Abstract
Microcirculatory dysfunction is a critical element of the pathogenesis of severe sepsis and septic shock. In this Bench-to-Bedside review, we present: (1) the central role of the microcirculation in the pathophysiology of sepsis; (2) new translational research techniques of in vivo videomicroscopy for assessment of microcirculatory flow in human subjects; (3) clinical investigations that reported associations between microcirculatory dysfunction and outcome in septic patients; (4) the potential role of novel agents to "rescue" the microcirculation in sepsis; (5) current challenges facing this emerging field of clinical investigation; and (6) a framework for the design of future clinical trials aimed to determine the impact of novel agents on microcirculatory flow and organ failure in patients with sepsis. We specifically focus this review on the central role and vital importance of the nitric oxide molecule in maintaining microcirculatory homeostasis and patency, especially when the microcirculation sustains an insult (as with sepsis), and we present the scientific rationale for clinical trials of exogenous nitric oxide administration to treat microcirculatory dysfunction and augment microcirculatory blood flow in early sepsis therapy.
Keywords: microcirculation, sepsis, severe sepsis, septic shock, resuscitation, endothelium, nitric oxide
INTRODUCTION
Sepsis is a common and devastating disease that is responsible for 215,000 deaths annually in the United States and is the leading cause of death in critically ill patients.1, 2 This disease is now recognized to be a time-sensitive emergency, as patients stand the best chance for survival when effective therapeutic interventions are delivered as early as possible.3, 4 Early protocol-driven resuscitation (e.g. early goal-directed therapy)4 targeting optimization of global hemodynamic parameters has been associated with the largest mortality benefit to date in sepsis randomized controlled trials.4, 5 However, severe sepsis and septic shock still carry a high mortality rate (21–28%), even after effective interventions to optimize global (i.e. macrocirculatory) hemodynamics have been applied.6 The persistently high mortality rate despite early aggressive resuscitation suggests a need for novel therapeutic interventions to further improve survival.
After aggressive resuscitation of the septic patient, a normal or high cardiac output is typically achieved, yet tissue perfusion can remain markedly impaired. Tissue hypoxia can persist despite achievement of normal or supranormal global oxygen delivery. Clinically, this may manifest with persistent acidosis, mottled skin, or progressive multi-organ failure. Therefore, limiting goal-directed resuscitation solely to macrocirculatory perfusion indices alone (e.g. cardiac filling pressure, mean arterial pressure, cardiac output, or mixed/central venous oxygen saturation) may not be sufficient to optimize blood flow to tissues in many patients. The microcirculation (blood vessels <100µm in diameter) is the principal site of oxygen exchange between blood and underlying tissues, and there is abundant data indicating profound disruption of the microcirculation in sepsis. Future clinical trials designed to go beyond global hemodynamic optimization and test novel therapeutic strategies to augment microvessel blood flow may contribute important new information to our understanding of optimal resuscitation in patients with sepsis.
This paper is intended as a focused review of the role of microcirculatory perturbation in the pathogenesis of sepsis, with special emphasis on the importance of nitric oxide (NO) in maintaining microcirculatory homeostasis. We review the available techniques for monitoring the microcirculation in human subjects, and important considerations for designing clinical trials of therapeutic agents to rescue the microcirculation in patients with sepsis-induced tissue hypoperfusion.
SEPSIS IS A DISORDER OF THE MICROCIRCULATION
The microcirculation is an integrated functional system that ensures tissue oxygen delivery meets cellular oxygen demand throughout the body. When this system becomes unhinged, maldistribution of blood flow and tissue hypoxia may result. Although microcirculatory dysfunction may occur to some degree in most shock states (e.g. cardiogenic shock and ischemia-reperfusion injury), microcirculatory failure appears to be a hallmark of the septic state and central to sepsis pathophysiology.
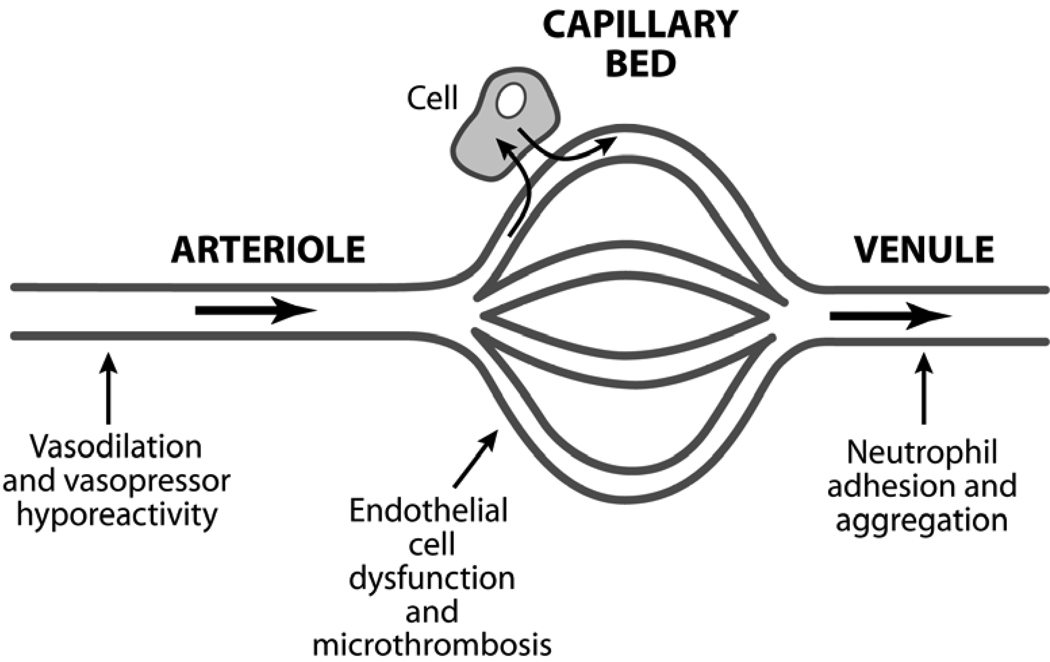
The microcirculatory unit – comprised of the arteriole, capillary bed, and post-capillary venule – is the landscape where most of the pivotal events of sepsis pathogenesis take place, including loss of vasomotor reactivity, endothelial cell injury, activation of coagulation, and disordered leukocyte trafficking (Figure 1). In rat models of cecal ligation and puncture, investigators have used intravital videomicroscopy to demonstrate that sepsis is characterized by decreased microcirculatory flow velocity, an abundance of stopped-flow microvessels, increased heterogeneity of microcirculatory flow, and low density of perfused capillaries.7–10 As these microcirculatory flow alterations can occur in the absence of global hemodynamic derangements (e.g. absence of arterial hypotension),8, 10–12 microcirculatory dysfunction largely reflects intrinsic events occurring in the microvessels. The ensuing microcirculatory “failure” can cause marked impairment of tissue oxygen transport resulting in tissue hypoxia (Figure 2).7
Figure 1.
Sepsis is a disorder of the microcirculation. Much of the pathophysiology of sepsis can be explained within the microcirculatory unit – the terminal arteriole, capillary bed, and the post-capillary venule. The arteriole is where the characteristic vasodilation and vasopressor hyporesponsiveness of sepsis occurs. The capillary bed is where the effects of endothelial cell activation/dysfunction are most pronounced and microvascular thromboses are formed. The post-capillary venule is where leukocyte trafficking is most disordered – leukocytes adhere to the vessel wall, aggregate, and further impair flow through the microcirculation.
