Abstract
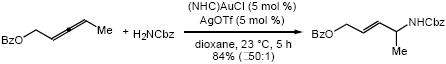
Reaction of 2,3-pentadienyl benzoate and benzyl carbamate with a catalytic 1:1 mixture of (NHC)AuCl and AgOTf in dioxane at 23 °C for 5 h led to isolation of (E)-4-(benzyloxycarbonylamino)-2-pentenyl benzoate in 84% yield as a single regio- and diastereomer. Gold(I)-catalyzed hydroamination was effective for a number of N-unsubstituted carbamates and a range of substituted allenes.
Allylic amines are components of many naturally occurring and biologically active molecules and are versatile building blocks for the synthesis of complex nitrogen-containing molecules. As a result, considerable effort has been directed toward the development of general and selective methods for the synthesis of allylic amines.1,2 The transition metal-catalyzed addition of the N–H bond of an amine or carboxamide derivative across the C=C bond of an allene represents an attractive and atom economical approach to the synthesis of allylic amines.3 However, whereas general and efficient methods for the intramolecular hydroamination of allenes have been developed,4,5 the intermolecular hydroamination of allenes remains problematic and no methods are availavble that effectively employ ammonia or ammonia equivalents as nucleophiles.6,7 Here we describe a general, regio- and stereoselective gold(I)-catalyzed protocol for the intermolecular hydroamination of allenes that employs N-unsubstituted carbamates as ammonia surrogates.
We have developed an effective protocol for the intramolecular hydroamination of N- γ- and δ-allenyl carbamates catalyzed by a mixture of the gold phosphine complex (1)AuCl [1 = Pt-Bu2o-biphenyl]5 and AgOTf and a protocol for the intermolecular hydroalkoxylation of allenes with alcohols catalyzed by a mixture of the gold (NHC) complex (2)AuCl [2 = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidine] and AgOTf (Scheme 1).8,9 We therefore targeted (1)AuCl and (2) AuCl as precatalysts for the intermolecular hydroamination of allenes with N-unsubstituted carbamates. In an initial experiment, reaction of 3-methyl-1,2-butadiene (3) and benzyl carbamate catalyzed by a 1:1 mixture of (1)AuCl and AgOTf in dioxane at 23 °C for 24 h led to 57% conversion to form the N-tertiary allylic carbamate 4a as the exclusive product (Table 1, entry 1). Longer reaction time or employment of related gold phosphine catalysts led to no significant improvement in conversion (Table 1, entries 2-5). Conversely, reaction of 3 and benzyl carbamate with a catalytic mixture of (2)AuCl and AgOTf at 23 °C for 24 h led to 96% conversion to form 4a as the exclusive product (Table 1, entry 6). Allylic carbamate 4a was isolated in 93% yield from the corresponding preparative-scale reaction (Table 2, entry 1).
Scheme 1.
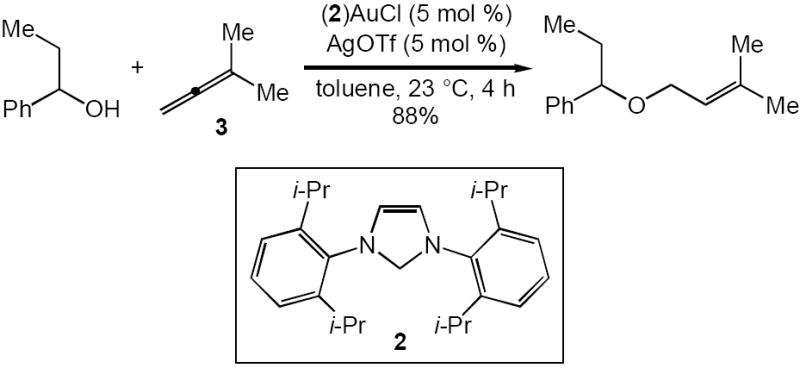
Table 1.
Gold(I)-Catalyzed Hydroamination of 3-Methyl-1,2-butadiene (3) with Benzyl Carbamate as a Function of Supporting Ligand.
 | ||
|---|---|---|
| entry | L | convn (%)a |
| 1 | 1 | 57 |
| 2 | PCy2o-biphenyl | 58 |
| 3 | PCy2{2-[2,5-(OMe)2C6H3]C6H4} | 47 |
| 4 | Pt-Bu2[2-(2-NMe2C6H3)C6H4] | 0 |
| 5 | P(4-MeOC6H4)3 | 25 |
| 6 | 2 | 96 |
Conversion determined by GC analysis of the crude reaction mixture versus hexadecane internal standard.
