that FR inhibited other components of the immune system, namely, the complement system, which is critical for memory B cell generation, priming, and localization (Klaus and Humphrey 1977; Klaus 1979; Cutler et al. 1998). Domestic mice lacking two complement components (C3 and C1q) exhibit abnormal B memory cell proliferation (Klaus and Humphrey 1977; Cutler et al. 1998).
FR had no differential effect on antibody isotype predominance. Two isotypes were measured in this study to determine whether FR more dramatically affected Th2- or Th1-directed immune responses (Pruett and Fan 2001), IgG1 and IgG2a, respectively. Both antibody variants increased after primary and secondary KLH challenges, but neither the subclass titer 5 d following the secondary KLH challenge nor the magnitude of secondary antibody responses (change day 0–day 5) of each subclass differed significantly between FR and AL mice. Conservatively, this outcome may indicate that 70% FR effects on IgG production in deer mice are more modest than previously indicated (Martin et al. 2007). On the other hand, effects of FR on KLH-specific IgG did not manifest until 7 d after the secondary challenge in the previous study (Martin et al. 2007). Spleens in the current study were collected at day 5 to ensure that any effects of FR on memory B cells could be detected before mice could compensate by refeeding when returned to an AL diet. For these reasons, future studies should replicate our work and quantify antibodies and memory B cell numbers after a longer intervals post–secondary challenge.
We expect that our results are relevant in natural contexts. Oftentimes, compensation for food scarcity may be achieved in the wild by preferentially investing resources in only those physiological processes critical for immediate survival, which likely would not include maintenance of immune memory. This phenomenon motivated the day length manipulation component of the study. Photoperiod is a salient, error-free cue that organisms use to adjust their phenotypes to prevailing or impending changes in environment (Prendergast et al. 2001). Because photoperiod extensively affects rodent immune defenses (Nelson 2004), we predicted that prior natural selection for appropriate resource allocation to immunity versus other physiological processes would have also influenced how photoperiod and FR affected splenic B cell numbers, but this was not the case. Instead, our results indicate that immediate availability of food directly affected B cell population sizes; deer mice appear unable to prime themselves for FR, at least when restricted to 70% of their AL diet. Perhaps during times of low energy demands, they and other wild organisms favor less costly types of immune defenses (Lochmiller and Deerenberg 2000), but this possibility requires further study.
One final aspect of this study that must be emphasized is its potential relevance to human health. Infections are more frequent and tend to be chronic in malnourished children (Cunningham-Rundles et al. 2005), and the consequences of some infections are dramatically (e.g., pneumonia, bacterial and viral diarrhea, measles, and tuberculosis) or moderately (e.g., influenza and HIV) magnified by malnutrition (Chandra 1999). For some infections, malnutrition may compromise the ability of individuals to produce antibodies against vaccines. Just after the drought of 2000 in the Gombe region of Ethiopia, field efficacy of measles vaccine (an index of the ability of vaccine to prevent measles in vaccinated versus unvaccinated individuals) was only 66.3%; under ideal conditions, a similar vaccination regime produces 85% efficacy (Talley and Salama 2003). Other factors may also have mitigated the low success rate in that study, but work on historic epidemics (e.g., whooping cough in eighteenth- and nineteenth-century Liverpool) suggests that human mortality to infection is often strongly influenced by food availability (Duncan et al. 1996). Likewise, anorectic individuals in modern populations suffer from decreased circulating IgG and compromised T cell coordination of B cell activities (Allende et al. 1998). In many cases, refeeding can eliminate such immune deficiencies.
In most of these studies and ours, the specific contribution of calories versus protein versus micronutrients (Chandra 1999) was unidentified but would be worthy of future study. Further, it would be useful to determine to what extent leptin, a regulatory peptide secreted by adipose tissue (Lord et al. 1998), orchestrates decreases in memory B cell numbers and subsequent IgG production. Previous work in another rodent species indicated that the suppressive effects of fat tissue ablation on antibody production could be reversed by leptin treatment (Demas and Sakaria 2005). Finally, it would be intriguing to determine how FR affects proliferation of new memory B cells versus maintenance of previously generated B cell populations (Demas and Nelson 1998a; Buchanan et al. 2003; Gasparini et al. 2006). At present, it is unknown whether FR inhibited one or both processes; imposing FR without administering second KLH challenges could reconcile these alternatives.
Acknowledgments
We thank Stephanie Kidder, Eric Johnson, Jonathan Wade, and Zachary Weil for help with animal care, assays, and data discussion. This work was supported by National Institutes of Health grants MH 57535 and MH 66144 and by National Science Foundation grant IBN 04-16897 to R.J.N. K.J.N. was supported by National Research Service Award T32 AI055411.