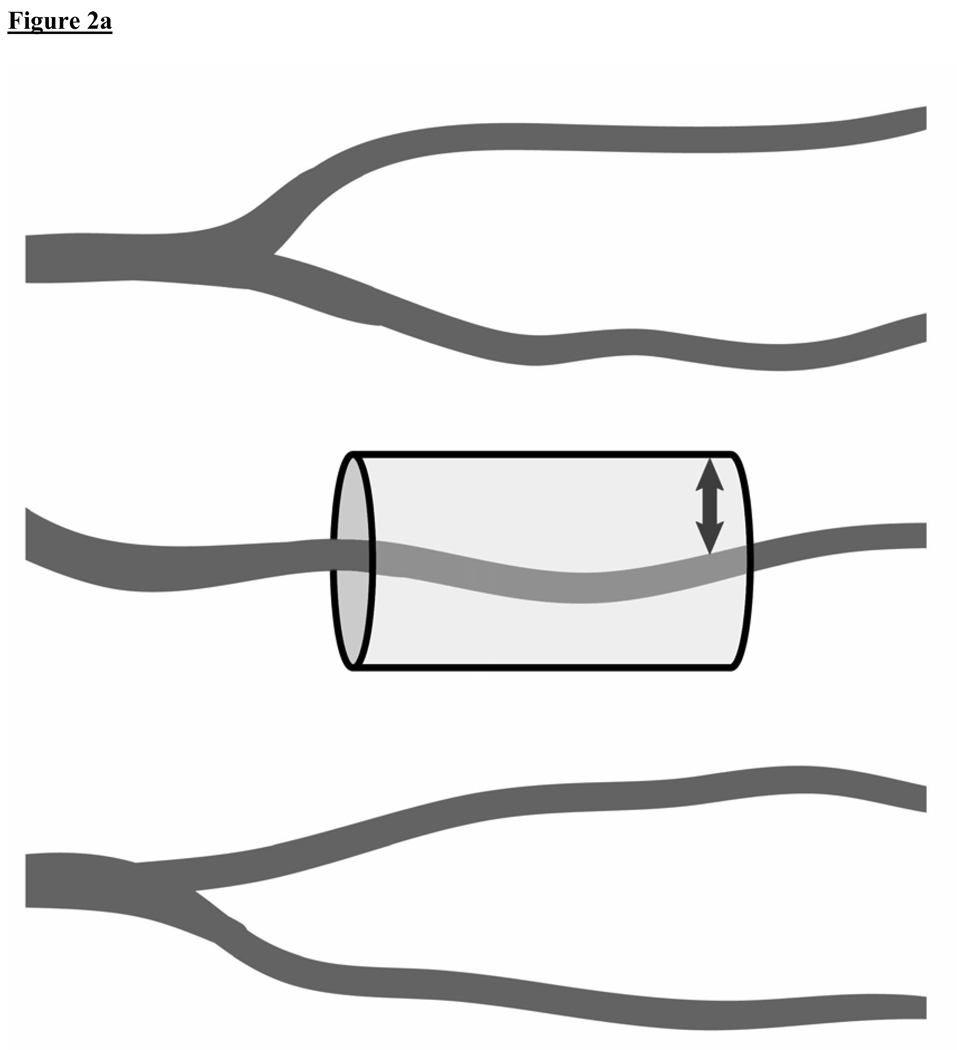
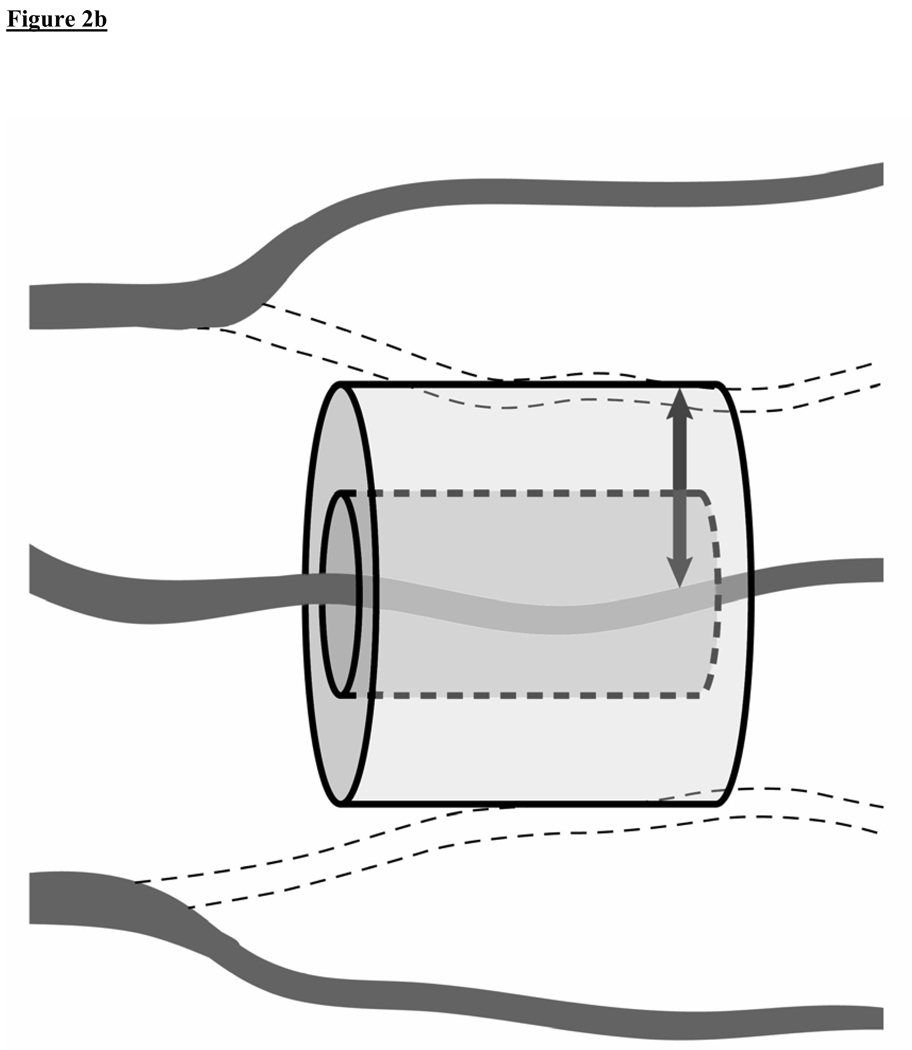
Figure 2A and 2B.
A conceptual model of oxygen diffusion from capillaries. These figures illustrate how sepsis-induced microcirculatory dysfunction can play a key role in the impairment of tissue oxygen transport and contribute to tissue hypoxia. (2A)Healthy state: A cylinder represents the area of tissue that is supplied with oxygen by an individual capillary. The diffusion distance for oxygen in the tissues is shown (small arrow). (2B)Sepsis: Intrinsic microcirculatory dysfunction results in non-perfused capillaries (dotted line vessels). This decreases the density of perfused vessels, increasing the diffusion distance for oxygen in the tissues (large arrow).
Role of the Endothelium in Sepsis-Induced Microcirculatory Dysfunction
The endothelium is a single layer of cells lining all blood vessels, numbering ~1013 cells, approximately 4,000–7,000 m2 in an average adult. The endothelium is a highly active organ that dynamically regulates microvessel thrombosis, profibrinolysis, leukocyte adhesion/migration, microvascular tone, permeability, and blood flow in both health and disease.13 During normal function, endothelial cells (ECs) in microcirculatory networks function as an integrated system actively autoregulating vasomotor tone and upstream microvessel recruitment via cell-cell signaling based on conditions downstream in the capillary bed,14 and recruiting blood flow primarily via local release of vasodilators, most notably NO.15 Endotoxemia can disrupt these cell-cell signal transduction pathways resulting in maldistribution of blood flow.14 The endothelium also protects vascular integrity in the micrcocirculation by actively maintaining tight junctions between cells.
The endothelium contributes in fundamental ways to the hemostatic balance by expressing multiple anticoagulant and procoagulant proteins. For example, ECs express thrombomodulin, which converts protein C to its activated form. Once activated, protein C cleaves and inactivates factors Va and VIIIa. In addition, the endothelium expresses tissue factor pathway inhibitor, which blocks the extrinsic coagulation pathway; synthesizes heparan, a cofactor for antithrombin III; and releases tissue-type plasminogen activator and plasminogen activator inhibitor-1. These activities all work together to maintain the anticoagulant nature of the EC surface in the healthy state.16
Endothelial cells are highly responsive to changes in their extracellular milieu, and sense a myriad of biomechanical and biochemical forces. They integrate these signals and respond in ways that are usually adaptive (endothelial activation), but are at times maladaptive and harmful to the host (endothelial dysfunction). Endothelial activation also describes a phenotypic response to an inflammatory stimulus that may be mediated by cytokines [interleukin (IL)-1, IL-6, or tumor necrosis factor (TNF)-alfa] or exposure to oxidative stress,17 either directly or indirectly via activation of nuclear factor-kappa B.18 The endothelial activation phenotype is characterized by a pro-coagulant and pro-adhesive cell surface, dysregulation of vasomotor tone, and compromised barrier function.
The endothelial contribution to the procoagulant state in the activation phenotype is primarily related to the loss of its role in anticoagulation. There is an increase in EC tissue factor expression, decreased EC surface expression of thrombomodulin, and decreased protein C activation, the severity of which has been associated with poor outcome in sepsis.19 Moreover, activated ECs amplify the local inflammatory response by releasing their own complement of pro-inflammatory cytokines that can propogate focal and ongoing microvascular injury in a perpetuating cycle. This injury cycle disrupts EC tight junctions, causing tissue edema that can further impair oxygen delivery to tissues.20 Under hypoxic conditions, hypoxia inducible factor (HIF)-1 gene can be upregulated, increasing vascular endothelial growth factor (VEGF) expression, which has been associated with high severity, organ failure, and death in sepsis.21, 22
Activated ECs mediate leukocyte trafficking through a highly regulated multi-step adhesion cascade that involves selectin-mediated attachment and rolling (P-selectin on platelets and ECs and E-selectin on endothelium), and cell adhesion molecule-dependent firm adhesion to the endothelial surface mediated by intercellular cell adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1. The pro-adhesive activated endothelial phenotype is compounded by sepsis-induced changes in circulating cells, comprising not only leukocyte activation, but changes in red blood cells (RBCs) including impaired deformability causing increased viscosity,23, 24 aggregation, and adherence.25 The end result of EC activation/dysfunction in sepsis is a multifaceted disruption of microcirculatory homeostasis. If uncorrected, this disruption may impair oxygen transport culminating in cellular hypoxia, acute organ dysfunction, and death.26
The Centrality of Nitric Oxide in Regulating Microcirculatory Homeostasis
Nitric oxide plays a pivotal and multifaceted role in the complex pathophysiology of sepsis. In the healthy state and under pathologic conditions, NO maintains microcirculatory homeostasis by regulating microvascular tone, leukocyte adhesion, platelet aggregation, microthrombi formation, and microvascular permeability.27–34 When the microvasculature sustains an insult (e.g. sepsis) the NO molecule becomes vital to maintaining microcirculatory patency, integrity and function.
