SUMMARY
Although IgA nephropathy is the most common form of primary glomerulopathy, the detailed mechanisms underlying its development remain uncertain.
In the present study, we used male high IgA strain of ddY (HIGA) mice as the IgA nephropathy model and age-matched male BALB/c mice as the control. Recent studies have demonstrated that reactive oxygen species (ROS)-dependent enhancement of the renin-angiotensin system (RAS) plays a potential role in the development and progression of renal injury. Therefore, in the present study we periodically measured the systolic blood pressure (SBP) of mice over the period 21-25 weeks of age and estimated markers for ROS, RAS and renal damage after mice had been killed at 25 weeks of age.
Markers for ROS (urinary 8-isoprostane excretion and renal 4-hydroxy-2-nonenal accumulation), RAS (renal angiotensinogen protein expression, urinary angiotensinogen excretion and renal angiotensin II) and renal damage (desmin-positive area and urinary protein excretion), as well as SBP, were significantly increased in HIGA mice compared with control BALB/c mice.
The data suggest that both ROS and the RAS are activated at an early phase in IgA nephropathy model mice.
Keywords: IgA nephropathy, reactive oxygen species, renin-angiotensin system
INTRODUCTION
Immunoglobulin A (IgA) nephropathy is characterized by the pre-dominant deposition of IgA in the glomerular mesangium.1 It is the most common form of primary glomerulopathy among all races from Europe,2-4 Asia5,6 and Australia.7 Nair et al. reported that the incidence of IgA nephropathy has increased and that in the US this condition accounts for a large proportion of young adults (20-39 years of age) with primary glomerulopathy.8 However, the detailed mechanism underlying IgA nephropathy remains unclear and a radical cure for this condition has yet to be established.
Recently, clinical and experimental studies have demonstrated that blockade of the renin-angiotensin system (RAS) successfully alleviates the symptoms of IgA nephropathy,9 suggesting a role for the activated RAS in the development and progression of IgA nephropathy. Recently, we demonstrated clinically that the immunoreactivity of intrarenal angiotensinogen (AGT)10 and intrarenal angiotensin (Ang) II was significantly increased in kidneys of IgA nephropathy patients compared with normal portions of surgically resected kidneys.11 We also showed that the immunoreactivity of intrarenal heme oxygenase (HO)-1 and 4-hydroxy-2-nonenal (4-HNE), markers of oxidative stress, was significantly greater in IgA nephropathy patients than in the control group.11
Recent studies have revealed that reactive oxygen species (ROS)-dependent enhancement of AGT may be involved in the development and progression of renal injury observed in salt-sensitive hypertension,12,13 as well as in diabetic nephropathy.14,15
These data imply that the activated intrarenal ROS-RAS axis may play an important role in the development of IgA nephropathy. However, it is difficult to elucidate more detailed mechanisms in clinical studies. Therefore, the present study was undertaken to test the hypothesis that activation of ROS and RAS is important during the early phase of IgA nephropathy in an experimental model.
METHODS
Experimental design
The experimental protocol used in the present study conforms to the guidelines of the Animal Care and Use Committee of Tulane University. BALB/c mice were used as controls and the high IgA strain of ddY (HIGA) mice was used as the experimental model of IgA nephropathy. Both mouse strains were purchased from Japan SLC (Hamamatsu, Japan; age: 21 weeks; n = 10 of each strain). All mice were maintained in a temperature-controlled room under a 12 h light-dark cycle and allowed free access to water. Systolic blood pressure (SBP) was measured once a week for 4 weeks by the tail-cuff method in conscious but restrained mice. At 25 weeks of age, all mice were killed by decapitation because anaesthesia is known to elevate RAS activity.16
Urinary measurements
Before mice were killed, 24 h urine samples were collected every week for 4 weeks. The urinary concentration of 8-isoprostane, a marker of oxidative stress, was determined by ELISA using a commercially available kit (Cayman Chemical, Ann Arbor, MI, USA). In addition, urinary protein excretion over 24 h was evaluated using a pyrogallol red-molybdate protein assay kit (Wako Pure Chemical Industries, Osaka, Japan). Urinary AGT excretion over 24 h was also measured using a sandwich-type ELISA (Immuno-Biological Laboratories, Takasaki, Japan) that we recently developed.17
Western blot analysis
Protein extraction from the kidney and western blot analysis for renal AGT and renal 4-HNE were performed using an infrared imaging system (LI-COR Biosciences, Lincoln, NE, USA), as described previously.18-20 Rabbit polyclonal anti-mouse/rat AGT antibody (Immuno-Biological Laboratories) and mouse monoclonal anti-4-HNE antibody (Japan Institute for the Control of Ageing, Fukuroi, Japan) were used. Mouse monoclonal anti-GAPDH antibody was purchased from Sigma (St Louis, MO, USA). The data obtained from western blot analysis of AGT protein levels and 4-HNE accumulation were normalized against GAPDH protein levels.
