Abstract
Tissue engineering remains a promising therapeutic strategy for the repair or regeneration of diseased or damaged tissues. Previous approaches have typically focused on combining cells and bioactive molecules (e.g., growth factors, cytokines, and DNA fragments) with a biomaterial scaffold that function as a template to control the geometry of the newly formed tissue, while facilitating the attachment, proliferation, and differentiation of embedded cells. Biomaterial scaffolds also play a crucial role in determining the functional properties of engineered tissues, including biomechanical characteristics such as inhomogeneity, anisotropy, nonlinearity, or viscoelasticity. While single-phase, homogenous materials have been used extensively to create numerous types of tissue constructs, there continue to be significant challenges in the development of scaffolds that can provide the functional properties of load-bearing tissues such as articular cartilage. In an attempt to create more complex scaffolds that promote the regeneration of functional engineered tissues, composite scaffolds comprising two or more distinct materials have been developed. This paper reviews various studies on the development and testing of composite scaffolds for the tissue engineering of articular cartilage, using techniques such as embedded fibers and textiles for reinforcement, embedded solid structures, multi-layered designs, or three-dimensionally woven composite materials. In many cases, the use of composite scaffolds can provide unique biomechanical and biological properties for the development of functional tissue engineering scaffolds.
Keywords: articular cartilage, tissue engineering, stem cell, hydrogel, fiber, biomaterial
1. INTRODUCTION
Articular cartilage is the avascular and aneural tissue that functions as a nearly frictionless, load-bearing surface covering the ends of long bones in diarthrodial joints. Healthy articular cartilage is able to withstand decades of repetitive loading that regularly exceeds several times body weight [1]. Its unique behavior is attributed to the multiphasic nature of the extracellular matrix (ECM), which can be described as a charged, porous-permeable solid that is saturated with water and dissolved ions [2, 3]. Collagen, the primary component within the solid phase of the ECM, forms a fibrous network that is predominantly responsible for determining the tensile and shear mechanical characteristics of the tissue. Intermingled throughout this collagen microstructure are large, negatively charged proteoglycan macromolecules that give cartilage the ability to imbibe water and swell the tissue in an attempt to maintain physicochemical equilibrium [4–6]. This swelling effect, which is constrained by the collagen network, pressurizes the tissue and contributes significantly to its load bearing and low-friction properties [7, 8].
Osteoarthritis (OA), the most common form of degenerative joint disease affecting articular cartilage, is a chronic condition influenced by a combination of both biochemical and biomechanical factors [9, 10]. OA is characterized by the progressive, irreversible breakdown of cartilage through pathologic processes that may take over 20 or 30 years [11, 12]. The most common and successful surgical treatment for persons suffering from OA of the hip, knee, or shoulder is total joint replacement [13, 14].
Unfortunately, the materials used in these procedures have a limited lifespan in vivo (i.e., 10 to 20 years) and as a result, are not suitable for younger or more active patients [15]. Existing clinical treatments for full-thickness lesions that attempt to promote cartilage repair (i.e., microfracture), or repair defects using healthy transplanted tissue (i.e., autograft or allograft transplantation) have yielded variable success [16]. To date, one approved cell-based therapy – autologous chondrocyte transplantation – has shown good initial results [17, 18], but recent controlled trials suggest similar outcomes as compared to the microfracture technique [19]. Despite the short- and mid-term benefits afforded by these repair methods, an effective long-term treatment for cartilage pathology does not currently exist.
Tissue engineering remains one of the most promising therapeutic strategies aimed at repairing diseased or damaged biological tissues. Previous attempts have typically focused on combining cells and bioactive molecules (e.g., growth factors, cytokines, and DNA fragments) with a biomaterial scaffold whose primary function is to control the shape and volume of the newly formed tissue, while at the same time facilitating the attachment, proliferation, and differentiation of embedded cells [20–23]. In addition to influencing the shape and metabolic activity of the engineered construct, scaffolds must also be capable of withstanding the mechanical environment of the native tissue that is to be replaced [24, 25]. To satisfy these criteria, one strategy that has been investigated is the development of specialized scaffolds formed by combining two or more dissimilar materials. The result is a composite structure that possesses unique properties that cannot be replicated by a single material alone. Here we review various techniques that have been previously used to construct multi-material scaffolds for the tissue engineering of articular cartilage using techniques such as embedded fibers and textiles for reinforcement, embedded solid structures, multi-layered designs, or three-dimensionally woven composite materials. We conclude with a brief discussion of our ongoing development of a 3-D woven composite scaffold system engineered to recapitulate the complex biomechanical properties of native cartilage.
2. EMBEDDED REINFORCEMENT
2.1 Embedded fibers and textiles
One method that has been used to form composite scaffolds for cartilage tissue engineering has been to embed textile materials, either as randomly dispersed fibers or higher-order structures, within hydrogels or bulk polymers. The rationale behind forming these types of structures has varied; however, the main advantage of this approach is that the constituent fibers provide reinforcement to the scaffolds and improve their mechanical characteristics.