Although the sepsis pro-inflammatory response triggers a sharp increase in systemic NO production,35 the upregulation of inducible nitric oxide synthase (iNOS) is heterogeneously expressed between and within organ systems 35, 36 and NO can be consumed by reactive oxygen species, giving the potential for localized areas of relative NO deficiency in microvascular beds despite a state of total body NO “excess”.36, 37 This can be a major factor in the heterogeneity of tissue perfusion that characterizes both experimental and human sepsis,8, 10, 38 and may also help explain pathologic microcirculatory shunting in sepsis – the diversion of blood flow away from distressed microvascular units via opening of arteriovenous shunts within capillary beds.39
Clinical Investigations of the Microcirculation in Sepsis
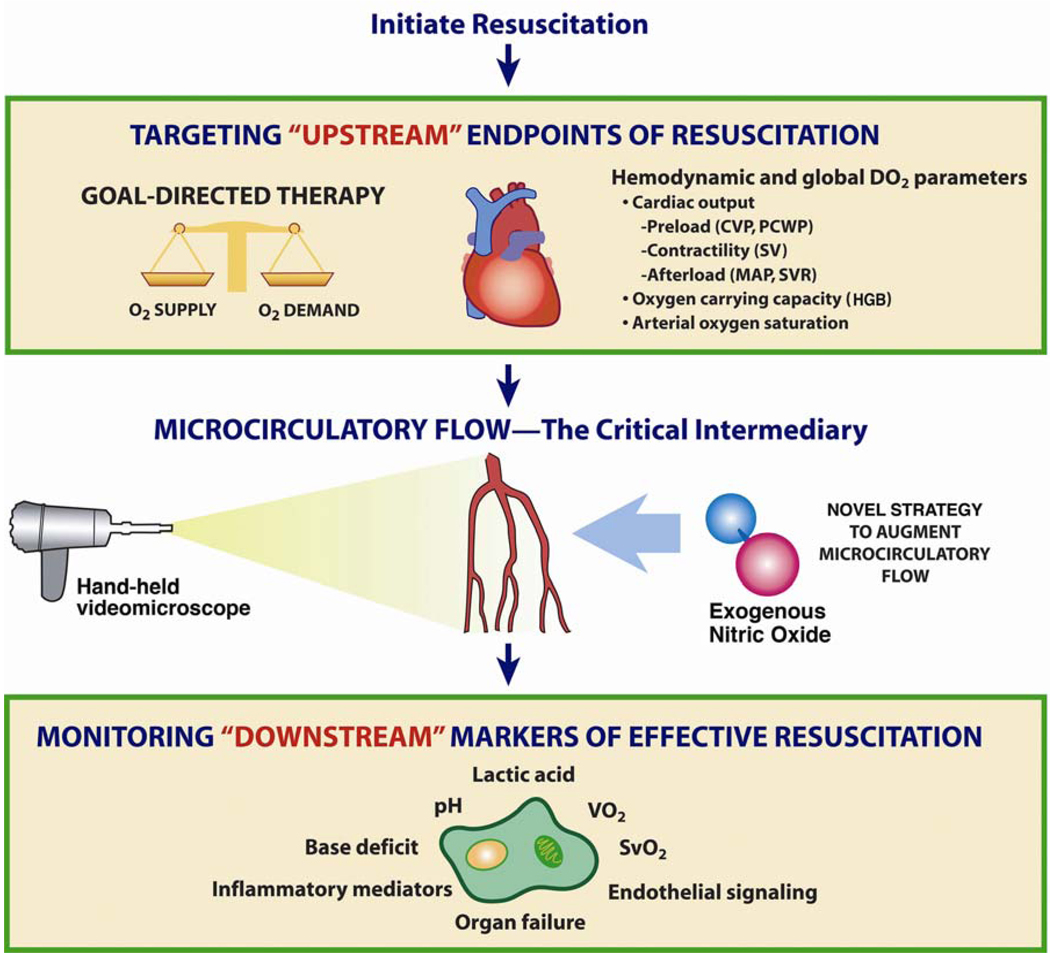
Although clinical investigations in sepsis cardiovascular support have traditionally focused on macrocirculatory hemodynamics (i.e. heart and large arteries) that reflect the distribution of blood flow globally throughout the body, a functional microcirculation is a critical component of the cardiovascular system that is necessary for effective blood flow to tissues. This conceptual framework in the context of shock and resuscitation is shown in Figure 3. With the advent of new in vivo videomicroscopy techniques, it is now possible to visualize the microcirculation in human subjects. Although a shift of research focus from global hemodynamic parameters to indices of microvessel perfusion could potentially be viewed as a major departure for the clinical research mission, the microcirculation may actually prove to be a logical next frontier in understanding the full scope of circulatory failure in sepsis.
Figure 3.
Conceptual framework of the importance of the microcirculation in septic shock and resuscitation. Conventional resuscitation targets optimization of “upstream” (i.e. macrocirculatory) hemodynamic parameters (e.g. mean arterial pressure, cardiac output), with monitoring of “downstream” markers of tissue perfusion (e.g. acidosis, organ function) to determine the effectiveness of resuscitation efforts. The microcirculation represents a critical intermediary. Although the macrocirculation circulates blood throughout the body, an intact and functional microcirculation is necessary for effective blood flow to tissues. Therefore, intrinsic microcirculatory failure may contribute to sepsis-associated tissue hypoperfusion. Sublingual microcirculatory blood flow can now be visualized directly in sepsis clinical research using a hand-held videomicroscope (shown on left). In this paper, we present a scientific rationale for a clinical trial of a novel agent (e.g. exogenous nitric oxide administration, shown on right) to reduce microcirculatory dysfunction and augment microcirculatory blood flow in sepsis resuscitation. [CVP = central venous pressure; PCWP = pulmonary capillary wedge pressure; SV = stroke volume; MAP = mean arterial pressure; SVR = systemic vascular resistance; HGB = hemoglobin; VO2 = oxygen consumption; SvO2 = mixed venous oxygen saturation] Adapted from: Trzeciak S, Dellinger RP, Parrillo JE, Septic Shock, In: Parrillo JE and Dellinger RP (3rd Edition) Critical Care Medicine: Principles of Diagnosis and Management in the Adult. (2008) Philadelphia, PA: Mosby Elsevier.
In septic patients, microcirculatory failure appears to be a major perturbation with prognostic significance.11, 12, 38 Severe derangements of microcirculatory flow, including the severity of initial derangements in the early resuscitation phase of therapy as well as the persistence of microcirculatory derangements over time, have been associated with lower survival.11, 12, 38 Impairment of microcirculatory blood flow may be an early triggering event in the development of sepsis-induced multi-organ failure,12, 40–42 which is known to be a critical (and early) determinant of sepsis mortality.43, 44 A lack of improvement in microcirculatory flow indices early in the ICU course has been associated with multi-organ failure, suggesting that the capacity to impact outcome via restoration of microcirculatory flow may be time-sensitive.12 Table 1 summarizes recent published clinical investigations that used in vivo videomicroscopy to study the association between microcirculatory flow impairment and outcome in patients with sepsis.
Table 1.
Published manuscripts that used in vivo videomicroscopy to examine the association between microcirculatory perfusion indices and clinical outcome in patients with severe sepsis and/or septic shock. [ED = Emergency Department; ICU = Intensive Care Unit]
| AUTHORS | COUNTRY | YEAR | n | SUBJECTS | SETTING | PRIMARY OUTCOME MEASURE(S) | MAIN FINDINGS |
|---|---|---|---|---|---|---|---|
| De Backer et al 11 | Belgium | 2002 | 50 | Severe sepsis | ICU | Mortality | Microcirculatory perfusion impairment was more severe in sepsis non-survivors compared to survivors, independent of global hemodynamics. |
| Sakr et al 12 | Belgium | 2004 | 46 | Septic shock | ICU | Multi-organ failure and mortality | With longitudinal (daily) measurements of microcirculatory perfusion in sepsis patients, failure to improve microcirculatory perfusion was strongly associated with the development of multi-organ failure and death. |
| Trzeciak et al 38 | USA | 2007 | 26 | Severe sepsis and septic shock | ED and ICU | Mortality | During the early resuscitation phase of sepsis therapy, microcirculatory flow velocity was more severely impaired and more heterogeneous in patients that ultimately did not survive, compared to survivors. |
TRANSLATIONAL VIDEOMICROSCOPY TECHNIQUES FOR ASSESSING THE MICROCIRCULATION IN HUMAN SUBJECTS
Intravital videomicroscopy in animal models has historically required a tissue dissection; however, new minimally-invasive videomicroscopy techniques permit direct visualization of the microcirculatory network beneath thin mucosal surfaces using a hand-held instrument [Orthogonal Polarization Spectral (OPS) or Sidestream Dark Field (SDF) imaging], making microcirculatory assessment possible in human subjects (Figure 4). These techniques have been validated in experimental models and human subjects.45–49
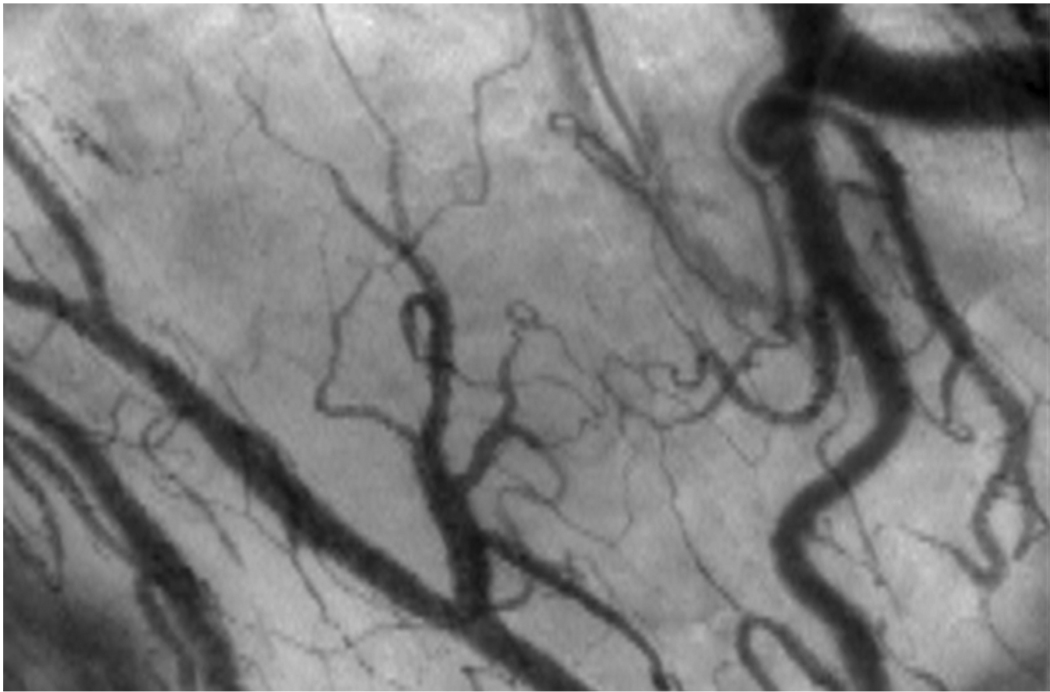
Figure 4.