Evaluation of glomerular lesions
Kidney tissue was fixed in zinc-saturated formalin and embedded in paraffin. Tissue sections (3 μm) were stained with periodic acid-Schiff (PAS). The extent of mesangial matrix deposition was evaluated quantitatively in PAS-stained sections using automatic image analysis of individual glomeruli (Mass Histology Service, Worcester, MA, USA), as described previously.21 The affected areas were, in turn, divided by the total area of the glomerulus using Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA). Twenty glomeruli were examined for each mouse and the average percentage of affected area was obtained for each mouse.
Immunohistochemical analysis
The extent of the IgA-positive area in glomeruli was examined by immunohistochemistry using a commercially available antibody against IgA (Dakocytomation, Glostrup, Denmark), as described previously.11,21,22 Immunohistochemistry was performed using a robotic system (Autostainer; Dakocytomation) and samples were counterstained with haematoxylin. The fraction of glomeruli in which IgA was present was determined using Image-Pro Plus software (Media Cybernetics). For each glomerulus, the IgA-positive area (brown) was calculated automatically by the software and this area was, in turn, divided by the total area of the glomerulus. Twenty glomeruli were examined for each mouse and the average percentage of IgA-positive areas was determined for each mouse. A ratio of IgA-positive areas was calculated relative to control.
Similarly, the intensity of immunoreactivity for glomerular fibrinogen (brown) was examined by immunohistochemistry with a commercially available polyclonal rabbit anti-human fibrinogen antibody (Dakocytomation). Desmin-positive areas (red) in the glomeruli were examined by immunohistochemistry with a commercially available monoclonal mouse anti-human desmin antibody (Dakocytomation). The intensity of AGT immunoreactivity was examined by immunohistochemistry with a rabbit polyclonal anti-mouse/rat AGT antibody (Immuno-Biological Laboratories), as described previously.11 Immunohistochemistry was performed in a similar manner as for IgA. Tubules in 20 consecutive microscopic fields were examined for each mouse and the intensity of AGT immunoreactivity (brown) was calculated. The average intensity of AGT immunoreactivity was then obtained for each mouse. In a similar manner, the intensity of AngII immunoreactivity (brown) was examined by immunohistochemistry with commercially available rabbit anti-AngII serum (Phoenix Pharmaceuticals, Burlingame, CA, USA).11
All quantitative analyses were performed in a blinded manner to avoid any bias.
Statistical analysis
Statistical analyses were performed using Student’s t-test with Statview (Abacus Concepts, Berkeley, CA, USA). All data are presented as the mean±SEM. P < 0.05 was considered statistically significant.
RESULTS
Deposition of IgA in mesangial areas
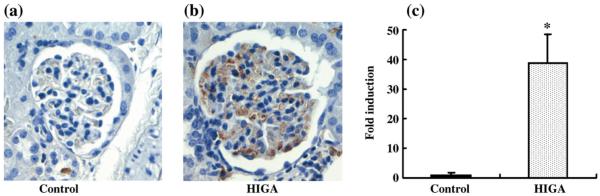
After the HIGA mice had been killed at 25 weeks of age, IgA deposition in the mesangial areas was estimated in order to confirm establishment of the IgA nephropathy model. The deposition of IgA was significantly greater in HIGA mice compared with control mice and IgA nephropathy was completely established in the HIGA mice (Fig. 1).
Fig. 1.
Immunoglobulin A deposition in mesangial areas in control and high IgA strain of ddY (HIGA) mice after harvest at 25 weeks of age. (a,b) Representative photomicrographs of IgA deposition in mesangial areas in control (a) and HIGA (b) mice at 25 weeks of age. Deposition of IgA deposition was detected in mesangial areas and was increased significantly in HIGA mice. (Original magnification ×400.) (c) Semiquantitative analysis of IgA deposition in mesangial areas in control and HIGA mice at 25 weeks of age. Twenty glomeruli were examined for each mouse and an average percentage of IgA-positive areas was obtained for each mouse and used to calculate a ratio relative to the control. Data are the mean±SEM. *P < 0.01 compared with control.