For example, Marijnissen et al. studied the effects of using alginate as a chondrocyte-delivery substance to aid in the construction of tissue-engineered cartilage grafts [26]. Non-woven polyglactin (PLGA) fiber meshes were seeded directly with bovine chondrocytes, or bovine chondrocytes suspended in alginate to test the effects of using a hydrogel as a cell carrier for forming cartilage constructs. The authors reasoned that the alginate carrier could potentially improve seeding by allowing for even cell distribution while retaining the chondrocytes within the large pores of the non-woven scaffolds. After being implanted subcutaneously in nude mice for 8 weeks, both the alginate-PLGA constructs and the PLGA only constructs had synthesized similar amounts of glycosaminoglycans and collagen type II, however, the PLGA only constructs had a dense layer of cells concentrated along their outside borders while the cells in the alginate-PLGA constructs were more equally distributed. The PLGA-only constructs had contracted and warped from their original size and shape during the 8 week implantation period while the alginate-PLGA constructs had not. The results of this study showed that an alginate cell-carrier can increase seeding efficiency by retaining and uniformly dispersing cells throughout the pores of non-woven fiber meshes, without negatively affecting extracellular matrix protein synthesis. Additionally, by combining the non-woven meshes with a hydrogel carrier, initial construct geometry was maintained.
Using a similar two-material approach to more effectively retain seeded cells, Chen et al. developed a scaffold consisting of a web-like collagen microsponge formed upon a knitted PLGA fabric [27]. The knitted fabric provided the scaffold with mechanical integrity, while the type I collagen microsponge filled the large pores of the fabric and facilitated uniform cell distribution, cell attachment, and tissue formation (Figure 1). After being implanted in nude mice for a period of 8 weeks, bovine chondrocytes seeded within the composite scaffold were shown to have maintained their natural morphology while producing type II collagen and aggrecan extracellular matrix proteins.
Figure 1.
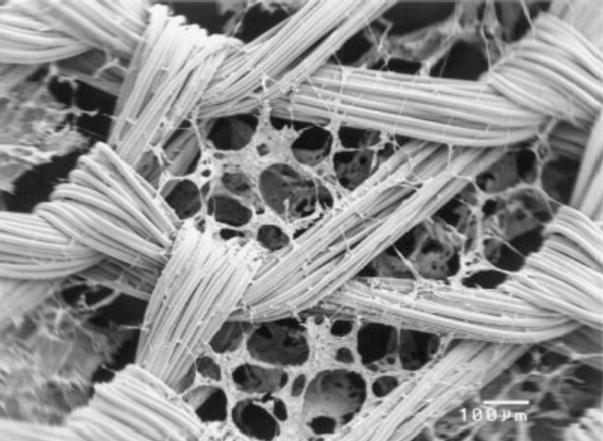
SEM photomicrograph of knitted PLGA/collagen composite scaffold. Reproduced with permission [63].
To examine the influence that different hydrogel carriers may have on cells embedded within non-woven fiber meshes, Hannouche et al. seeded PGA scaffolds with mesenchymal stem cells (MSCs) using type I collagen and alginate gels [28]. It was hypothesized by the authors that the hydrogel component of a composite scaffold would support the chondrogenic differentiation of rabbit MSCs, while the PGA mesh would provide for a solid scaffold architecture that could be secured into a chondral defect. After extended culture in a rotating bioreactor, it was observed that MSCs seeded in collagen/PGA constructs experienced rapid growth and chondrogenic differentiation within the first three weeks, while cells in alginate/PGA constructs had delayed proliferation and differentiation. After six weeks in culture, however, alginate/PGA constructs had accumulated higher amounts of glycosaminoglycans and lower contents of type I collagen. The results of this study demonstrated that the hydrogel component of a composite scaffold used for cartilage tissue engineering plays an important role in determining the phenotype of the seeded cells and ultimately the composition of the synthesized extracellular matrix.
Ameer et al. constructed biodegradable composite scaffolds to entrap isolated chondrocytes and potentially deliver them to a target injury site [29]. Using fibrin gel and non-woven PGA meshes, the authors formed constructs with improved physical integrity and handling characteristics over PGA-only scaffolds. While mechanical properties were not evaluated, in vitro culture for 28 days demonstrated that these scaffolds, when seeded with porcine chondrocytes, supported the chondrogenic phenotype and synthesis of new extracellular matrix.
As proof-of-concept, Mooney et al. produced reinforced foam PLGA by embedding short polyglycolic acid (PGA) fibers into the bulk polymer prior to foaming [30]. Image analysis revealed reinforcing fibers distributed throughout the solid phase of the scaffold, but mechanical properties were not evaluated. Using similar techniques, Slivka et al. demonstrated that the mechanical properties of these scaffolds could be tailored for potential use in articular cartilage repair by adjusting their relative material composition [31]. By varying the amount (up to 20% by weight) and orientation of the PGA fibers embedded in the foamed PLGA scaffolds, compressive modulus was shown to increase from 10 to 50 MPa, while yield strength increased from 0.7 to 2.5 MPa. The largest increases in mechanical properties were observed when testing scaffolds with uniaxially oriented fibers aligned parallel to the direction of compressive loading. The authors concluded that fiber reinforcement is a controllable variable that can be manipulated when engineering the mechanical and physical properties of biomaterial scaffolds to suit specific applications.
2.2 Embedded solid structures
Another technique used to form composite scaffolds for cartilage tissue engineering has been to combine cell-carrying hydrogels with rigid polymer materials such as foams or solid free-form fabricated structures. In these instances, hydrogels are typically used to improve cell seeding within the mechanically stiff scaffolds.