A still image of the human sublingual microcirculation as visualized with Orthogonal Polarization Spectral (OPS) videomicroscopy. The videomicroscope uses a 5X objective (167X magnification) giving a 940 × 1259 µm field of view. Real-time video of healthy and dysfunctional microcirculation is available for viewing or download at: http://www.cooperhealth.org/content/gme_fellowship_shock.htm
The sublingual site has emerged as the preferred site for microcirculatory assessment in human subjects with overt or impending shock. Beginning with the work of Weil and coworkers, numerous investigators have demonstrated that impaired sublingual perfusion can track impairment of splanchnic perfusion and can detect early systemic perfusion failure in shock states.50–53 Monitoring sublingual blood flow can yield important information for use in clinical studies of circulatory shock because (1) the sublingual mucosa shares the same embryologic (and therefore anatomic) origin as the splanchnic mucosa, (2) derangements in sublingual perfusion can reflect derangements in splanchnic blood flow,50, 51, 53–56 and (3) the sublingual space is easily accessible. Tracking splanchnic hypoperfusion can be clinically important because it is one of the earliest indicators of systemic hypoperfusion in circulatory shock;57–59 therefore, impaired sublingual blood flow may herald the onset of systemic hypoperfusion.52, 53 Although some discrepancy between sublingual and gut microvascular perfusion has been reported,60 clinical data have found sublingual flow to be independently prognostic.11, 12, 38
Details of our technique for sublingual image acquisition, processing, and analysis appear in an ONLINE DATA SUPPLEMENT [E1]. Our methodology is consistent with the recently published proceedings of a consensus conference on microcirculatory image analysis that was intended to help standardize analysis techniques among different groups of investigators.61 The consensus recommendations advocate the calculation of multiple microcirculatory indices including a semi-quantitative flow velocity index, the proportion of perfused vessels, perfused vessel density, and the heterogeneity of flow between different sublingual sites.61
POTENTIAL THERAPEUTIC STRATEGIES TO AUGMENT MICROCIRCULATORY FLOW IN SEPSIS RESUSCITATION
Therapeutic approaches to counteract microcirculatory failure could represent a novel strategy to help optimize tissue perfusion in sepsis resuscitation. An ideal agent to recruit the microcirculation in sepsis would most likely be either: (a) an endothelium modulator, (b) a vasodilator to “open” low-flow microcirculatory units, or (c) both. The concept of using pharmacotherapy to augment microcirculatory flow in critically ill patients originated in the 1980s with clinical trials of agents with vasodilatory properties (prostacyclin) or combined inotropic/vasodilatory properties (dobutamine).62, 63 These studies demonstrated increased systemic oxygen consumption (VO2) with drug administration, suggesting that successful microcirculatory recruitment had occurred. Recently, De Backer et al used OPS imaging in two studies of the effects of dobutamine and recombinant human activated protein C (rhAPC) on the microcirculation in septic patients.64, 65 Both agents were associated with increases in capillary perfusion independent of systemic hemodynamic effects, presumably via microvessel vasodilatory properties or rheologic effects in the case of dobutamine, and via modulation of leukocyte-endothelial cell interactions for rhAPC.64–67 Additional agents that may hold promise include anticoagulants (e.g. antithrombin III), other vasodilators (e.g. pentoxifylline), and antioxidants that may attenuate oxidative stress-induced endothelial activation and/or increase NO bioavailability by reducing NO consumption by reactive oxygen species (e.g. parenteral ascorbate, N-Acetyl-L-cysteine).68–70
The history of investigating NO modulation in sepsis has been largely driven by the hypothesis that NO is deleterious. The thought that NO is harmful in sepsis comes from its consequences on the macrocirculation (arterial hypotension), disregarding its possible beneficial effects on the microcirculation. Indeed, iNOS upregulation and the concomitant NO-induced relaxation of microvascular tone can produce arterial hypotension.71–74 However, although NOS inhibition is clearly effective at raising arterial pressure in sepsis,75–79 it can simultaneously worsen the impairment of microcirculatory perfusion and oxygen transport to tissues.28, 32, 80–88 Blocking NO production in sepsis worsens leukocyte adhesion,89, 90 platelet aggregation and microthrombosis,28, 32 and microvascular permeability,34, 82 causing decreased splanchnic28, 83–88 and myocardial81 blood flow and defects in tissue oxygenation that do not recover with fluid resuscitation alone.80 Although iNOS-deficient septic animals have less severe arteriolar hyporesponsiveness to adrenergic agents,73 they have increased endothelial adhesion molecule expression and leukocyte-endothelial interactions.91–93 Due to the dichotomy of macrocirculatory and microcirculatory effects, the concept of NOS inhibition in sepsis has been considered a “double-edged sword”.94 In human subjects, a phase III randomized controlled trial of nonspecific NOS inhibition was stopped early because of increased mortality in the NOS inhibition group.95
As NO preserves microcirculatory patency and function, upregulation of NO may be adaptive (rather than maladaptive) and in fact protective in sepsis. Taking a contrarian approach to studying NO modulation in septic patients, administration of exogenous NO could potentially improve tissue perfusion indices. Nitric oxide is especially attractive as a candidate therapy to treat microcirculatory dysfunction in sepsis because it could in theory recruit microcirculatory flow by two potential mechanisms of action – modulation of leukocyte-endothelial interactions and microvessel vasodilation – simultaneously. In experimental models, administration of a NO-donor decreased endothelial adhesion molecule expression and leukocyte adhesion,96 augmented splanchnic microcirculatory blood flow,97, 98 and optimized tissue oxygen transport.99, 100 In two clinical studies of sepsis patients utilizing OPS imaging, the sepsis-induced impairment of sublingual microcirculatory blood flow was reversed with (1) topical administration of acetylcholine (suggesting that the endothelium was still NO-responsive)11, and (2) intravenous nitroglycerin (an NO donor).101 Because administration of intravenous nitroglycerin could cause or exacerbate a drop in arterial pressure in septic patients,102 a clinical trial of exogenous NO administration to human subjects with sepsis would require an agent that would not be expected to induce or exacerbate arterial hypotension – e.g. inhaled nitric oxide (INO).103, 104
Although the classical view of NO metabolism assumed that the bioavailability of INO was limited to the lung due to rapid binding to heme iron, it is now recognized that the inhaled route can deliver NO to the systemic circulation and exert extrapulmonary effects via two mechanism: formation of nitrite and/or S-nitrosothiol (SNO).105 Under hypoxic conditions, RBCs can convert circulating nitrite to NO and release SNO bioavailability. As such, RBCs are capable of dilating microvessels and regulating of blood flow.106, 107 In human subjects, administration of INO effectively delivered SNO to extrapulmonary vascular beds and dilated the peripheral microvasculature.108–111 In studies of microcirculatory impairment induced by NOS inhibition, INO attenuated mesenteric vasoconstriction and leukocyte adhesion in experimental models and reversed distal extremity vasoconstriction in human subjects,112–115 indicating that INO administration can generate circulating molecules with NO-carrying capacity, exert distant (i.e. extrapulmonary) effects, and help maintain microvascular homeostasis. These data support the concept that INO could be a novel treatment for a disease characterized by systemic endothelial dysfunction. In previous studies (Table 2) INO improved microcirculatory homeostasis by multiple separate and distinct effects.
Table 2.
Mechanisms by which inhaled nitric oxide (INO) could potentially attenuate microcirculatory dysfunction and improve microcirculatory homeostasis in shock states.
| MECHANISMS | SUPPORTING EVIDENCE |
|---|---|
| Modulation of microvascular tone | In experimental models of ischemia/reperfusion injury, INO restored flow to ischemic myocardium116,117 and raised renal blood flow and glomerular filtration.118 |
| Preservation of microvascular integrity | In sepsis and endotoxemia models, INO attenuated neutrophil transmigration across the local (pulmonary) endothelial barrier119,120 as well as the influx of inflammatory cells into systemic (extrapulmonary) organs.121 In ischemia/reperfusion models, INO decreased microvascular injury and neutrophil transmigration, decreasing the tissue inflammatory reaction.112,122,123 |
| Endothelial-dependent effects | In models of endotoxemia, ischemia/reperfusion, and oxidative endothelial activation, INO demonstrated an anti-adhesive effect on distant (mesenteric) inflamed microvasculature by attenuating endothelial dysfunction and leukocyte adhesion with an increase in microcirculatory flow.112–114,121,124 In patients with acute respiratory distress syndrome, INO decreased endothelial adhesion molecule expression, platelet aggregation, and fibrinogen binding.125 |
| Leukocyte-dependent effects | INO may exert an indirect effect on the microcirculation by “pacifying” leukocytes in transit through the pulmonary circulation.126 In experimental models, INO attenuated the oxidative burst from activated neutrophils,119,120 prevented neutrophil-mediated, oxygen-radical dependent endothelial damage,127 and reduced neutrophil adhesion and sequestration into tissues by inhibiting integrin-mediated firm adhesion to the endothelium.126,128 |
| Direct anti-inflammatory effect | INO can decrease the amount of NF-kappa B available for binding to the regulatory region of genes that produce pro-inflammatory cytokines.129 130 |
It is notable that, although three randomized controlled trials of INO failed to improve outcome in patients with acute respiratory distress syndrome (ARDS),104, 131 132 only 4% of 742 total subjects had sepsis-associated ARDS. Therefore, the efficacy of INO in treating patients with sepsis has not yet been adequately tested.
CHALLENGES
There are important challenges (and limitations) to studying the microcirculation in critically ill patients. The main challenges to overcome in development of analysis techniques are the need for a fully quantitative (rather than semi-quantitative) measurement of microcirculatory blood flow velocity, and automated image analysis that can provide real-time readout at the bedside. Ideally, one software-based image analysis solution would satisfy both of these needs, and this represents a bioengineering opportunity. As OPS/SDF microcirculatory imaging requires focused training to become proficient in the technique, a broader challenge is that it remains unclear whether the technique can effectively translate to widespread use in critically ill patients, outside the confines of dedicated laboratories, or if its use will be limited to use only by experienced operators and investigators. If efficacy of microcirculation-directed therapies is demonstrated by dedicated labs in clinical trials, this question will be of paramount importance in subsequent trials testing effectiveness of these therapies when applied broadly.