Intrarenal ROS activation
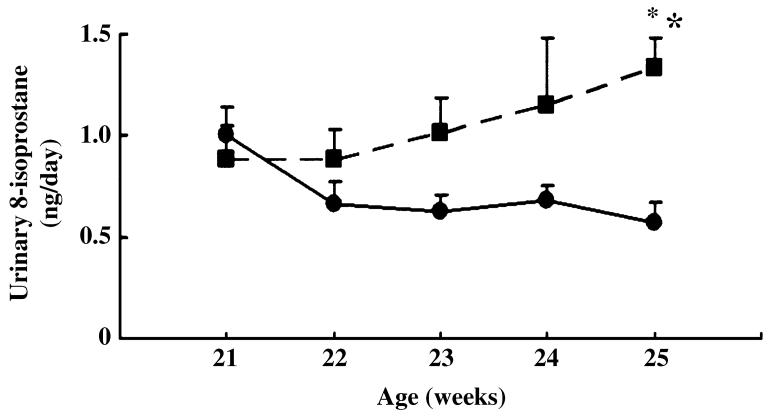
From 21 to 25 weeks of age, we monitored the urinary excretion of 8-isoprostane, the major urinary metabolite of F2-isoprostanes, which is formed non-enzymatically by the action of superoxide radicals on arachidonic acid and is used as a marker of oxidative stress.23 Gradual increases in the urinary excretion of 8-isoprostane were observed in HIGA mice compared with control mice and the difference became significant at 25 weeks of age (Fig. 2). In addition to the urinary excretion of 8-isoprostane, the accumulation of renal 4-HNE at 25 weeks of age was evaluated using western blotting. Of the aldehydes formed during lipid peroxidation, 4-HNE is the most abundant and is known to play a major role in tissue toxicity due to lipid peroxidation.24 The accumulation of renal 4-HNE was significantly greater in HIGA mice compared with control mice (Fig. 3).
Fig. 2.
Urinary excretion of 8-isoprostane in control (●) and high IgA strain of ddY (HIGA; ■) mice from 21 to 25 weeks of age. Data are the mean ± SEM. *P < 0.01 compared with control.
Fig. 3.
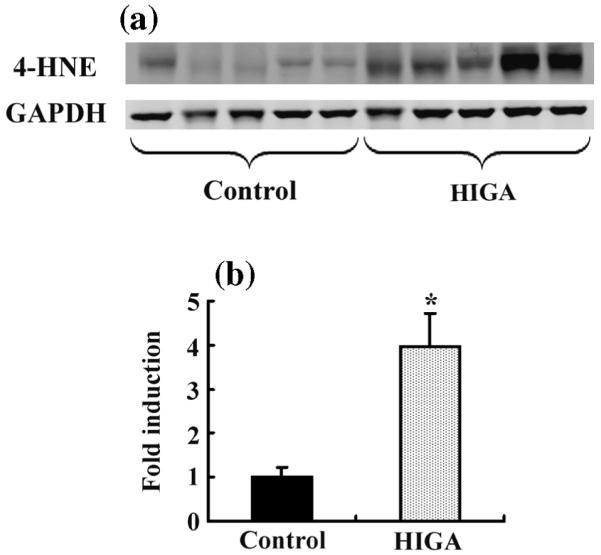
Accumulation of intrarenal 4-hydroxy-2-nonenal (4-HNE) determined by the western blot in control and high IgA strain of ddY (HIGA) mice after harvest in Week 25. (a) Accumulation of 4-HNE increased significantly in HIGA mice. (b) Densitometric ratios of 4-HNE bands normalized against GAPDH bands were calculated relative to the control. Data are the mean±SEM. *P < 0.01 compared with control.
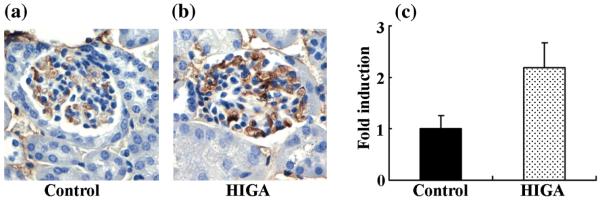
Intrarenal RAS activation
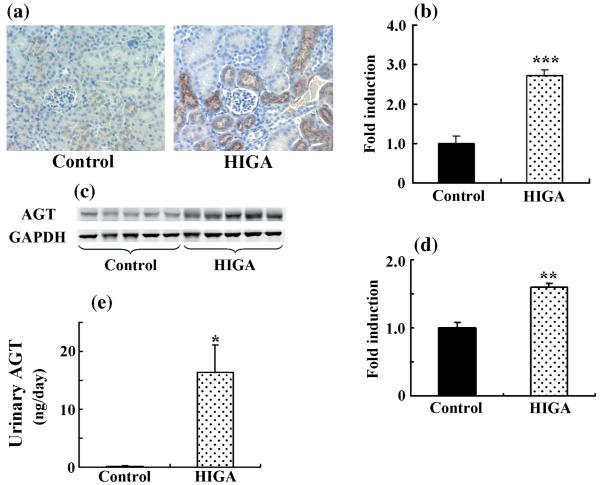
Weak immunoreactivity for AGT was noted mainly in some proximal tubular cells in control mice at 25 weeks of age. Angiotensinogen immunoreactivity in HIGA mice was significantly greater than in control mice at 25 weeks of age (Fig. 4a,b). Intrarenal AGT protein expression in control and HIGA mice was also determined in mice at 25 weeks of age using western blot. The levels of expression of AGT protein (Fig. 4c,d) and urinary AGT excretion (Fig. 4e) were significantly increased in HIGA mice compared with control mice.
Fig. 4.