Utilizing this approach, Caterson et al. seeded porous poly-L-lactic acid (PLA) foam blocks with bone marrow-derived stem cells (MSCs) suspended in alginate [32]. Constructs were cultured in chondrogenic media containing varying concentrations of recombinant human transforming growth factor-β1 (TGF-β1) in static conditions for up to 3 weeks. Histological and immunohistochemical analyses indicated that the TGF-β1- treated PLA/alginate composite construct showed development of a cartilaginous phenotype during the culture period. Expression of cartilage specific genes (e.g. aggrecan and collagen types II and IX) was also detected in the TGF-β1-treated groups. The authors claimed that the alginate gel, which was used to improve the seeding and retention of cells within the mechanically robust PLA, aided in the induction of chondrogenesis by maintaining the MSCs in an optimal rounded shape. They also suggested that the gel possibly improved the effectiveness of the added soluble growth factors by confining them to the space within the construct, thereby enhancing their interaction with the seeded cells.
In a follow-up study, MSC-seeded PLA/alginate constructs treated with TGF-β1 were implanted into cylindrical osteochondral defects in canine femoral condyles to evaluate their in vivo response [33]. Cell-loaded and cell-free composite constructs were implanted for a period of 6 weeks, after which they were assessed by histology, immunohistochemistry, and biomechanical testing. Defects repaired with cell-loaded constructs showed cartilage-like matrix development, cell distribution, and proteoglycan staining. Cell-free constructs displayed similar, yet less robust remodeling by host cells that had invaded the scaffold from the underlying blood supply. While both experimental groups were able to initiate some degree of repair at the defect site, in situ indentation testing indicated that the neo-tissue formed was mechanically inferior to the surrounding native cartilage.
Huang et al. performed a comparable study by implanting cell-free composite constructs loaded with TGF-β1 into various ectopic sites in rabbits [34]. Their objective was to determine if MSCs could be recruited into the construct to initiate cartilage formation. To this end, polycaprolactone (PCL) scaffolds were fabricated using fused deposition modeling (FDM), encapsulated with varying doses of TGF-β1-laded fibrin glue, and placed in subcutaneous, intramuscular, and subperiosteal sites for up to 6 weeks. Results showed that active cells invaded the PCL/fibrin/TGF-β1 constructs regardless of implantation site. Constructs located in subperiosteal sites began forming tissue with cartilage-like characteristics, whereas constructs placed subcutaneously and intramuscularly developed fibrous tissue within their pores. The authors hypothesized that these phenotypic differences may be due to site-dependent variations in the biochemical or biomechanical environment.
Image-based design (IBD) and solid free-form (SFF) fabrication techniques have been used to make custom-fit scaffolds with designated pore sizes and architectures from a variety of materials (Figure 2) [35, 36]. Using these manufacturing techniques, Schek et al. attempted to form integrated osteochondral constructs using multiple materials [37]. The focus of this work centered on developing a bi-layered cylindrical scaffold composed of a PLA sponge fixed to underlying SFF fabricated hydroxyapatite (HA) (Figure 3). In this construction, the upper polymer phase was directly seeded with porcine chondrocytes to support cartilage development, while the lower ceramic phase was seeded with BMP-7-transduced human gingival fibroblasts suspended in fibrin glue to support the formation of bone. Cell-loaded scaffolds were implanted into dorsal subcutaneous pockets formed in immunocompromised mice for 4 weeks, after which they were evaluated histologically for tissue growth. The authors reported that the development of cartilage, vascularized bone, and an organized bone-cartilage interface was observed within the composite scaffold. These findings suggest that SFF fabrication can be used to manufacture multiphase composites that could improve integration with host tissue at the site of an osteochondral defect. In addition, this research group has published further studies describing the use of hyaluronic acid and collagen type I hydrogels to improve cell seeding and retention within SFF fabricated composite scaffolds [38, 39].
Figure 2.
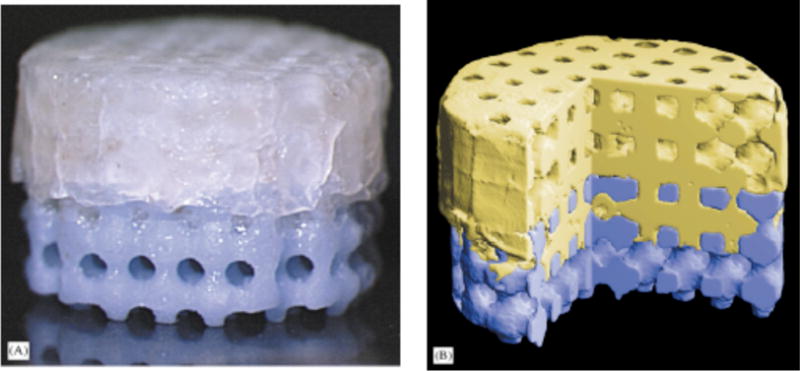
(A) Bilayered PLA/HA composite scaffold for osteochondral tissue engineering. (B) Colorized μCT of bilayered scaffold showing internal pore structure and integration of the two phases. Reproduced with permission [36].
Figure 3.