The most important limitation of studying microcirculatory dysfunction in general may be that circulatory failure only represents one of multiple complex mechanisms leading to cellular dysfunction in shock. Mechanisms at a cellular level such as mitochondrial failure (i.e. “cytopathic hypoxia”) and apoptosis are pivotal factors in the development of sepsis-associated cell death and organ failure.133–137 Although evaluation of the microcirculation might provide information on oxygen delivery to tissues that is not available from macrocirculatory parameters, imaging alone does not provide information on oxygen utilization by the cells. However, some authors have suggested that abnormalities at a cellular level are a late adaptive response that may be preceded (or perhaps triggered) by circulatory failure.138
FUTURE DIRECTIONS: IMPORTANT CONSIDERATIONS FOR CLINICAL TRIAL DESIGN
Going forward, it will be imperative to ascertain whether or not (or to what extent) the effects of microcirculation-directed therapies are clinically meaningful. This will be best addressed in a randomized controlled trial (RCT) design employing both microcirculatory and patient-oriented outcome measures. Choosing the optimal patient-oriented outcome measure in this context is vital.139 Although 28-day mortality is the typical outcome measure selected for sepsis randomized controlled trials, it captures no information on the biologic or physiologic activity of an intervention, or its capacity to modulate disease processes,139 and potentially could be confounded by a number of factors that are non-physiologic, not least of which, for example, may be family preferences for limitations of support later in the hospital course.
Alternatively, indices of acute organ system dysfunction (e.g. Sequential Organ Failure Assessment [SOFA] scores140, 141) are measures of morbidity that provide important serial assessments of physiology and response to treatment. Acute multi-organ dysfunction is a critical event in sepsis pathogenesis that is closely linked with survival.1, 44, 142 Early evidence of organ failure and early changes in organ function are especially strong mortality predictors,43, 44, 140, 141 whereas later changes in organ function have little predictive value.43 Serial SOFA scores, therefore, can be a dynamic index of disease progression and response to a novel therapy.139, 143
Table 3 is a framework that could be employed in designing clinical trials of new interventions to augment microcirculatory flow and reduce organ failure in sepsis. Using this framework, all of the possible study outcomes could yield important new information about the pathogenesis and treatment of sepsis.
Table 3.
A framework of possible outcomes for randomized controlled trials of novel interventions to augment microcirculatory flow and attenuate organ failure in sepsis. Using this framework for trial design would not only test the ability of a new intervention to (A) augment microcirculatory flow and (B) reduce sepsis-associated organ failure, but also could help test the hypothesis that microcirculatory perfusion is a key determinant of organ failure in patients with sepsis. Because it is also necessary to ascertain whether or not short-term administration of a therapy has sustainable benefit over the long-term, serial organ failure assessments well beyond 24 hours should also be incorporated.
| HYPOTHESIS A: Intervention X improves microcirculatory blood flow in sepsis resuscitation | HYPOTHESIS B: Intervention X during sepsis resuscitation reduces organ failure at 24 hours. | RESULTS INTERPRETATION |
|---|---|---|
| YES | YES | Intervention X during sepsis resuscitation improves microcirculatory perfusion and reduces organ failure. This supports the concept that organ failure in sepsis can be a perfusion-mediated phenomenon. |
| YES | NO | Intervention X improves microcirculatory perfusion in sepsis resuscitation; however, organ failure in early sepsis may not be a perfusion-mediated phenomenon. |
| NO | YES | Intervention X improves organ failure is sepsis but not through a perfusion-mediated mechanism. Possible explanations for the beneficial effects of intervention X on organ failure could be modulation of mitochondrial respiration or cellular apoptosis. |
| NO | NO | Intervention X does not improve microcirculatory perfusion and is not beneficial in sepsis. Other methods of resuscitating the microcirculation should be investigated. |
Overcoming heterogeneity among subjects in sepsis clinical trials
Historically, a plethora of clinical trials of novel agents for sepsis failed to demonstrate a benefit; this may be due (in part) to enrollment of highly heterogeneous populations of patients with nonspecific sepsis syndromes.144 As sepsis is characterized by activation of a multitude of different pathophysiologic pathways that are heterogeneously expressed, the capacity to respond to a novel agent may be a function of the degree of abnormal expression of a specific pathophysiologic mechanism at which the novel therapy is aimed (or some other subclinical phenotype that is a determinant of response to therapy). Failing to screen for expression of these factors prior to the decision to randomize may accrue a large volume of subjects in the sample with little or no capacity to respond to the new therapy, causing the clinical trial to be underpowered to show a treatment effect. For example, for a randomized clinical trial of a novel microcirculation-directed therapy in sepsis, having even a small percentage of subjects in the sample with no (or minimal) microcirculatory flow impairment could cause the trial to be underpowered. Therefore, screening sepsis subjects at the bedside for the presence or absence of microcirculatory dysfunction and limiting randomization only to those who manifest significant microcirculatory impairment despite aggressive conventional resuscitation should yield a more homogeneous sample and maximize the number of potential “responders” in the trial (Figure 5). This type of tailored clinical trial design could be considered analogous to a “personalized medicine” approach (i.e. driven by the phenotype of an individual patient). Another important consideration for clinical trials is the potential for genetic heterogeneity in response to microcirculation-directed agents.
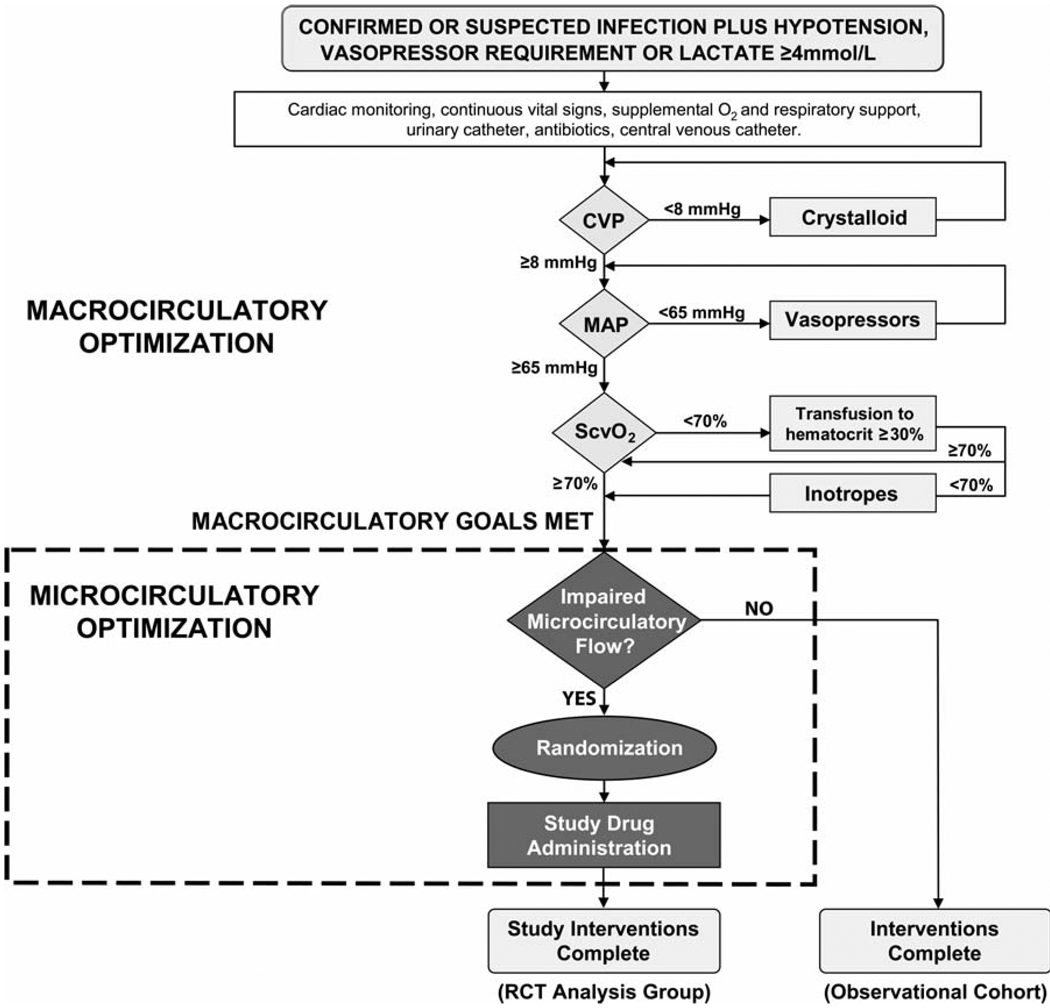
Figure 5.
A template for designing a randomized clinical trial of a novel agent to augment microcirculatory flow and improve outcome in sepsis resuscitation. Microcirculatory flow would be assessed with in vivo videomicroscopy at the bedside. Although clinically speaking, an agent that improves microcirculatory flow might optimally be initiated immediately at the time of severe sepsis identification, a requisite for this type of clinical trial would be early achievement of homogeneity in macrocirculatory hemodynamic optimization (e.g. early goal-directed therapy as per Rivers et al 4 or a similar resuscitation algorithm) in both the control and treatment subjects, in order to permit precise determination of the treatment effect of microcirculatory optimization on outcome. Because enrolling patients who do not manifest the microcirculatory dysfunction phenotype could cause the clinical trial to be underpowered to show a treatment effect, we advocate a “personalized” trial design employing a real-time assessment of microcirculatory flow prior to the decision to randomize (as shown). [CVP = central venous pressure; MAP = mean arterial pressure; ScvO2 = central venous oxygen saturation; RCT = randomized controlled trial]
Defining the control group interventions
Subjects in both control and treatment arms of RCTs of microcirculation-directed therapies should receive early protocol-directed hemodynamic optimization with standard interventions (i.e. intravenous fluids, vasopressors, etc.) targeting pre-defined quantitative resuscitation goals, in order to help ensure homogeneity in the adequacy of conventional resuscitation and normalization of global hemodynamic parameters. This homogeneity in macrocirculatory indices would be necessary in order for investigators to isolate (and better test hypotheses about) the impact of novel therapies on the microcirculation and determine the microcirculation-specific treatment effects (Figure 5).