Literature Cited
- Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. Saunders; New York: 2000. [Google Scholar]
- Allende LM, Corell A, Manzanares J, Madruga D, Marcos A, Madrono A, Lopez-Goyanes A, et al. Immunodeficiency associated with anorexia nervosa is secondary and improves after refeeding. Immunology. 1998;94:543–551. doi: 10.1046/j.1365-2567.1998.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am Nat. 2003;161:367–379. doi: 10.1086/346134. [DOI] [PubMed] [Google Scholar]
- Bourgeon S, Martinez J, Criscuolo F, Le Maho Y, Raclot T. Fasting-induced changes of immunological and stress indicators in breeding female eiders. Gen Comp Endocrinol. 2006;147:336–342. doi: 10.1016/j.ygcen.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Buchanan KL, Spencer KA, Goldsmith AR, Catchpole CK. Song as an honest signal of past developmental stress in the European starling (Sturnus vulgaris) Proc R Soc B. 2003;270:1149–1156. doi: 10.1098/rspb.2003.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra RK. Nutrition and immunology: from the clinic to cellular biology and back again. Proc Nutr Soc. 1999;58:681–683. doi: 10.1017/s0029665199000890. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–1128. doi: 10.1016/j.jaci.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Botto M, van Essen D, Rivi R, Davies KA, Gray D, Walport MJ. T cell–dependent immune response in C1q-deficient mice: defective interferon gamma production by antigen-specific T cells. J Exp Med. 1998;187:1789–1797. doi: 10.1084/jem.187.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc R Soc B. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Photoperiod and temperature interact to affect immune parameters in adult male deer mice (Peromyscus maniculatus) J Biol Rhythms. 1996;11:94–102. doi: 10.1177/074873049601100202. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Photoperiod, ambient temperature, and food availability interact to affect reproductive and immune function in adult male deer mice (Peromyscus maniculatus) J Biol Rhythms. 1998a;13:253–262. doi: 10.1177/074873098129000093. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Short-day enhancement of immune function is independent of steroid hormones in deer mice (Peromyscus maniculatus) J Comp Physiol B. 1998b;168:419–426. doi: 10.1007/s003600050161. [DOI] [PubMed] [Google Scholar]
- Demas GE, Sakaria S. Leptin regulates energetic trade-offs between body fat and humoural immunity. Proc R Soc B. 2005;272:1845–1850. doi: 10.1098/rspb.2005.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derting TL, Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Physiol Biochem Zool. 2003;76:744–752. doi: 10.1086/375662. [DOI] [PubMed] [Google Scholar]
- Duncan CJ, Duncan SR, Scott S. Whooping cough epidemics in London, 1701–1812: infection dynamics, seasonal forcing and the effects of malnutrition. Proc R Soc B. 1996;263:445–450. doi: 10.1098/rspb.1996.0067. [DOI] [PubMed] [Google Scholar]
- Epel D. The effects of carbon monoxide inhibition on ATP level and the rate of mitosis in the sea urchin egg. J Cell Biol. 1963;17:315–319. doi: 10.1083/jcb.17.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair JM, Myers OB. The ecological and physiological costs of lead shot and immunological challenge to developing western bluebirds. Ecotoxicology. 2002;11:199–208. doi: 10.1023/a:1015474832239. [DOI] [PubMed] [Google Scholar]
- Gasparini J, Roulin A, Gill VA, Hatch SA, Boulinier T. In kittiwakes food availability partially explains the seasonal decline in humoral immunocompetence. Funct Ecol. 2006;20:457–463. [Google Scholar]
- Ilmonen P, Taarna T, Hasselquist D. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc R Soc B. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing KC. The costs of immunity. Acta Zool Sin. 2004;50:961–969. [Google Scholar]
- Klasing KC, Calvert CC. The care and feeding of an immune system: an analysis of lysine needs. In: Lobley GE, White A, editors. Protein Metabolism and Nutrition. Wageningen Academic; Wageningen: 1999. pp. 253–264. [Google Scholar]
- Klaus GGB. Generation of memory cells. III. Antibody class requirements for the generation of B-memory cells by antigen-antibody complexes. Immunology. 1979;37:345–351. [PMC free article] [PubMed] [Google Scholar]
- Klaus GGB, Humphrey JH. The generation of memory cells. I. The role of C3 in the generation of B-memory cells. Immunology. 1977;33:31–40. [PMC free article] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- Lord GM, Matarese G, Howard LK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Martin LB, Navara KJ, Weil ZM, Nelson RJ. Immunological memory is compromised by food restriction in deer mice, Peromyscus maniculatus. Am J Physiol. 2007;292:R316–R320. doi: 10.1152/ajpregu.00386.2006. [DOI] [PubMed] [Google Scholar]
- Martin LB, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Refining approaches and diversifying directions in ecoimmunology. Integr Comp Biol. 2006;46:1030–1039. doi: 10.1093/icb/icl039. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88:2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philos Trans R Soc B. 2008;363:321–339. doi: 10.1098/rstb.2007.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Bilbo SD, Nelson RJ. Peripubertal immune challenges attenuate reproductive development in male Siberian hamsters (Phodopus sungorus) Biol Reprod. 2004;70:813–820. doi: 10.1095/biolreprod.103.023408. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Kriegsfeld LJ, Nelson RJ. Photoperiodic polyphenisms in rodents: neuroendocrine mechanisms, costs, and functions. Q Rev Biol. 2001;76:293–325. doi: 10.1086/393989. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan RP. Quantitative modeling of suppression of IgG1, IgG2a, IL-2, and IL-4 responses to antigen in mice treated with exogenous corticosterone or restraint stress. J Toxicol Environ Health. 2001;62:175–189. doi: 10.1080/009841001458299. [DOI] [PubMed] [Google Scholar]
- Råberg L, Vestberg M, Hasselquist D, Holmdahl R, Svensson E, Nilsson JA. Basal metabolic rate and the evolution of the adaptive immune system. Proc R Soc B. 2002;269:817–821. doi: 10.1098/rspb.2001.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley L, Salama P. Short report: assessing field vaccine efficacy for measles in famine-affected rural Ethiopia. Am J Trop Med Hyg. 2003;68:545–546. doi: 10.4269/ajtmh.2003.68.545. [DOI] [PubMed] [Google Scholar]
- Verhulst S, Dieleman SJ, Parmentier HK. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc Natl Acad Sci USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]