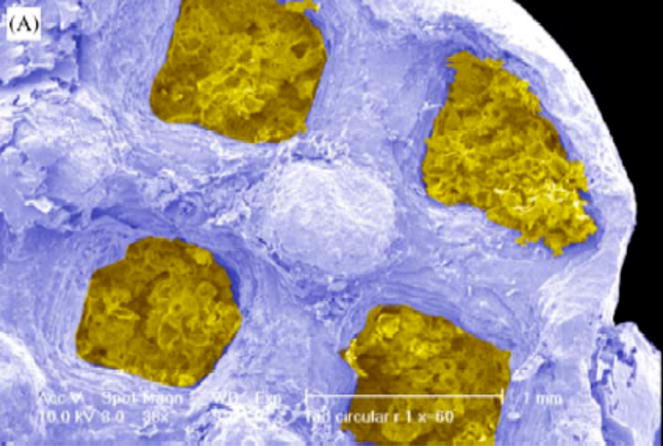
Colorized SEM photomicrograph showing PLA foam sponge (yellow) within SFF fabricated HA scaffold (blue). Reproduced with permission [39].
3. MULTI-LAYERED DESIGNS
Numerous studies have focused on developing multi-layered composite scaffolds for use in the tissue engineering of articular cartilage. The benefits of using this type of scaffold system include: (1) the potential for recreating the depth-dependent, zonal characteristics of native cartilage; and (2) the potential for creating osteochondral constructs consisting of a layer of cartilage integrally bound to a layer of underlying bone.
3.1 Multilayered cartilage constructs
Normal articular cartilage exhibits a distinct zonal structure, with different tissue composition and structure with depth from the tissue surface that are associated with distinct subpopulations of chondrocytes [40, 41]. In a study by Ng et al., a layer-by-layer casting approach was taken to form a stratified construct that could potentially mimic the depth-dependent material properties of native cartilage [42]. A chondrocyte-laden, stacked hydrogel system was constructed using 2% agarose as a top layer and 3% agarose as a bottom layer, and maintained in free-swelling culture conditions for 28 days. Unconfined compression testing revealed a significantly higher Young’s modulus in the bottom layer than in the top layer at Day 0. With increasing time in culture, however, this inhomogeneity became less pronounced as new extracellular matrix was formed by the embedded cells.
3.2 Layered osteochondral constructs
The implantation of tissue-engineered osteochondral constructs is commonly viewed as one of the most promising techniques for repairing full-thickness lesions of articular cartilage. Since bone-to-bone interfaces have been shown to integrate better and faster than cartilage-to-bone interfaces [43–46], numerous attempts have been made to develop cartilage-bone bilayered constructs by combining two distinct scaffold materials. In one such study, Hung et al. developed anatomically-shaped osteochondral constructs by casting a layer of chondrocyte-seeded agarose gel on top of devitalized trabecular bone of the patella (Figure 4) [47]. While cartilage-like matrix accumulation was observed in both phases of the composite constructs after 42 days in static culture, the peak compressive material properties only reached 1/8 those of native tissue over the same period of time. Schaefer et al. formed bilayered constructs by suturing engineered cartilage grown for 6 weeks on non-woven PGA scaffolds to a subchondral support made of Collagraft sponge [45]. Constructs were implanted into osteochondral defects in rabbit knees and allowed to remodel over a period of 6 months. Histological analysis revealed good integration with host bone, but poor integration with host cartilage using this technique.
Figure 4.
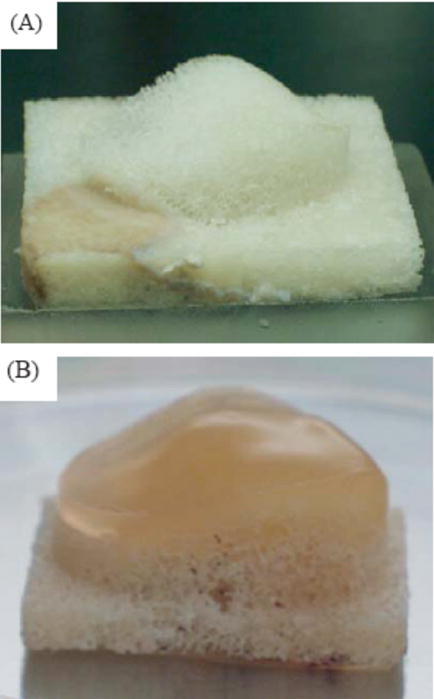
(A) Patella-shaped natural bovine trabecular bone substrate. (B) Patellar osteochondral construct after 14 days in culture. Reproduced with permission [47].
Similarly, Gao et al. assembled a bilayered scaffold using a hyaluronan sponge (HYAFF®-11) for cartilage regeneration and a calcium phosphate ceramic sponge for bone regeneration [48]. Each layer was seeded individually with MSCs prior to being bonded together using fibrin glue. After 6 weeks of in vivo culture in syngeneic rats, the authors reported finding bone ingrowth in the lower layer and immature fibrocartilage in the upper layer. Sherwood et al. used a 3-D printing system (TheriForm™) to form a composite scaffold consisting of a PLGA/PLA mix in the upper cartilage region, and a PLGA/tricalcium phosphate (TCP) mix in the lower bone region [49]. Results from this study showed that these scaffolds possessed robust initial mechanical properties in the bone region, while over time supporting the production of cartilage-like matrix proteins from seeded chondrocytes in the upper cartilage region.