Timing of microcirculation-directed therapies
The concept of using the resuscitation phase of therapy to investigate microcirculation-directed therapies in sepsis is based on the understanding that timing of interventions for circulatory optimization is a critical determinant of the capacity to impact outcome. On the continuum of sepsis treatment, early phase and late phase sepsis appear to be physiologically different because earlier interventions to optimize hemodynamics have been shown to be beneficial,4, 5, 145–147 whereas later interventions have not.146, 148, 149 Furthermore, organ failure in early sepsis is thought to be perfusion-mediated to a greater extent than the organ failure associated with late-phase sepsis, which may relate more closely to mitochondrial failure.134, 135, 138 Therefore, for future clinical trials of microcirculation-directed interventions, the resuscitation phase of therapy appears to be the greatest window of opportunity for demonstrating a treatment effect.
SUMMARY
Microcirculatory dysfunction is a pivotal event in the development of sepsis, and a critical component of sepsis-induced circulatory failure. Although there are still important challenges to overcome for translation of microcirculation imaging techniques to practice, obtaining microcirculatory perfusion indices may yield physiologic information that macrocirculatory indices cannot. Novel agents to “rescue” the microcirculation may prove to be a cutting-edge strategy to optimize tissue perfusion in sepsis resuscitation. As the NO molecule is vital to microcirculatory homeostasis, it appears to protect microcirculatory patency when the microcirculation sustains the septic insult. Exogenous NO administration may improve microcirculatory perfusion in sepsis, and we submit that there is sufficient scientific rationale and safety data for a clinical trial of exogenous INO administration in sepsis. In designing clinical trials to find novel microcirculation-directed therapies, the resuscitation phase of therapy appears to be the best window of opportunity for impact. Ultimately, the aim for this line of clinical investigation would be to give clinicians a novel intervention in their armamentarium to optimize tissue perfusion in the acute-phase management of sepsis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Microcirculatory Alterations in Resuscitation and Shock (MARS) team of investigators, especially Michael Rizzuto, RN, Sergio Zanotti, MD, and our past Shock Research Fellows, Jonathan V. McCoy, MD and Nicole L. Abate, MD, for their commitment to advancing microcirculation research in patients with severe sepsis and septic shock.
Funding: The authors’ microcirculation research has been supported by grants from the Emergency Medicine Foundation and the American Heart Association (0530152N) to Dr. Trzeciak. Dr. Trzeciak is now supported by a grant from the National Institutes of Health/National Institute of General Medical Sciences (K23GM083211).
Footnotes
Disclosures: Dr. Trzeciak will receive material support (drug and delivery system) from INO Therapeutics for a forthcoming investigator-initiated randomized clinical trial of inhaled nitric oxide to augment microcirculatory blood flow in sepsis resuscitation. Dr. Trzeciak has received research support from Eli Lilly for a multi-center clinical trial of the effect of recombinant human activated protein C on global and microcirculatory perfusion indices in sepsis. Dr. Dellinger receives research support from INO Therapeutics for an investigator-initiated pilot study of inhaled nitric oxide in patients with sepsis.
REFERENCES
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001 Jul;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003 Jan 9;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006 Jun;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Lin SM, Huang CD, Lin HC, Liu CY, Wang CH, Kuo HP. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006 Dec;26(6):551–557. doi: 10.1097/01.shk.0000232271.09440.8f. [DOI] [PubMed] [Google Scholar]
- 6.Otero RM, Nguyen HB, Huang DT, et al. Early goal-directed therapy in severe sepsis and septic shock revisited: concepts, controversies, and contemporary findings. Chest. 2006 Nov;130(5):1579–1595. doi: 10.1378/chest.130.5.1579. [DOI] [PubMed] [Google Scholar]
- 7.Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. Am J Physiol Heart Circ Physiol. 2002 Jan;282(1):H156–H164. doi: 10.1152/ajpheart.2002.282.1.H156. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar I, Martin CM, Lam C, Potter R, Ellis CG, Sibbald WJ. Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J Surg Res. 1996 Feb 15;61(1):190–196. doi: 10.1006/jsre.1996.0103. [DOI] [PubMed] [Google Scholar]
- 9.Fries M, Weil MH, Sun S, et al. Increases in tissue Pco2 during circulatory shock reflect selective decreases in capillary blood flow. Crit Care Med. 2006 Feb;34(2):446–452. doi: 10.1097/01.ccm.0000196205.23674.23. [DOI] [PubMed] [Google Scholar]
- 10.Lam C, Tyml K, Martin C, Sibbald W. Microvascular perfusion is impaired in a rat model of normotensive sepsis. J Clin Invest. 1994 Nov;94(5):2077–2083. doi: 10.1172/JCI117562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002 Jul 1;166(1):98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 12.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004 Sep;32(9):1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 13.Aird WC. Endothelium as an organ system. Crit Care Med. 2004 May;32(5 Suppl):S271–S279. doi: 10.1097/01.ccm.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- 14.Tyml K, Wang X, Lidington D, Ouellette Y. Lipopolysaccharide reduces intercellular coupling in vitro and arteriolar conducted response in vivo. Am J Physiol Heart Circ Physiol. 2001 Sep;281(3):H1397–H1406. doi: 10.1152/ajpheart.2001.281.3.H1397. [DOI] [PubMed] [Google Scholar]
- 15.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11–17;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 16.Aird WC. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med. 2001 Jul;29(7 Suppl):S28–S34. doi: 10.1097/00003246-200107001-00013. discussion S34–25. [DOI] [PubMed] [Google Scholar]
- 17.Terada LS, Hybertson BM, Connelly KG, Weill D, Piermattei D, Repine JE. XO increases neutrophil adherence to endothelial cells by a dual ICAM-1 and P-selectin-mediated mechanism. J Appl Physiol. 1997 Mar;82(3):866–873. doi: 10.1152/jappl.1997.82.3.866. [DOI] [PubMed] [Google Scholar]
- 18.Marumo T, Schini-Kerth VB, Fisslthaler B, Busse R. Platelet-derived growth factor-stimulated superoxide anion production modulates activation of transcription factor NF-kappaB and expression of monocyte chemoattractant protein 1 in human aortic smooth muscle cells. Circulation. 1997 Oct 7;96(7):2361–2367. doi: 10.1161/01.cir.96.7.2361. [DOI] [PubMed] [Google Scholar]
- 19.Yan SB, Helterbrand JD, Hartman DL, Wright TJ, Bernard GR. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001 Sep;120(3):915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- 20.van den Berg BM, Vink H, Spaan JA. The endothelial glycocalyx protects against myocardial edema. Circ Res. 2003 Apr 4;92(6):592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- 21.Yano K, Liaw PC, Mullington JM, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006 Jun 12;203(6):1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro NI, Yano K, Okada H, et al. A Prospective, Observational Study of Soluble Flt-1 and Vascular Endothelial Growth Factor in Sepsis. Shock. 2007 Aug 16; doi: 10.1097/shk.0b013e31815072c1. Publish Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Condon MR, Kim JE, Deitch EA, Machiedo GW, Spolarics Z. Appearance of an erythrocyte population with decreased deformability and hemoglobin content following sepsis. Am J Physiol Heart Circ Physiol. 2003 Jun;284(6):H2177–H2184. doi: 10.1152/ajpheart.01069.2002. [DOI] [PubMed] [Google Scholar]
- 24.Bateman RM, Jagger JE, Sharpe MD, Ellsworth ML, Mehta S, Ellis CG. Erythrocyte deformability is a nitric oxide-mediated factor in decreased capillary density during sepsis. Am J Physiol Heart Circ Physiol. 2001 Jun;280(6):H2848–H2856. doi: 10.1152/ajpheart.2001.280.6.H2848. [DOI] [PubMed] [Google Scholar]
- 25.Kempe DS, Akel A, Lang PA, et al. Suicidal erythrocyte death in sepsis. J Mol Med. 2007 Mar;85(3):273–281. doi: 10.1007/s00109-006-0123-8. [DOI] [PubMed] [Google Scholar]
- 26.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003 May 15;101(10):3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 27.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shultz PJ, Raij L. Endogenously synthesized nitric oxide prevents endotoxin-induced glomerular thrombosis. J Clin Invest. 1992 Nov;90(5):1718–1725. doi: 10.1172/JCI116045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radomski MW, Palmer RM, Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- 30.Azuma H, Ishikawa M, Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendelsohn ME, O'Neill S, George D, Loscalzo J. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J Biol Chem. 1990 Nov 5;265(31):19028–19034. [PubMed] [Google Scholar]