Intrarenal angiotensinogen (AGT) protein levels and urinary AGT excretion in control and high IgA strain of ddY (HIGA) mice at 25 weeks of age. (a) Representative photomicrographs of immunoreactivity for AGT in control and HIGA mice at 25 weeks of age. Weak immunoreactivity for AGT was noted mainly in some proximal tubular cells in the control, whereas AGT immunoreactivity increased significantly in HIGA mice. (Original magnification ×200.) (b) Semiquantitative analysis of the intensity of intrarenal AGT immunoreactivity in control and HIGA mice at 25 weeks of age. Twenty tubulo-interstitial lesions were examined for each mouse and the averaged intensity of AGT immunoreactivity for each mouse was used to calculate the ratio relative to control. (c) Intrarenal AGT protein expression determined by western blot in control and HIGA mice at 25 weeks of age. Levels of AGT protein expression, normalized against GAPDH, increased significantly in HIGA mice. (d) Densitometric ratios of AGT bands normalized against GAPDH bands were calculated relative to control. (e) Urinary AGT excretion over 24 h in control and HIGA mice at 25 weeks of age. Data are the mean±SEM. *P < 0.01, **P < 0.001, ***P < 0.0001 compared with control.
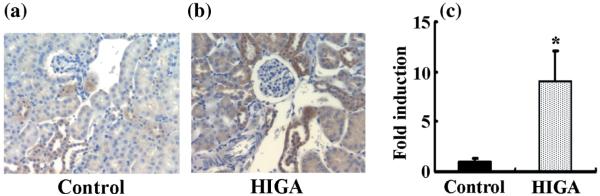
Angiotensin II immunoreactivity was detected mainly in some distal tubular cells in control mice at 25 weeks of age. In addition, in control mice some proximal tubular cells were also weakly positive for AngII. In HIGA mice, AngII staining increased significantly in proximal and distal tubular cells at 25 weeks of age (Fig. 5).
Fig. 5.
Intrarenal angiotensin (Ang) II protein levels in control and high IgA strain of ddY (HIGA) mice after harvest at 25 weeks of age. (a,b) Representative photomicrographs of immunoreactivity for AngII in control (a) and HIGA (b) mice. Immunoreactivity for AngII was noted mainly in some distal tubular cells in control mice and some proximal tubular cells were weakly positive for AngII. In HIGA mice, AngII staining increased significantly in proximal and distal tubular cells at 25 weeks of age. (c) Semiquantitative analysis of the intensity of intrarenal AngII immunoreactivity in control and HIGA mice at 25 weeks of age. Twenty tubulointerstitial lesions were examined for each mouse and the average intensity of AngII immunoreactivity for each mouse was used to calculate the ratio relative to control. Data are the mean±SEM. *P < 0.05 compared with control.
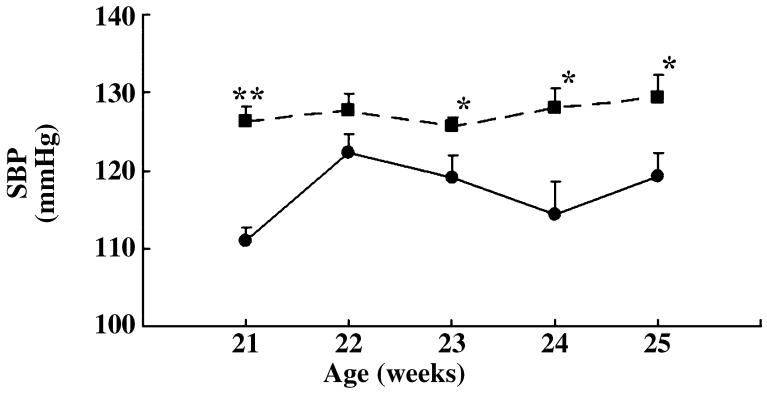
Systolic blood pressure
Systolic blood pressure was measured in mice using the tail-cuff method once a week for 4 weeks from 21 weeks of age. The SBP was significantly higher in HIGA mice compared with control mice from 21 weeks of age onwards (Fig. 6).
Fig. 6.
Systolic blood pressure (SBP) in control (●) and high IgA strain of ddY (HIGA; ■) mice from 21 to 25 weeks of age. Data are the mean±SEM. *P < 0.05, **P < 0.0001 compared with control.
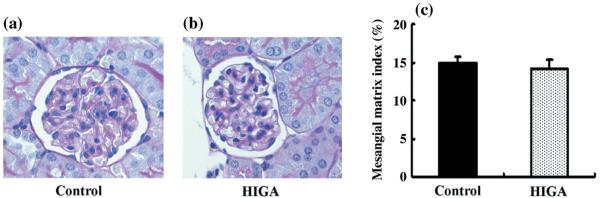
Renal histology and proteinuria
The degree of mesangial matrix expansion was estimated using PAS staining in both control and HIGA mice at 25 weeks of age; no significant differences were observed (Fig. 7). The intensity of glomerular fibrinogen immunoreactivity was also estimated in both control and HIGA mice at 25 weeks of age and weak immunoreactivity for fibrinogen was noted in the control mice. Although there were no significant differences in the level of glomerular fibrinogen immunoreactivity between control and HIGA mice, staining for glomerular fibrinogen tended to be increased mainly in the mesangial areas in HIGA mice (P = 0.065; Fig. 8).