In other studies, Tognana and co-workers have used multilayered concentric constructs as model systems to examine the integrative repair of engineered cartilage-bone composites [50, 51]. In these studies, engineered constructs were made using bovine calf chondrocytes seeded on hyaluronan benzyl ester non-woven mesh, which were the press-fit into adjacent tissue rings made of articular cartilage, devitalized bone, or vital bone. The constructs were then cultured in rotating bioreactors for up to 8 weeks, at which point their structure, biomechanical properties, and composition were assessed. The type of adjacent tissue was found to significantly affect construct adhesion, modulus, proteoglycan content, and collagen content, with the strongest integrative repair observed for constructs cultured adjacent to bone, rather than cartilage [50]. When implanted in vivo, these constructs showed similar behavior, although construct properties (e.g., moduli, proteoglycan content) were higher in vivo than in vitro, and certain histological characteristics, such chondrocyte alignment and bone remodeling were observed only in vivo [51]. Such composite systems provide novel models system to test the potential integrative properties of engineered constructs with native tissues in vitro or in vivo.
3.3 Multi-layered constructs from homogenous scaffolds
It is worth noting that multi-layered cartilage and osteochondral constructs have also been formed using scaffolds composed of a single homogenous material. For example, in an effort to recreate the native zonal architecture and phenotypic expression of cartilage, Kim et al. encapsulated articular chondrocytes within multi-layered photopolymerizing hydrogels of poly(ethylene glycol) diacrylate [52]. After 3 weeks of culture, cells encapsulated in each of the three layers of the hydrogel remained viable and remained in the respective layer in which they were encapsulated. Importantly, each zone of multilayered constructs exhibited histologic characteristics similar to those of native articular cartilage.
Kandel and co-workers have developed method to form a cartilage implant in vitro consisting of a layer of cartilaginous tissue overlying a porous, biodegradable calcium polyphosphate (CPP) substrate to improve anchorage of the cartilage once implanted in vivo [53]. Chondrocytes culture for 8 weeks on CPP substrates formed a continuous layer of cartilaginous tissue that integrated with the subsurface of the substrate. The in vitro-formed tissue exhibited a similar thickness to native articular cartilage and contained large proteoglycans and type II collagen. In subsequent studies, similar constructs formed in vitro were implanted for 3 and 9 months into 4mm diameter full thickness osteochondral defects in the trochlear groove of the sheep knee [54]. Control implants (ceramic only) had fibrous tissue on the articulating surface after implantation, while composite constructs maintained both cellularity and proteoglycan content over the 9 months period, while also exhibiting increased collagen content and mechanical properties during this time.
4. 3-D WOVEN COMPOSITE SCAFFOLDS
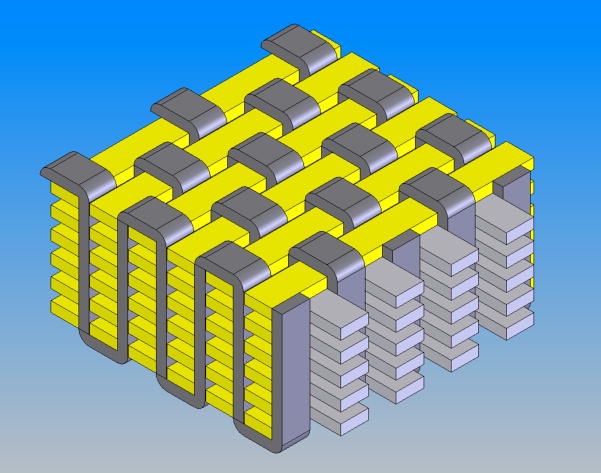
Recently, we reported on the physical and mechanical characterization of a novel, fiber-reinforced composite scaffold for the functional tissue engineering of articular cartilage [55]. The goal of this study was to create a scaffold whose initial mechanical properties mimicked those of native articular cartilage. Specifically, we endeavored to replicate the complex biomechanical behavior of the tissue using PGA fibers and biocompatible hydrogels known to support chondrogenesis. The basis of this work was a microscale three-dimensional (3-D) weaving technique that arranges multiple layers of continuous fibers in three orthogonal directions; fibers are arranged axially (x-direction), transversely (y-direction), and vertically (z-direction) within the architecture (Figure 5). The woven porous structures were consolidated with a cell-supporting hydrogel (i.e., agarose or fibrin) to form the composite scaffold. Tensile, compressive, and shear testing of acellular composites revealed properties that were of the same order of magnitude as native cartilage, with the exception of tensile moduli that were significantly higher. Moreover, the 3-D woven composite scaffolds displayed anisotropy, viscoelasticity, and tension-compression nonlinearity similar to articular cartilage.
Figure 5.
Schematic diagram of a 3-D orthogonally woven structure. Reproduced with permission [55].