Table 2.
Intermolecular Hydroamination of Allenes with N-Unsubstituted Carbamates Catalyzed by a Mixture of (2)AuCl (5 mol %) and AgOTf (5 mol %) in Dioxane at 23 °C for 24 h.
| entry | allene | R of H2NR | allylic carbamate | compd | yield (%)a |
|---|---|---|---|---|---|
| 1 | 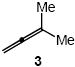 |
Cbz | 4a | 93 | |
| 2 | Fmoc | 4b | 94 | ||
| 3 | CO2Me | 4c | 87 | ||
| 4 | 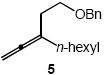 |
Cbz | 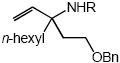 |
7a | 58 |
| 5 | CO2Me | 7b | 58 | ||
| 6 | 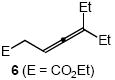 |
Cbz | 8 | 61 | |
| 7 | Cbz | 10 | 75 | ||
| 8 | 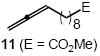 |
Cbz | 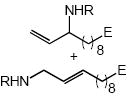 |
88(1:1) | |
| 9 | 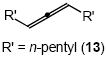 |
Cbz | 17 | 83 | |
| 10 | Cbz | 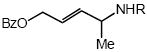 |
18 | 84 | |
| 11b | Cbz | 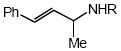 |
19 | 80 | |
| 12 | 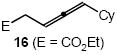 |
Cbz | 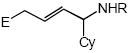 |
20 | 81 |
| 13 | 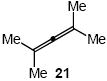 |
CO2Me | 22 | 61 |
Yields refer to isolated material of >95% purity. All N-allylic carbamates were formed in ≥98% regio- and diastereoisomeric purity unless noted otherwise.
Employment of enantiomerically enriched (S)-15 (76% ee) formed racemic 19 in 78% yield.
In addition to benzyl carbamate, 9-fluorenylmethyl carbamate and methyl carbamate reacted with 3 in the presence of (2)AuCl/AgOTf to form N-tertiary allylic carbamates 4b and 4c, respectively (Table 2, entries 2 and 3). Both the differentially 1,1-disubstituted allene 5 and trisubstituted allene 6 underwent intermolecular hydroamination to form the corresponding N-tertiary allylic carbamates (7-8) in modest yield as single regioisomers (Table 2, entries 4-6). Whereas hydroamination of the electron-deficient monosubstituted allene 9 led to exclusive formation of the N-primary (E)-allylic carbamate 10 (Table 2, entry 7), hydroamination of mono-alkyl-substituted allene 11 formed a 1:1 mixture of N-primary (12a) and N-secondary (12b) allylic carbamates (Table 2, entry 8). 1,3-Disubstituted allenes 13-16 underwent hydroamination in good yield, with high E-selectivity, and, in the case of differentially-substituted allenes 14-16, with exclusive attack of carbamate at the more electron-rich allene terminus (Table 2, entries 9-12). Reaction of enantiomericaly enriched allene (S)-15 (76% ee) with benzyl carbamate led to isolation of racemic 19 in 78% yield. This outcome is not surprising given the rapid (≤10 min) racemization of (S)-15 under reaction conditions.8 Hydroamination of tetrasubstituted allene 21 formed N-tertiary allylic carbamate 22 in modest yield (Table 2, entry 13).
Noteworthy is the contrasting regioselectivity of the (2)AuCl/AgOTf-catalyzed intermolecular hydroamination and hydroalkoxylation of allenes. While both transformations favor addition of the nucleophile to the more electron-rich terminus of differentially 1,3-disubstituted allenes such as 14 and 15, hydroalkoxylation displays much greater sensitivity to steric hinderance than does hydroamination, leading to preferential attack of alcohol at the less-substituted terminus of 1,1-disubstituted allenes such as 3 and trisubstituted allenes, as opposed to selective attack of carbamate at the more substituted allene terminus.10
Stereochemical analysis of the gold(I)-catalyzed hydrofunctionalization of C–C multiple bonds has consistently supported outer-sphere pathways for C–X (X = N, O, C) bond formation.5,8,11 It therefore appears likely that the intermolecular hydroamination of allenes catalyzed by (2)AuCl/AgOTf occurs via outer-sphere attack of the carbamate on gold π-allene complex I to initially form the cationic gold σ-alkenyl complex II that loses a proton to form II (Scheme 2). Protonolysis of the Au–C bond of III then releases the N-allylic carbamate with regeneration of the cationic Au(I) catalyst.12 Available evidence regarding the gold(I)-catalyzed hydrofunctionalization of allenes points to rapid and reversible formation of one or more gold π-allene complex followed by irreversible C–X bond formation.5,8,13 Therefore, the regioselectivity of gold(I)-catalyzed intermolecular hydroamination and hydroalkoxylation is presumably established via kinetic trapping of gold(I) π-allene complexes I and Ia with carbamate and alcohol, respectively, under Curtin-Hammett conditions (Scheme 2). However, the origins of this nucleophile-dependent selectivity remain unclear.10
Scheme 2.