Fig. 7.
Glomerular changes in control and high IgA strain of ddY (HIGA) mice after harvest at 25 weeks of age. (a,b) Representative photomicrographs of glomeruli stained with periodic-acid Schiff (PAS) in control (a) and HIGA (b) mice. There were no significant differences in mesangial cell proliferation and matrix accumulation between the two groups. (Original magnification ×400.) (c) Semiquantitative analysis of mesangial matrix expansion to each glomerulus in control and HIGA mice at 25 weeks of age. Twenty glomeruli were examined for each mouse and the average percentage of mesangial matrix expansion was obtained for each mouse. Data are the mean±SEM.
Fig. 8.
Glomerular fibrinogen immunoreactivity in control and high IgA strain of ddY (HIGA) mice after harvest at 25 weeks of age. (a,b) Representative photomicrographs of glomerular lesions stained with fibrinogen in control (a) and HIGA (b) mice at 25 weeks of age. In control mice, weak immunoreactivity for fibrinogen was noted. Although fibrinogen staining tended to increase mainly in mesangial areas in HIGA mice, there were no significant differences between the two groups (P = 0.065). (Original magnification ×400.) (c) Semiquantitative analysis of the intensity of glomerular fibrinogen immunoreactivity in control and HIGA mice at 25 weeks of age. Twenty glomeruli were examined for each mouse and the average intensity of fibrinogen immunoreactivity for each mouse was used to calculate the ratio relative to control. Data are the mean±SEM.
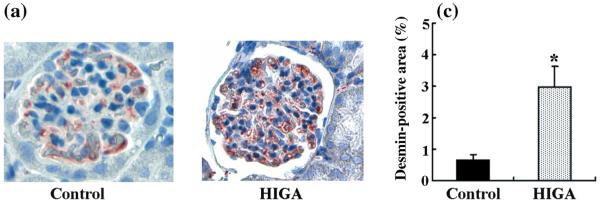
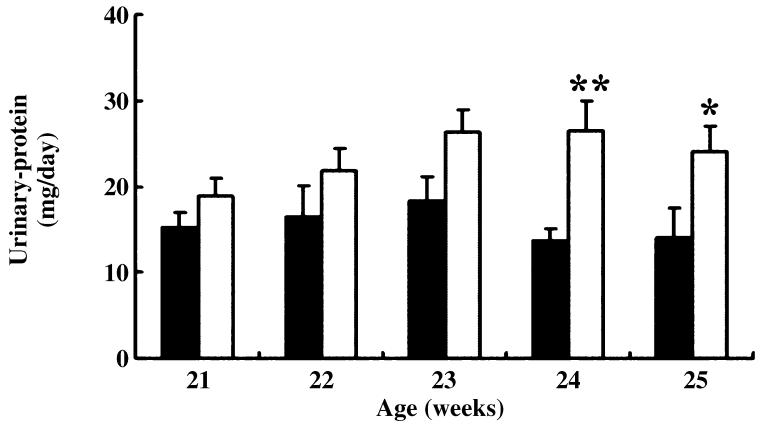
Staining for desmin was also positive in both control and HIGA mice at 25 weeks of age. In control mice, weak immunoreactivity for desmin was noted in some podocytes and mesangial cells, whereas in HIGA mice desmin staining was increased significantly in the podocytes and mesangial cells at 25 weeks of age (Fig. 9). The lesions observed coincided with the lesions that have been reported previously in mice.25 In addition to the histological changes, urinary protein excretion was compared between control and HIGA mice from 21 to 25 weeks of age. Persistently increasing proteinuria was noted in HIGA mice and significant differences were observed from 24 weeks of age (Fig. 10).
Fig. 9.
Desmin-positive areas in glomeruli in control and high IgA strain of ddY (HIGA) mice after harvest at 25 weeks of age. (a,b) Representative photomicrographs of desmin-positive areas in glomeruli in control (a) and HIGA (b) mice at 25 weeks of age. In control mice, weak immunoreactivity for desmin was noted in some podocytes and mesangial cells. In HIGA mice, desmin staining increased significantly in podocytes and mesangial cells. (Original magnification ×400.) (c) Semiquantitative analysis of -positive areas in control and HIGA mice at 25 weeks of age. Twenty glomeruli were examined for each mouse and the average percentage of desmin-positive areas was obtained for each mouse. Data are the mean±SEM. *P < 0.05 compared with control.
Fig. 10.
Urinary protein excretion over 24 h in control (■) and high IgA strain of ddY (HIGA; □) mice from 21 to 25 weeks of age. Data are the mean±SEM. *P < 0.05, **P < 0.01 compared with control.