- 32.Radomski MW, Vallance P, Whitley G, Foxwell N, Moncada S. Platelet adhesion to human vascular endothelium is modulated by constitutive and cytokine induced nitric oxide. Cardiovasc Res. 1993 Jul;27(7):1380–1382. doi: 10.1093/cvr/27.7.1380. [DOI] [PubMed] [Google Scholar]
- 33.Cambien B, Bergmeier W, Saffaripour S, Mitchell HA, Wagner DD. Antithrombotic activity of TNF-alpha. J Clin Invest. 2003 Nov;112(10):1589–1596. doi: 10.1172/JCI19284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutcheson IR, Whittle BJ, Boughton-Smith NK. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha FQ, Assreuy J, Moss DW, et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology. 1994 Feb;81(2):211–215. [PMC free article] [PubMed] [Google Scholar]
- 36.Morin MJ, Unno N, Hodin RA, Fink MP. Differential expression of inducible nitric oxide synthase messenger RNA along the longitudinal and crypt-villus axes of the intestine in endotoxemic rats. Crit Care Med. 1998 Jul;26(7):1258–1264. doi: 10.1097/00003246-199807000-00031. [DOI] [PubMed] [Google Scholar]
- 37.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–S19. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trzeciak S, Dellinger RP, Parrillo JE, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007 Jan;49(1):88–98. doi: 10.1016/j.annemergmed.2006.08.021. 98 e81–82. [DOI] [PubMed] [Google Scholar]
- 39.Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. 1999 Jul;27(7):1369–1377. doi: 10.1097/00003246-199907000-00031. [DOI] [PubMed] [Google Scholar]
- 40.McCoy J, Trzeciak S, Parrillo J, et al. Improved organ function at 24 hours is associated with increased microcirculatory flow during the early resuscitation of patients with sepsis (abstract) (Acad Emerg Med).2007 May;14(5 Suppl 1):S10–S11. [Google Scholar]
- 41.Vincent JL, De Backer D. Microvascular dysfunction as a cause of organ dysfunction in severe sepsis. Crit Care. 2005;9(Suppl 4):S9–S12. doi: 10.1186/cc3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doerschug KC, Delsing AS, Schmidt GA, Haynes WG. Impairments in microvascular reactivity are related to organ failure in human sepsis. Am J Physiol Heart Circ Physiol. 2007 Aug;293(2):H1065–H1071. doi: 10.1152/ajpheart.01237.2006. [DOI] [PubMed] [Google Scholar]
- 43.Levy MM, Macias WL, Vincent JL, et al. Early changes in organ function predict eventual survival in severe sepsis. Crit Care Med. 2005 Oct;33(10):2194–2201. doi: 10.1097/01.ccm.0000182798.39709.84. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D. The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med. 2006 Nov;48(5):583–590. doi: 10.1016/j.annemergmed.2006.07.007. 590 e581. [DOI] [PubMed] [Google Scholar]
- 45.Groner W, Winkelman JW, Harris AG, et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. 1999 Oct;5(10):1209–1212. doi: 10.1038/13529. [DOI] [PubMed] [Google Scholar]
- 46.Harris AG, Sinitsina I, Messmer K. The Cytoscan Model E-II, a new reflectance microscope for intravital microscopy: comparison with the standard fluorescence method. J Vasc Res. 2000 Nov-Dec;37(6):469–476. doi: 10.1159/000054079. [DOI] [PubMed] [Google Scholar]
- 47.Mathura KR, Vollebregt KC, Boer K, De Graaff JC, Ubbink DT, Ince C. Comparison of OPS imaging and conventional capillary microscopy to study the human microcirculation. J Appl Physiol. 2001 Jul;91(1):74–78. doi: 10.1152/jappl.2001.91.1.74. [DOI] [PubMed] [Google Scholar]
- 48.Mazzarelli J, Guglielmi M, Ross F, Parrillo JE, Hollenberg SM. Leukocyte dynamics measured by orthogonal polarization spectral imaging correlate with videomicroscopy (abstract) Shock. 2005;23(Supplement 3):122. [Google Scholar]
- 49.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. 2007;15:15101–15114. doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 50.Marik PE. Sublingual capnography: a clinical validation study. Chest. 2001 Sep;120(3):923–927. doi: 10.1378/chest.120.3.923. [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa Y, Weil MH, Tang W, et al. Sublingual capnometry for diagnosis and quantitation of circulatory shock. Am J Respir Crit Care Med. 1998 Jun;157(6 Pt 1):1838–1843. doi: 10.1164/ajrccm.157.6.9710029. [DOI] [PubMed] [Google Scholar]
- 52.Weil MH, Nakagawa Y, Tang W, et al. Sublingual capnometry: a new noninvasive measurement for diagnosis and quantitation of severity of circulatory shock. Crit Care Med. 1999 Jul;27(7):1225–1229. doi: 10.1097/00003246-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 53.Creteur J, De Backer D, Sakr Y, Koch M, Vincent JL. Sublingual capnometry tracks microcirculatory changes in septic patients. Intensive Care Med. 2006 Apr;32(4):516–523. doi: 10.1007/s00134-006-0070-4. [DOI] [PubMed] [Google Scholar]
- 54.Jin X, Weil MH, Sun S, Tang W, Bisera J, Mason EJ. Decreases in organ blood flows associated with increases in sublingual PCO2 during hemorrhagic shock. J Appl Physiol. 1998 Dec;85(6):2360–2364. doi: 10.1152/jappl.1998.85.6.2360. [DOI] [PubMed] [Google Scholar]
- 55.Povoas HP, Weil MH, Tang W, Moran B, Kamohara T, Bisera J. Comparisons between sublingual and gastric tonometry during hemorrhagic shock. Chest. 2000 Oct;118(4):1127–1132. doi: 10.1378/chest.118.4.1127. [DOI] [PubMed] [Google Scholar]
- 56.Povoas HP, Weil MH, Tang W, Sun S, Kamohara T, Bisera J. Decreases in mesenteric blood flow associated with increases in sublingual PCO2 during hemorrhagic shock. Shock. 2001 May;15(5):398–402. doi: 10.1097/00024382-200115050-00011. [DOI] [PubMed] [Google Scholar]
- 57.Guzman JA, Lacoma FJ, Kruse JA. Relationship between systemic oxygen supply dependency and gastric intramucosal PCO2 during progressive hemorrhage. J Trauma. 1998 Apr;44(4):696–700. doi: 10.1097/00005373-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 58.De Backer D, Creteur J, Noordally O, Smail N, Gulbis B, Vincent JL. Does hepato-splanchnic VO2/DO2 dependency exist in critically ill septic patients? Am J Respir Crit Care Med. 1998 Apr;157(4 Pt 1):1219–1225. doi: 10.1164/ajrccm.157.4.9705075. [DOI] [PubMed] [Google Scholar]
- 59.Nelson DP, Beyer C, Samsel RW, Wood LD, Schumacker PT. Pathological supply dependence of O2 uptake during bacteremia in dogs. J Appl Physiol. 1987 Oct;63(4):1487–1492. doi: 10.1152/jappl.1987.63.4.1487. [DOI] [PubMed] [Google Scholar]
- 60.Boerma EC, van der Voort PH, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. 2007 Apr;35(4):1055–1060. doi: 10.1097/01.CCM.0000259527.89927.F9. [DOI] [PubMed] [Google Scholar]
- 61.De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference; Crit Care; 2007. Sep 10, p. R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bihari D, Smithies M, Gimson A, Tinker J. The effects of vasodilation with prostacyclin on oxygen delivery and uptake in critically ill patients. N Engl J Med. 1987 Aug 13;317(7):397–403. doi: 10.1056/NEJM198708133170701. [DOI] [PubMed] [Google Scholar]
- 63.Shoemaker WC, Appel PL, Kram HB, Duarte D, Harrier HD, Ocampo HA. Comparison of hemodynamic and oxygen transport effects of dopamine and dobutamine in critically ill surgical patients. Chest. 1989 Jul;96(1):120–126. doi: 10.1378/chest.96.1.120. [DOI] [PubMed] [Google Scholar]
- 64.De Backer D, Creteur J, Dubois MJ, et al. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects. Crit Care Med. 2006 Feb;34(2):403–408. doi: 10.1097/01.ccm.0000198107.61493.5a. [DOI] [PubMed] [Google Scholar]
- 65.De Backer D, Verdant C, Chierego M, Koch M, Gullo A, Vincent JL. Effects of drotrecogin alfa activated on microcirculatory alterations in patients with severe sepsis. Crit Care Med. 2006 Jul;34(7):1918–1924. doi: 10.1097/01.CCM.0000220498.48773.3C. [DOI] [PubMed] [Google Scholar]
- 66.Hoffmann JN, Vollmar B, Laschke MW, Fertmann JM, Jauch KW, Menger MD. Microcirculatory alterations in ischemia-reperfusion injury and sepsis: effects of activated protein C and thrombin inhibition. Crit Care. 2005;9(Suppl 4):S33–S37. doi: 10.1186/cc3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iba T, Kidokoro A, Fukunaga M, Nagakari K, Shirahama A, Ida Y. Activated protein C improves the visceral microcirculation by attenuating the leukocyte-endothelial interaction in a rat lipopolysaccharide model. Crit Care Med. 2005 Feb;33(2):368–372. doi: 10.1097/01.ccm.0000153415.04995.88. [DOI] [PubMed] [Google Scholar]
- 68.Buwalda M. Ince C. Opening the microcirculation: can vasodilators be useful in sepsis? Intensive Care Med. 2002 Sep;28(9):1208–1217. doi: 10.1007/s00134-002-1407-2. [DOI] [PubMed] [Google Scholar]
- 69.Pleiner J, Mittermayer F, Schaller G, MacAllister RJ, Wolzt M. High doses of vitamin C reverse Escherichia coli endotoxin-induced hyporeactivity to acetylcholine in the human forearm. Circulation. 2002 Sep 17;106(12):1460–1464. doi: 10.1161/01.cir.0000030184.70207.ff. [DOI] [PubMed] [Google Scholar]
- 70.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit Care Med. 2005 Aug;33(8):1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 71.Baker CH, Wilmoth FR. Microvascular responses to E coli endotoxin with altered adrenergic activity. Circ Shock. 1984;12(3):165–176. [PubMed] [Google Scholar]