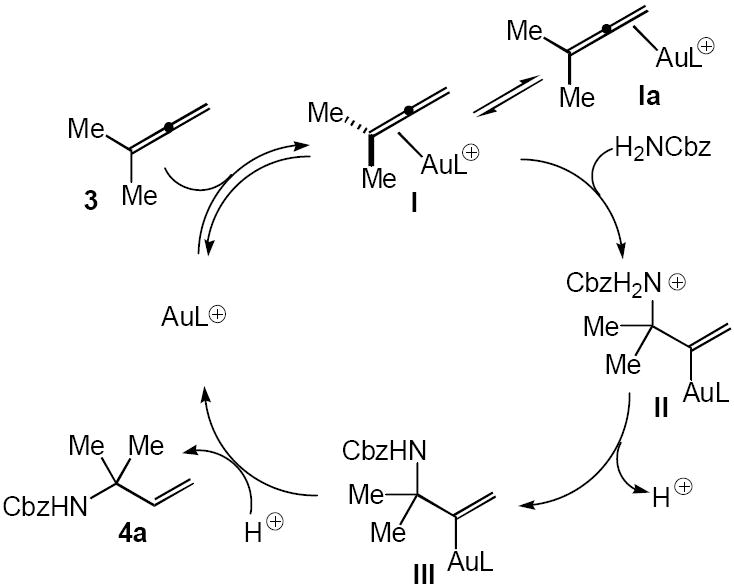
In summary, we have developed a gold(I)-catalyzed protocol for the intermolecular hydroamination of allenes. The protocol was effective for a number of N-unsubstituted carbamates and was effective for monosubstituted, 1,1- and 1,3-disubstituted, trisubsituted, and tetrasubstituted allenes. We are currently working toward the development of enantioselective intermolecular allene hydroamination protocols and toward an understanding of the nucleophile-dependent regioselectivity of gold(I)-catalyzed allene hydrofunctionalization.
Supplementary Material
Acknowledgments
Acknowledgment is made to the NSF (CHE-0555425), NIH (GM-080422), and Johnson&Johnson for support of this research.
Footnotes
Supporting Information Available: Experimental procedures and scans of NMR spectra for N-allylic carbamates (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Johannsen M, Jørgensen KA. Chem Rev. 1998;98:1689. doi: 10.1021/cr970343o. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Van Vranken DL. Chem Rev. 1996;96:395. doi: 10.1021/cr9409804. [DOI] [PubMed] [Google Scholar]; (c) Hollis TK, Overman LE. J Organomet Chem. 1999;576:290. [Google Scholar]; (d) Basavaiah D, Rao AJ, Satyanarayana T. Chem Rev. 2003;103:811. doi: 10.1021/cr010043d. [DOI] [PubMed] [Google Scholar]
- 2.For recent efforts directed toward the synthesis of allylic amines see: Singh OV, Han H. J Am Chem Soc. 2007;129:774. doi: 10.1021/ja067966g.Polet D, Alexakis A, Tissot-Croset K, Corminboeuf C, Ditrich K. Chem Eur J. 2006;12:3596. doi: 10.1002/chem.200501180.Defieber C, Ariger MA, Moriel P, Carreira EM. Angew Chem Int Ed. 2007;46:3139. doi: 10.1002/anie.200700159.Weiss ME, Fischer DF, Xin Z-q, Jautze S, Schweizer WB, Peters R. Angew Chem Int Ed. 2006;45:5694. doi: 10.1002/anie.200601731.Jautze S, Seiler P, Peters R. Angew Chem Int Ed. 2007;46:1260. doi: 10.1002/anie.200603568.Skucas E, Ngai M, Komanduri V, Krische MJ. Acc Chem Res. 2007;40:1394. doi: 10.1021/ar7001123.Reed SA, White MC. J Am Chem Soc. 2008;130:3316. doi: 10.1021/ja710206u.Fiori KW, Du Bois J. J Am Chem Soc. 2007;129:562. doi: 10.1021/ja0650450.Liang C, Collet F, Robert-Peillard F, Muller P, Dodd RH, Dauban P. J Am Chem Soc. 2008;130:343. doi: 10.1021/ja076519d.Matsui K, Takizawa S, Sasai H. J Am Chem Soc. 2005;127:3680. doi: 10.1021/ja0500254.Shi Y-L, Shi M. Eur J Org Chem. 2007:2905.