DISCUSSION
To examine the detailed mechanisms underlying the development of IgA nephropathy, we used 25-week-old male HIGA mice as an experimental model in the present study. The HIGA mice are an inbred strain with high serum IgA levels of ddY mice, which are an outbred strain with IgA nephropathy like characteristics.26 The HIGA mice exhibit high serum IgA levels from a young age,27 specifically deposition of IgA in mesangial areas, augmentation of mesangial cells and high urinary protein excretion, which are similar to findings in patients with IgA nephropathy.26 These symptoms appear in the mice at around 25 weeks of age and, by 40 weeks of age, are getting worse.26,27 Thus, the 25-week-old HIGA mice used in the present study are an experimental model of the early phase of IgA nephropathy; however, these mice do not develop haematuria, which is one of the characteristics of IgA nephropathy.26 In the present study, age-matched male BALB/c mice were used as controls. Because BALB/c mice have low serum IgA levels and no renal damage, they are frequently used as controls in experiments on HIGA mice.27
In the present study, we demonstrated that urinary 8-isoprostane excretion and renal 4-HNE accumulation, markers of ROS, are significantly greater in HIGA mice than in control mice. The origin of 8-isoprostane in the kidneys was not determined in the present study. However, Montero et al. reported that 8-isoprostanes are synthesised by glomerular endothelial and mesangial cells under ambient high-glucose conditions.28 Therefore, 8-isoprostane may have been secreted from mesangial cells that were initially damaged in the IgA nephropathy mice. We also showed that renal AGT protein expression, urinary AGT excretion and immunoreactivity for renal AngII, markers of the RAS, were significantly increased in HIGA mice compared with control mice. Urinary AGT is known to reflect intrarenal AGT levels.29 Recently, we developed a new ELISA system to determine urinary AGT excretion levels.17 Although no significant differences were observed in levels of mesangial matrix expansion between HIGA and control mice in the present study, the intensity of the immunoreactivity of glomerular fibrinogen, a marker of renal damage, tended to be greater in HIGA mice. In addition, we found that the desmin-positive area and urinary protein excretion were significantly greater in HIGA mice than in control mice. These data suggest that both ROS and the RAS were activated during the early phase of IgA nephropathy in the HIGA mice. The augmentation of desmin expression, which was noted in the present study, suggests development of IgA nephropathy. This is because mesangial cell proliferation, mesangial matrix accumulation and podocyte injury, including detachment from glomerular capillaries, occur during the course of IgA nephropathy1 and renal damage is known to increase desmin immunoreactivity in the mesangial cells and podocytes of mice.25 It is possible that significant differences in mesangial matrix expansion and fibrinogen staining were not detected. It is possible that the 25-week-old HIGA mice were in such an early phase of IgA nephropathy that significant differences could not be detected using PAS and fibrinogen staining, as reported by Muso et al. previously.26,27
It has been reported that when proximal tubular epithelial cells are cultured in a medium conditioned with human mesangial cells activated with IgA, intratubular AngII production is upregulated.30 Angiotensin II increases AGT expression in vitro and in vivo18,21,31,32 and upregulates renin.33 Activated AGT and renin increase AngII expression in the kidney, particularly in proximal tubular cells. This explains why the intrarenal RAS was activated, especially in the tubular compartments, when only glomerular damage was induced in the present experimental model of IgA nephropathy. In addition, it is known that AngII activates the transforming growth factor-β-fibrogenic loop at the tubulointerstitium in IgA nephropathy.34 Periglomerular fibrosis, a common fibrotic change, plays an important contributory role in IgA nephropathy by occluding the glomerulo-tubular junction, resulting in the formation of atubular glomeruli.35 Thus, tubular RAS activation plays a role in glomerular injury via tubulointerstitial changes and gradually worsens IgA nephropathy.
Recently, cell-specific RAS activation was observed in mesangial cells and podocytes. Renin, AGT and angiotensin-converting enzyme (ACE) were detected in the mesangial cells and podocytes in samples of kidney tissues collected from patients with IgA nephropathy.36 Kashem et al.37 reported that in renal biopsy specimens obtained from patients with IgA nephropathy and non-IgA mesangial proliferative glomerulonephritis, the activity of the copper and zinc forms of superoxide dismutase (SOD) at both the protein and mRNA levels was lower in moderately or severely damaged tissues compared with normal or mildly damaged tissues. Low SOD activity may decrease the scavenger reaction of superoxide, thus rendering tissues more vulnerable to oxidative stress.37 In addition, Lemley et al. reported that podocyte injury was associated with an increase in the severity of IgA nephropathy and may contribute to progressive proteinuria, glomerulosclerosis and filtration failure.38 Another clinical study also showed that podocyte injury correlated with disease severity and provided additional prognostic information in patients with IgA nephropathy.39 These data may account, in part, for the renoprotective effects of AngII AT1 receptor antagonists and ACE inhibitors in the early phase of IgA nephropathy observed in clinical studies.40,41
In the present study, SBP was significantly higher in HIGA mice than in control mice at 21 weeks of age. Sachetelli et al. reported that the overproduction of AGT in the kidneys alone increased SBP and induced proteinuria and renal injury.42 This report may support our finding of significantly higher SBP in HIGA mice, whose intrarenal RAS activity was increased, compared with the SBP in control mice.