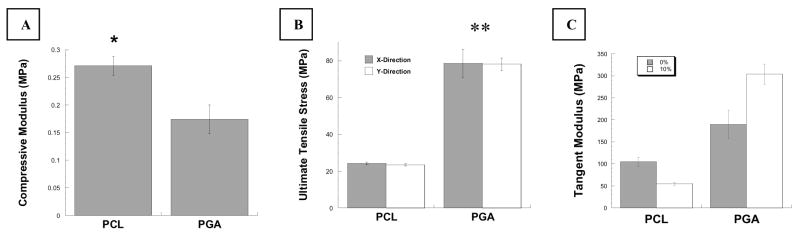
In continuation of this study, we have utilized an alternative constituent fiber – poly (ε-caprolactone) (PCL) – as the basis for our 3-D woven scaffolds. PCL is a slowly degrading aliphatic polyester that has been shown to remain in vivo for up to 3 years [56]. It is an FDA approved biomaterial with good physical characteristics and has been used extensively to produce scaffolds using a variety of manufacturing methods including electrospinning [57, 58], fused deposition modeling [34, 59, 60], and traditional textile processes [61, 62]. PCL yarns with approximately 150 μm diameters (EMS/Griltech, Domat, Switzerland) were woven using the same technique described above in our previous work for PGA scaffolds. Baseline mechanical properties of these scaffolds were then assessed using tensile and compressive mechanical tests. The results of these preliminary studies demonstrated that, similar to PGA scaffolds, 3-D woven PCL scaffolds could be produced with defined biomimetic properties a priori (Figure 6). The mechanical properties of the PCL scaffolds were markedly different than those of the PGA scaffolds even though they shared the same woven structure. This result is attributable to the physical and mechanical differences in the constituent yarns. For example, the PCL yarns were of larger diameter and had a higher degree of twist than did the PGA yarns. These properties gave the PCL-based scaffolds a more tightly woven architecture, which in turn, resulted in an increased compressive Young’s modulus (Figure 6A). Furthermore, since PCL is not as strong or stiff as PGA, the measured tensile properties of the PCL scaffolds were significantly lower (Figures 6B and 6C).
Figure 6.
Mechanical properties of PCL vs. PGA scaffolds. (A) Compressive Young’s modulus (E) as determined by unconfined compression. (B) PGA scaffolds had significantly higher ultimate tensile stress than did PCL scaffolds. (C) Tangent moduli (at 0 and 10%ε) were higher for PGA scaffolds than for PCL scaffolds. Data presented are mean ± SEM. Statistical significance determined by ANOVA with Fisher’s PLSD, * p < 0.05, **p < 0.0001.
5. CONCLUSIONS
Articular cartilage can be described as a fluid-saturated multiphasic material with inhomogeneous, anisotropic, nonlinear, and viscoelastic mechanical properties. These characteristics give healthy joints the ability to repeatedly bear applied loads, dissipate energy, and provide lubrication to the articulating joint surfaces over a lifetime. Early attempts at regenerating cartilage ex vivo have typically been performed using single, homogeneous biomaterials as scaffolds. However, these materials have not shown long-term success in the development of engineered cartilage with all the physical and biomechanical characteristics of the native tissue. The development of composite scaffolds by combining multiple materials with different properties may provide unique characteristics to promote the repair or regeneration of load-bearing tissues. Our findings suggest that a 3-D woven fiber-reinforced composite scaffold that possesses initial mechanical properties that are nonlinear, anisotropic, and viscoelastic properties may provide advantages in forming cartilage construct capable of load-bearing without the need for extended in vitro cultivation. Such scaffolds, or other composite techniques, could provide biomimetic mechanical characteristics, while simultaneously providing an appropriate physiologic environment for embedded cells to sustain chondrogenesis.
Acknowledgments
Supported by NIH grants AR49294, AR50245, AG15768, and AR48852, NASA grant NNJ04HC72G, the Wallace H. Coulter Foundation, and the Duke Translational Research Institute. We thank Dr. Bill Tawil of Baxter Biosurgery for providing Tisseel™, and Dr. Lisa Freed for many helpful discussions.