- 3.The intermolecular hydroamination of 1,3-dienes has also been investigated as a route to allylic amines. Qin H, Yamagiwa N, Matsunaga S, Shibasaki M. J Am Chem Soc. 2006;128:1611. doi: 10.1021/ja056112d.Brouwer C, He C. Angew Chem Int Ed. 2006;45:1744. doi: 10.1002/anie.200504495.Johns AM, Liu Z, Hartwig JF. Angew Chem Int Ed. 2007;46:7259. doi: 10.1002/anie.200701899.Johns AM, Utsunomiya M, Incarvito CD, Hartwig JF. J Am Chem Soc. 2006;128:1828. doi: 10.1021/ja056003z.Lober O, Kawatsura M, Hartwig JF. J Am Chem Soc. 2001;123:4366. doi: 10.1021/ja005881o.Baker R, Onions A, Popplestone RJ, Smith TN. J Chem Soc Perkin Trans. 1975;2:1133.Pawlas J, Nakao Y, Kawatsura M, Hartwig JF. J Am Chem Soc. 2002;124:3669. doi: 10.1021/ja017575w.Kiji J, Sasakawa E, Yamamoto K, Furukawa J. J Organomet Chem. 1974;77:125.
- 4.(a) Volz F, Krause N. Org Biomol Chem. 2007;5:1519. doi: 10.1039/b703995f. [DOI] [PubMed] [Google Scholar]; (b) Morita N, Krause N. Eur J Org Chem. 2006:4634. [Google Scholar]; (c) Morita N, Krause N. Org Lett. 2004;6:4121. doi: 10.1021/ol0481838. [DOI] [PubMed] [Google Scholar]; (d) Hoover JM, Petersen JR, Pikul JH, Johnson AR. Organometallics. 2004;23:4614. [Google Scholar]; (e) Zhang Z, Bender CF, Widenhoefer RA. Org Lett. 2007;9:2887. doi: 10.1021/ol071108n. [DOI] [PubMed] [Google Scholar]; (f) LaLonde RL, Sherry BD, Kang EJ, Toste FD. J Am Chem Soc. 2007;129:2452. doi: 10.1021/ja068819l. [DOI] [PubMed] [Google Scholar]; (g) Lee PH, Kim H, Lee K, Kim M, Noh K, Kim H, Seomoon D. Angew Chem Int Ed. 2005;44:1840. doi: 10.1002/anie.200462512. [DOI] [PubMed] [Google Scholar]; (h) Meguro M, Yamamoto Y. Tetrahedron Lett. 1998;39:5421. [Google Scholar]; (i) Patil NT, Lutete LM, Nishina N, Yamamoto Y. Tetrahedron Lett. 2006;47:4749. [Google Scholar]; (j) Claesson A, Sahlberg C, Luthman K. Acta Chem Scand. 1979:309. [Google Scholar]; (k) Huby NJS, Kinsman RG, Lathbury D, Vernon PG, Gallagher T. J Chem Soc Perkin Trans 1. 1991:145. [Google Scholar]; (l) Gallagher T, Jones SW, Mahon MF, Molloy KC. J Chem Soc Perkin Trans 1. 1991:2193. [Google Scholar]; (m) Ha JD, Cha JK. J Am Chem Soc. 1999;121:10012. [Google Scholar]; (n) Arredondo VM, McDonald FE, Marks TJ. J Am Chem Soc. 1998;120:4871. [Google Scholar]; (o) Hong S, Kawaoka AM, Marks TJ. J Am Chem Soc. 2003;125:15878. doi: 10.1021/ja036266y. [DOI] [PubMed] [Google Scholar]; (p) Ackermann L, Bergman RG. Org Lett. 2002;4:1475. doi: 10.1021/ol0256724. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Ackermann L, Bergman RG, Loy RN. J Am Chem Soc. 2003;125:11956. doi: 10.1021/ja0361547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Liu C, Kinder RE, Han X, Qian H, Widenhoefer RA. J Am Chem Soc. 2006;128:9066. doi: 10.1021/ja062045r. [DOI] [PubMed] [Google Scholar]