Both ROS and the RAS were undoubtedly activated in the early phase of IgA nephropathy in the present study. We reported previously that tempol (an SOD mimetic) decreases the levels of activated intrarenal AGT in rats with salt-sensitive hypertension.13 Moreover, we also demonstrated that after an increment of urinary 8-isoprostane, intrarenal AGT and AngII increased in diabetic nephropathy rats.15 In addition, it has been reported that ROS induced by high glucose concentrations stimulates AGT expression in immortalized renal proximal tubular cells.43 These data suggest that the intrarenal RAS activated by ROS plays an important role in the development of IgA nephropathy. However, we could not clearly determine whether both ROS and RAS activation induced renal damage. In the near future, we will conduct an interventional study in the experimental model used in the present study to determine whether ROS and RAS activation produce renal damage.
In conclusion, we demonstrated for the first time in the present study that augmented oxidative stress, intrarenal AGT and intrarenal AngII are involved in the development of renal injury in the early phase of IgA nephropathy in HIGA mice. These data suggest that both ROS and RAS activation are involved in the development of IgA nephropathy in this experimental model of the disease from an early phase.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408). The authors acknowledge critical reviews and valuable comments from Drs Romer A Gonzalez-Villalobos and L Gabriel Navar (Tulane University). The authors also acknowledge the excellent technical assistance of My-Linh Rauv and Duy V Tran (Tulane University).
REFERENCES
- 1.Barratt J, Feehally J. IgA nephropathy. J. Am. Soc. Nephrol. 2005;16:2088–97. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 2.Rivera F, Lopez-Gomez JM, Perez-Garcia R. Frequency of renal pathology in Spain 1994-1999. Nephrol. Dial. Transplant. 2002;17:1594–602. doi: 10.1093/ndt/17.9.1594. [DOI] [PubMed] [Google Scholar]
- 3.Schena FP, The Italian Group of Renal Immunopathology Survey of the Italian registry of renal biopsies. Frequency of the renal diseases for 7 consecutive years. Nephrol. Dial. Transplant. 1997;12:418–26. doi: 10.1093/ndt/12.3.418. [DOI] [PubMed] [Google Scholar]
- 4.Simon P, Ramee MP, Boulahrouz R, et al. Epidemiologic data of primary glomerular diseases in western France. Kidney Int. 2004;66:905–8. doi: 10.1111/j.1523-1755.2004.00834.x. [DOI] [PubMed] [Google Scholar]
- 5.Research Group on Progressive Chronic Renal Disease Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1850 biopsied cases. Nephron. 1999;82:205–13. doi: 10.1159/000045404. [DOI] [PubMed] [Google Scholar]
- 6.Li LS, Liu ZH. Epidemiologic data of renal diseases from a single unit in China. Analysis based on 13 519 renal biopsies. Kidney Int. 2004;66:920–3. doi: 10.1111/j.1523-1755.2004.00837.x. [DOI] [PubMed] [Google Scholar]
- 7.Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol. Dial. Transplant. 2001;16:1364–7. doi: 10.1093/ndt/16.7.1364. [DOI] [PubMed] [Google Scholar]
- 8.Nair R, Walker PD. Is IgA nephropathy the commonest primary glomerulopathy among young adults in the USA? Kidney Int. 2006;69:1455–8. doi: 10.1038/sj.ki.5000292. [DOI] [PubMed] [Google Scholar]
- 9.Dillon JJ. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for IgA nephropathy. Semin. Nephrol. 2004;24:218–24. doi: 10.1016/j.semnephrol.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Kobori H, Ozawa Y, Suzaki Y, et al. Young Scholars Award Lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am. J. Hypertens. 2006;19:541–50. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobori H, Katsurada A, Ozawa Y, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem. Biophys. Res. Commun. 2007;358:156–63. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl-salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–7. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in Dahl salt-sensitive rats. Biochem. Biophys. Res. Commun. 2004;315:746–50. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in Zucker diabetic fatty rats. Int. J. Biol. Sci. 2007;3:40–6. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin angiotensin system axis in renal injury of type 2 diabetic rats. Clin. Exp. Pharmacol. Physiol. 2008;35:922–7. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker LA, Gellai M, Valtin H. Renal response to pentobarbital anesthesia in rats: Effect of interrupting the renin-angiotensin system. J. Pharmacol. Exp. Ther. 1986;236:721–8. [PubMed] [Google Scholar]