References
- 1.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13:67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 2.Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annu Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 3.Mow VC, Ateshian GA. Lubrication and Wear of Diarthrodial Joints. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. 2. Lippincott-Raven; Philadelphia: 1997. pp. 275–315. [Google Scholar]
- 4.Maroudas A, Ziv I, Weisman N, Venn M. Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology. 1985;22:159–169. doi: 10.3233/bir-1985-22206. [DOI] [PubMed] [Google Scholar]
- 5.Urban JP, Maroudas A, Bayliss MT, Dillon J. Swelling pressures of proteoglycans at the concentrations found in cartilaginous tissues. Biorheology. 1979;16:447–464. doi: 10.3233/bir-1979-16609. [DOI] [PubMed] [Google Scholar]
- 6.Lai WM, Hou JS, Mow VC. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991;113:245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soltz MA, Basalo IM, Ateshian GA. Hydrostatic pressurization and depletion of trapped lubricant pool during creep contact of a rippled indenter against a biphasic articular cartilage layer. J Biomech Eng. 2003;125:585–593. doi: 10.1115/1.1610020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, Sowers M, McAlindon T, Spector TD, Poole AR, Yanovski SZ, Ateshian G, Sharma L, Buckwalter JA, Brandt KD, Fries JF. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 11.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004:S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 12.Poole A, Guilak F, Abramson SB. Etiopathogenesis of Osteoarthritis. In: Moskowitz R, Howell DS, Goldberg VM, Mankin HJ, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4. Lippincott; Philadelphia: 2006. pp. 27–49. [Google Scholar]
- 13.Felson DT, Lawrence RC, Hochberg MC, McAlindon T, Dieppe PA, Minor MA, Blair SN, Berman BM, Fries JF, Weinberger M, Lorig KR, Jacobs JJ, Goldberg V. Osteoarthritis: new insights. Part 2: treatment approaches. Ann Intern Med. 2000;133:726–737. doi: 10.7326/0003-4819-133-9-200011070-00015. [DOI] [PubMed] [Google Scholar]
- 14.Font-Rodriguez DE, Scuderi GR, Insall JN. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997:79–86. [PubMed] [Google Scholar]
- 15.Callaghan JJ, Forest EE, Olejniczak JP, Goetz DD, Johnston RC. Charnley total hip arthroplasty in patients less than fifty years old. A twenty to twenty-five-year follow-up note. J Bone Joint Surg Am. 1998;80:704–714. doi: 10.2106/00004623-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443–460. doi: 10.1177/0363546505274578. [DOI] [PubMed] [Google Scholar]
- 17.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 18.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455–464. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17:93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 21.Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991;113:143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- 22.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y) 1994;12:689–693. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 23.Mikos AG, Bao Y, Cima LG, Ingber DE, Vacanti JP, Langer R. Preparation of poly(glycolic acid) bonded fiber structures for cell attachment and transplantation. J Biomed Mater Res. 1993;27:183–189. doi: 10.1002/jbm.820270207. [DOI] [PubMed] [Google Scholar]
- 24.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–575. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 25.Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001:S295–305. [PubMed] [Google Scholar]
- 26.Marijnissen WJ, van Osch GJ, Aigner J, van der Veen SW, Hollander AP, Verwoerd-Verhoef HL, Verhaar JA. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 2002;23:1511–1517. doi: 10.1016/s0142-9612(01)00281-2. [DOI] [PubMed] [Google Scholar]
- 27.Chen GP, Sato T, Ushida T, Hirochika R, Shirasaki Y, Ochiai N, Tateishi T. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. Journal of Biomedical Materials Research Part A. 2003;67A:1170–1180. doi: 10.1002/jbm.a.10164. [DOI] [PubMed] [Google Scholar]
- 28.Hannouche D, Terai H, Fuchs JR, Terada S, Zand S, Nasseri BA, Petite H, Sedel L, Vacanti JP. Engineering of implantable cartilaginous structures from bone marrow-derived mesenchymal stem cells. Tissue Eng. 2007;13:87–99. doi: 10.1089/ten.2006.0067. [DOI] [PubMed] [Google Scholar]
- 29.Ameer GA, Mahmood TA, Langer R. A biodegradable composite scaffold for cell transplantation. J Orthop Res. 2002;20:16–19. doi: 10.1016/S0736-0266(01)00074-2. [DOI] [PubMed] [Google Scholar]
- 30.Mooney DJ, Baldwin DF, Suh NP, Vacanti LP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 31.Slivka MA, Leatherbury NC, Kieswetter K, Niederauer GG. Porous, resorbable, fiber-reinforced scaffolds tailored for articular cartilage repair. Tissue Eng. 2001;7:767–780. doi: 10.1089/107632701753337717. [DOI] [PubMed] [Google Scholar]
- 32.Caterson EJ, Nesti LJ, Li WJ, Danielson KG, Albert TJ, Vaccaro AR, Tuan RS. Three-dimensional cartilage formation by bone marrow-derived cells seeded ion polylactide/alginate amalgam. J Biomed Mater Res. 2001;57:394–403. doi: 10.1002/1097-4636(20011205)57:3<394::aid-jbm1182>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Wayne JS, McDowell CL, Shields KJ, Tuan RS. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11:953–963. doi: 10.1089/ten.2005.11.953. [DOI] [PubMed] [Google Scholar]
- 34.Huang Q, Hutmacher DW, Lee EH. In vivo mesenchymal cell recruitment by a scaffold loaded with transforming growth factor beta 1 and the potential for in situ chondrogenesis. Tissue Eng. 2002;8:469–482. doi: 10.1089/107632702760184727. [DOI] [PubMed] [Google Scholar]
- 35.Hollister SJ, Levy RA, Chu TM, Halloran JW, Feinberg SE. An image-based approach for designing and manufacturing craniofacial scaffolds. Int J Oral Maxillofac Surg. 2000;29:67–71. doi: 10.1034/j.1399-0020.2000.290115.x. [DOI] [PubMed] [Google Scholar]