- 6.(a) Johnson JS, Bergman RG. J Am Chem Soc. 2001;123:2923. doi: 10.1021/ja005685h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ayinla RO, Schafer LL. Inorg Chim Acta. 2006;359:3097. [Google Scholar]; (c) Besson L, Gore J, Cazes B. Tetrahedron Lett. 1995;36:3857. [Google Scholar]; (d) Nishina N, Yamamoto Y. Angew Chem Int Ed. 2006;45:3314. doi: 10.1002/anie.200600331. [DOI] [PubMed] [Google Scholar]; (e) Nishina N, Yamamoto Y. Synlett. 2007:1767. [Google Scholar]
- 7.Yamamoto has reported the Pd(0)-catalyzed hydroamination of allenes with dibenzylamine and sulfonamide, but these transformations were restricted to monosubstituted aryl allenes and, in the case of TsNH2, led to formation of mixtures of mono- and bisaddition products: Al-Masum M, Meguro M, Yamamoto Y. Tetrahedron Lett. 1997;38:6071.
- 8.Zhang Z, Widenhoefer RA. Org Lett. 2008;10:2079. doi: 10.1021/ol800646h. [DOI] [PubMed] [Google Scholar]
- 9.For additional examples of intermolecular allene hydrofunctionalization see: Patil NT, Pahadi NK, Yamamoto Y. Can J Chem. 2005;83:569. doi: 10.1021/jo051524q.Kim IS, Krische M. J Org Lett. 2008;10:513. doi: 10.1021/ol702914p.Schulz M, Teles JH. Chem Abstr (BASF AG), WO-A1 9721648. 1997;127:121499.Trost BM, Gerusz VJ. J Am Chem Soc. 1995;117:5156.Yamamoto Y, Al-Masum M, Asao N. J Am Chem Soc. 1994;116:6019.Yamamoto Y, Al-Masum M, Fujiwara N, Asao N. Tetrahedron Lett. 1995;36:2811.Yamamoto Y, Al-Masum M. Synlett. 1995:969.Yamamoto Y, Al-Masum M, Takeda A. Chem Commun. 1996:831.Yamamoto Y, Al-Masum M, Fujiwara N. Chem Commun. 1996:381.Besson L, Gore J, Cazes B. Tetrahedron Lett. 1995;36:3853.Al-Masum M, Yamamoto Y. J Am Chem Soc. 1998;120:3809.
- 10.It appears unlikely that these regiochemical differences can be attributed soley to the steric profile of the respective nucleophiles as reaction of 3 with 2-phenyl-1-ethanol catalyzed by (2)AuCl/AgOTf led to formation of a 4.4:1 mixture of 3-methyl-1-phenethoxy-2-butene and 1,1-dimethyl-1-phenethoxy-2-propene: Zhang Z, Widenhoefer RA. unpublished results.
- 11.(a) Kennedy-Smith JJ, Staben ST, Toste FD. J Am Chem Soc. 2004;126:4526. doi: 10.1021/ja049487s. [DOI] [PubMed] [Google Scholar]; (b) Zhang J, Yang C-G, He C. J Am Chem Soc. 2006;128:1798. doi: 10.1021/ja053864z. [DOI] [PubMed] [Google Scholar]; (c) Hashmi ASK, Weyrauch JP, Frey W, Bats JW. Org Lett. 2004;6:4391. doi: 10.1021/ol0480067. [DOI] [PubMed] [Google Scholar]; (d) Liu Y, Song F, Song Z, Liu M, Yan B. Org Lett. 2005;7:5409. doi: 10.1021/ol052160r. [DOI] [PubMed] [Google Scholar]
- 12.Kovács G, Ujaque G, Lledós A. J Am Chem Soc. 2008;130:853. doi: 10.1021/ja073578i. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z, Bender CF, Widenhoefer RA. J Am Chem Soc. 2007;129:14148. doi: 10.1021/ja0760731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