- 17.Kobori H, Katsurada A, Miyata K, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am. J. Physiol. Renal Physiol. 2008;294:F1257–63. doi: 10.1152/ajprenal.00588.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobori H, Ozawa Y, Satou R, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in genetargeted mice. Am. J. Physiol. Renal Physiol. 2007;293:F938–45. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am. J. Physiol. Renal Physiol. 2007;293:F956–60. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-kappaB axis in angiotensin II-induced renal injury. Am. J. Physiol. Renal Physiol. 2007;293:F100–9. doi: 10.1152/ajprenal.00520.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J. Am. Soc. Nephrol. 2005;16:2073–80. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am. J. Physiol. Renal Physiol. 2007;292:F330–9. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrono C, FitzGerald GA. Isoprostanes: Potential markers of oxidant stress in atherothrombotic disease. Arterioscler. Thromb. Vasc. Biol. 1997;17:2309–15. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- 24.Ji C, Kozak KR, Marnett LJ. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001;276:18 223–8. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 25.Barisoni L, Madaio MP, Eraso M, Gasser DL, Nelson PJ. The kd/kd mouse is a model of collapsing glomerulopathy. J. Am. Soc. Nephrol. 2005;16:2847–51. doi: 10.1681/ASN.2005050494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyawaki S, Muso E, Takeuchi E, et al. Selective breeding for high serum IgA levels from noninbred ddY mice: Isolation of a strain with an early onset of glomerular IgA deposition. Nephron. 1997;76:201–7. doi: 10.1159/000190169. [DOI] [PubMed] [Google Scholar]
- 27.Muso E, Yoshida H, Takeuchi E, et al. Enhanced production of glomerular extracellular matrix in a new mouse strain of high serum IgA ddY mice. Kidney Int. 1996;50:1946–57. doi: 10.1038/ki.1996.517. [DOI] [PubMed] [Google Scholar]
- 28.Montero A, Munger KA, Khan RZ, et al. F2-isoprostanes mediate high glucose-induced TGF-beta synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int. 2000;58:1963–72. doi: 10.1111/j.1523-1755.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 29.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–85. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan LY, Leung JC, Tang SC, Choy CB, Lai KN. Tubular expression of angiotensin II receptors and their regulation in IgA nephropathy. J. Am. Soc. Nephrol. 2005;16:2306–17. doi: 10.1681/ASN.2004121117. [DOI] [PubMed] [Google Scholar]
- 31.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–35. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective SV40 DNA: Autocrine ANG II feedback. Am. J. Physiol. 1999;276:F218–27. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 33.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–9. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Prete D, Gambaro G, Lupo A, et al. Precocious activation of genes of the renin-angiotensin system and the fibrogenic cascade in IgA glomerulonephritis. Kidney Int. 2003;64:149–59. doi: 10.1046/j.1523-1755.2003.00065.x. [DOI] [PubMed] [Google Scholar]
- 35.Sato M, Hotta O, Taguma Y. Glomerulo-tubular junction stenosis as a factor contributing to glomerular obsolescence in IgA nephropathy. J. Pathol. 2002;197:14–19. doi: 10.1002/path.1085. [DOI] [PubMed] [Google Scholar]
- 36.Lai KN, Leung JC, Lai KB, To WY, Yeung VT, Lai FM. Gene expression of the renin-angiotensin system in human kidney. J. Hypertens. 1998;16:91–102. doi: 10.1097/00004872-199816010-00014. [DOI] [PubMed] [Google Scholar]
- 37.Kashem A, Endoh M, Yamauchi F, et al. Superoxide dismutase activity in human glomerulonephritis. Am. J. Kidney Dis. 1996;28:14–22. doi: 10.1016/s0272-6386(96)90125-0. [DOI] [PubMed] [Google Scholar]
- 38.Lemley KV, Lafayette RA, Safai M, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–85. doi: 10.1046/j.1523-1755.2002.00269.x. [DOI] [PubMed] [Google Scholar]
- 39.Hishiki T, Shirato I, Takahashi Y, Funabiki K, Horikoshi S, Tomino Y. Podocyte injury predicts prognosis in patients with IgA nephropathy using a small amount of renal biopsy tissue. Kidney Blood Press. Res. 2001;24:99–104. doi: 10.1159/000054214. [DOI] [PubMed] [Google Scholar]
- 40.Maschio G, Cagnoli L, Claroni F, et al. ACE inhibition reduces proteinuria in normotensive patients with IgA nephropathy: A multicentre, randomized, placebo-controlled study. Nephrol. Dial. Transplant. 1994;9:265–9. [PubMed] [Google Scholar]
- 41.Russo D, Pisani A, Balletta MM, et al. Additive antiproteinuric effect of converting enzyme inhibitor and losartan in normotensive patients with IgA nephropathy. Am. J. Kidney Dis. 1999;33:851–6. doi: 10.1016/s0272-6386(99)70416-6. [DOI] [PubMed] [Google Scholar]
- 42.Sachetelli S, Liu Q, Zhang SL, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–23. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002;143:2975–85. doi: 10.1210/endo.143.8.8931. [DOI] [PubMed] [Google Scholar]