- 36.Taboas JM, Maddox RD, Krebsbach PH, Hollister SJ. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials. 2003;24:181–194. doi: 10.1016/s0142-9612(02)00276-4. [DOI] [PubMed] [Google Scholar]
- 37.Schek RM, Taboas JM, Segvich SJ, Hollister SJ, Krebsbach PH. Engineered osteochondral grafts using biphasic composite solid free-form fabricated scaffolds. Tissue Eng. 2004;10:1376–1385. doi: 10.1089/ten.2004.10.1376. [DOI] [PubMed] [Google Scholar]
- 38.Liao E, Yaszemski M, Krebsbach P, Hollister S. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13:537–550. doi: 10.1089/ten.2006.0117. [DOI] [PubMed] [Google Scholar]
- 39.Schek RM, Wilke EN, Hollister SJ, Krebsbach PH. Combined use of designed scaffolds and adenoviral gene therapy for skeletal tissue engineering. Biomaterials. 2006;27:1160–1166. doi: 10.1016/j.biomaterials.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37:271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 41.Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- 42.Ng KW, Wang CC, Mauck RL, Kelly TA, Chahine NO, Costa KD, Ateshian GA, Hung CT. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs. J Orthop Res. 2005;23:134–141. doi: 10.1016/j.orthres.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997:254–269. [PubMed] [Google Scholar]
- 44.Goldberg VM, Caplan AI. Biologic restoration of articular surfaces. Instr Course Lect. 1999;48:623–627. [PubMed] [Google Scholar]
- 45.Schaefer D, Martin I, Jundt G, Seidel J, Heberer M, Grodzinsky A, Bergin I, Vunjak-Novakovic G, Freed LE. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46:2524–2534. doi: 10.1002/art.10493. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer D, Martin I, Shastri P, Padera RF, Langer R, Freed LE, Vunjak-Novakovic G. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599–2606. doi: 10.1016/s0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 47.Hung CT, Lima EG, Mauck RL, Taki E, LeRoux MA, Lu HH, Stark RG, Guo XE, Ateshian GA. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853–1864. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 48.Gao J, Dennis JE, Solchaga LA, Awadallah AS, Goldberg VM, Caplan AI. Tissue-engineered fabrication of an osteochondral composite graft using rat bone marrow-derived mesenchymal stem cells. Tissue Eng. 2001;7:363–371. doi: 10.1089/10763270152436427. [DOI] [PubMed] [Google Scholar]
- 49.Sherwood JK, Riley SL, Palazzolo R, Brown SC, Monkhouse DC, Coates M, Griffith LG, Landeen LK, Ratcliffe A. A three-dimensional osteochondral composite scaffold for articular cartilage repair. Biomaterials. 2002;23:4739–4751. doi: 10.1016/s0142-9612(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 50.Tognana E, Chen F, Padera RF, Leddy HA, Christensen SE, Guilak F, Vunjak-Novakovic G, Freed LE. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthritis Cartilage. 2005;13:129–138. doi: 10.1016/j.joca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 51.Tognana E, Padera RF, Chen F, Vunjak-Novakovic G, Freed LE. Development and remodeling of engineered cartilage-explant composites in vitro and in vivo. Osteoarthritis Cartilage. 2005;13:896–905. doi: 10.1016/j.joca.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Kim TK, Sharma B, Williams CG, Ruffner MA, Malik A, McFarland EG, Elisseeff JH. Experimental model for cartilage tissue engineering to regenerate the zonal organization of articular cartilage. Osteoarthritis Cartilage. 2003;11:653–664. doi: 10.1016/s1063-4584(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 53.Waldman SD, Grynpas MD, Pilliar RM, Kandel RA. Characterization of cartilagenous tissue formed on calcium polyphosphate substrates in vitro. J Biomed Mater Res. 2002;62:323–330. doi: 10.1002/jbm.10235. [DOI] [PubMed] [Google Scholar]
- 54.Kandel RA, Grynpas M, Pilliar R, Lee J, Wang J, Waldman S, Zalzal P, Hurtig M. Repair of osteochondral defects with biphasic cartilage-calcium polyphosphate constructs in a sheep model. Biomaterials. 2006;27:4120–4131. doi: 10.1016/j.biomaterials.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6:162–167. doi: 10.1038/nmat1822. [DOI] [PubMed] [Google Scholar]
- 56.Huang MH, Li SM, Hutmacher DW, Coudane J, Vert M. Degradation characteristics of poly(epsilon-caprolactone)-based copolymers and blends. Journal of Applied Polymer Science. 2006;102:1681–1687. [Google Scholar]
- 57.Chew SY, Wen Y, Dzenis Y, Leong KW. The role of electrospinning in the emerging field of nanomedicine. Curr Pharm Des. 2006;12:4751–4770. doi: 10.2174/138161206779026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li WJ, Cooper JA, Jr, Mauck RL, Tuan RS. Fabrication and characterization of six electrospun poly(alpha-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006;2:377–385. doi: 10.1016/j.actbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Hutmacher DW, Schantz T, Zein I, Ng KW, Teoh SH, Tan KC. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J Biomed Mater Res. 2001;55:203–216. doi: 10.1002/1097-4636(200105)55:2<203::aid-jbm1007>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 60.Zein I, Hutmacher DW, Tan KC, Teoh SH. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials. 2002;23:1169–1185. doi: 10.1016/s0142-9612(01)00232-0. [DOI] [PubMed] [Google Scholar]
- 61.Ng KW, Khor HL, Hutmacher DW. In vitro characterization of natural and synthetic dermal matrices cultured with human dermal fibroblasts. Biomaterials. 2004;25:2807–2818. doi: 10.1016/j.biomaterials.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 62.Van Lieshout M, Peters G, Rutten M, Baaijens F. A knitted, fibrin-covered polycaprolactone scaffold for tissue engineering of the aortic valve. Tissue Eng. 2006;12:481–487. doi: 10.1089/ten.2006.12.481. [DOI] [PubMed] [Google Scholar]