- 72.Price SA, Spain DA, Wilson MA, Harris PD, Garrison RN. Subacute sepsis impairs vascular smooth muscle contractile machinery and alters vasoconstrictor and dilator mechanisms. J Surg Res. 1999 May 1;83(1):75–80. doi: 10.1006/jsre.1998.5568. [DOI] [PubMed] [Google Scholar]
- 73.Hollenberg SM, Broussard M, Osman J, Parrillo JE. Increased microvascular reactivity and improved mortality in septic mice lacking inducible nitric oxide synthase. Circ Res. 2000 Apr 14;86(7):774–778. doi: 10.1161/01.res.86.7.774. [DOI] [PubMed] [Google Scholar]
- 74.Hollenberg SM, Tangora JJ, Piotrowski MJ, Easington C, Parrillo JE. Impaired microvascular vasoconstrictive responses to vasopressin in septic rats. Crit Care Med. 1997 May;25(5):869–873. doi: 10.1097/00003246-199705000-00025. [DOI] [PubMed] [Google Scholar]
- 75.Hollenberg SM, Cunnion RE, Zimmerberg J. Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to catecholamines in septic rats. Am J Physiol. 1993 Feb;264(2 Pt 2):H660–H663. doi: 10.1152/ajpheart.1993.264.2.H660. [DOI] [PubMed] [Google Scholar]
- 76.Hollenberg SM, Easington CR, Osman J, Broussard M, Parrillo JE. Effects of nitric oxide synthase inhibition on microvascular reactivity in septic mice. Shock. 1999 Oct;12(4):262–267. doi: 10.1097/00024382-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 77.Hollenberg SM, Piotrowski MJ, Parrillo JE. Nitric oxide synthase inhibition reverses arteriolar hyporesponsiveness to endothelin-1 in septic rats. Am J Physiol. 1997 Mar;272(3 Pt 2):R969–R974. doi: 10.1152/ajpregu.1997.272.3.R969. [DOI] [PubMed] [Google Scholar]
- 78.Bakker J, Grover R, McLuckie A, et al. Administration of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of arandomized, double-blind, placebo-controlled multicenter study (study no. 144–002) Crit Care Med. 2004 Jan;32(1):1–12. doi: 10.1097/01.CCM.0000105118.66983.19. [DOI] [PubMed] [Google Scholar]
- 79.Watson D, Grover R, Anzueto A, et al. Cardiovascular effects of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) in patients with septic shock: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002) Crit Care Med. 2004 Jan;32(1):13–20. doi: 10.1097/01.CCM.0000104209.07273.FC. [DOI] [PubMed] [Google Scholar]
- 80.Anning PB, Sair M, Winlove CP, Evans TW. Abnormal tissue oxygenation and cardiovascular changes in endotoxemia. Am J Respir Crit Care Med. 1999 Jun;159(6):1710–1715. doi: 10.1164/ajrccm.159.6.9801124. [DOI] [PubMed] [Google Scholar]
- 81.Avontuur JA, Bruining HA, Ince C. Inhibition of nitric oxide synthesis causes myocardial ischemia in endotoxemic rats. Circ Res. 1995 Mar;76(3):418–425. doi: 10.1161/01.res.76.3.418. [DOI] [PubMed] [Google Scholar]
- 82.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 83.Nishida J, McCuskey RS, McDonnell D, Fox ES. Protective role of NO in hepatic microcirculatory dysfunction during endotoxemia. Am J Physiol. Dec. 1994;267(6 Pt 1):G1135–G1141. doi: 10.1152/ajpgi.1994.267.6.G1135. [DOI] [PubMed] [Google Scholar]
- 84.Spain DA, Wilson MA, Bar-Natan MF, Garrison RN. Nitric oxide synthase inhibition aggravates intestinal microvascular vasoconstriction and hypoperfusion of bacteremia. J Trauma. 1994 May;36(5):720–725. doi: 10.1097/00005373-199405000-00021. [DOI] [PubMed] [Google Scholar]
- 85.Spain DA, Wilson MA, Bloom IT, Garrison RN. Renal microvascular responses to sepsis are dependent on nitric oxide. J Surg Res. 1994 Jun;56(6):524–529. doi: 10.1006/jsre.1994.1084. [DOI] [PubMed] [Google Scholar]
- 86.Spain DA, Wilson MA, Garrison RN. Nitric oxide synthase inhibition exacerbates sepsis-induced renal hypoperfusion. Surgery. 1994 Aug;116(2):322–330. discussion 330–321. [PubMed] [Google Scholar]
- 87.Tribl B, Bateman RM, Milkovich S, Sibbald WJ, Ellis CG. Effect of nitric oxide on capillary hemodynamics and cell injury in the pancreas during Pseudomonas pneumonia-induced sepsis. Am J Physiol Heart Circ Physiol. 2004 Jan;286(1):H340–H345. doi: 10.1152/ajpheart.00234.2003. [DOI] [PubMed] [Google Scholar]
- 88.Corso CO, Gundersen Y, Dorger M, Lilleaasen P, Aasen AO, Messmer K. Effects of the nitric oxide synthase inhibitors N(G)-nitro-L-arginine methyl ester and aminoethyl-isothiourea on the liver microcirculation in rat endotoxemia. J Hepatol. 1998 Jan;28(1):61–69. doi: 10.1016/s0168-8278(98)80203-1. [DOI] [PubMed] [Google Scholar]
- 89.Sundrani R, Easington CR, Mattoo A, Parrillo JE, Hollenberg SM. Nitric oxide synthase inhibition increases venular leukocyte rolling and adhesion in septic rats. Crit Care Med. 2000 Aug;28(8):2898–2903. doi: 10.1097/00003246-200008000-00035. [DOI] [PubMed] [Google Scholar]
- 90.Binion DG, Fu S, Ramanujam KS, et al. iNOS expression in human intestinal microvascular endothelial cells inhibits leukocyte adhesion. Am J Physiol. 1998 Sep;275(3 Pt 1):G592–G603. doi: 10.1152/ajpgi.1998.275.3.G592. [DOI] [PubMed] [Google Scholar]
- 91.Lush CW, Cepinskas G, Sibbald WJ, Kvietys PR. Endothelial E- and P-selectin expression in iNOS- deficient mice exposed to polymicrobial sepsis. Am J Physiol Gastrointest Liver Physiol. 2001 Feb;280(2):G291–G297. doi: 10.1152/ajpgi.2001.280.2.G291. [DOI] [PubMed] [Google Scholar]
- 92.Hickey MJ, Granger DN, Kubes P. Inducible nitric oxide synthase (iNOS) and regulation of leucocyte/endothelial cell interactions: studies in iNOS-deficient mice. Acta Physiol Scand. 2001 Sep;173(1):119–126. doi: 10.1046/j.1365-201X.2001.00892.x. [DOI] [PubMed] [Google Scholar]
- 93.Hickey MJ, Sharkey KA, Sihota EG, et al. Inducible nitric oxide synthase-deficient mice have enhanced leukocyte-endothelium interactions in endotoxemia. Faseb J. 1997 Oct;11(12):955–964. doi: 10.1096/fasebj.11.12.9337148. [DOI] [PubMed] [Google Scholar]
- 94.Dellinger RP, Parrillo JE. Mediator modulation therapy of severe sepsis and septic shock: does it work? Crit Care Med. 2004 Jan;32(1):282–286. doi: 10.1097/01.CCM.0000105423.06091.8E. [DOI] [PubMed] [Google Scholar]
- 95.Lopez A, Lorente JA, Steingrub J, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. 2004 Jan;32(1):21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 96.De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995 Jul;96(1):60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gundersen Y, Corso CO, Leiderer R, et al. The nitric oxide donor sodium nitroprusside protects against hepatic microcirculatory dysfunction in early endotoxaemia. Intensive Care Med. 1998 Dec;24(12):1257–1263. doi: 10.1007/s001340050759. [DOI] [PubMed] [Google Scholar]
- 98.Boughton-Smith NK, Hutcheson IR, Deakin AM, Whittle BJ, Moncada S. Protective effect of S-nitroso-N-acetyl-penicillamine in endotoxin-induced acute intestinal damage in the rat. Eur J Pharmacol. 1990 Dec 4;191(3):485–488. doi: 10.1016/0014-2999(90)94185-z. [DOI] [PubMed] [Google Scholar]
- 99.Zhang H, Rogiers P, Smail N, et al. Effects of nitric oxide on blood flow distribution and O2 extraction capabilities during endotoxic shock. J Appl Physiol. 1997 Oct;83(4):1164–1173. doi: 10.1152/jappl.1997.83.4.1164. [DOI] [PubMed] [Google Scholar]
- 100.Siegemund M, Van Bommel J, Sinaasappel M, et al. The NO donor SIN-1 improves intestinal-arterial P(CO(2)) gap in experimental endotoxemia: an animal study. Acta Anaesthesiol Scand. 2007 Jul;51(6):693–700. doi: 10.1111/j.1399-6576.2007.01334.x. [DOI] [PubMed] [Google Scholar]
- 101.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DF. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002 Nov 2;360(9343):1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 102.Spronk P, Ince C, Gardien M, Mathura K, Zandstra D. Nitroglycerin for septic shock. Lancet. 2003;361(9360):880. [Google Scholar]
- 103.Hataishi R, Zapol WM, Bloch KD, Ichinose F. Inhaled nitric oxide does not reduce systemic vascular resistance in mice. Am J Physiol Heart Circ Physiol. 2006 May;290(5):H1826–H1829. doi: 10.1152/ajpheart.00938.2005. [DOI] [PubMed] [Google Scholar]
- 104.Dellinger RP, Zimmerman JL, Taylor RW, et al. Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med. 1998 Jan;26(1):15–23. doi: 10.1097/00003246-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 105.Goldfarb RD, Cinel I. Inhaled nitric oxide therapy for sepsis: more than just lung. Crit Care Med. 2007 Jan;35(1):290–292. doi: 10.1097/01.CCM.0000251290.41866.2B. [DOI] [PubMed] [Google Scholar]
- 106.Cosby K, Partovi KS, Crawford JH, et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003 Dec;9(12):1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 107.Gladwin MT, Shelhamer JH, Schechter AN, et al. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11482–11487. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gow AJ. The biological chemistry of nitric oxide as it pertains to the extrapulmonary effects of inhaled nitric oxide. Proc Am Thorac Soc. 2006 Apr;3(2):150–152. doi: 10.1513/pats.200506-058BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mannick JB. Immunoregulatory and antimicrobial effects of nitrogen oxides. Proc Am Thorac Soc. 2006 Apr;3(2):161–165. doi: 10.1513/pats.200505-048BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006 Apr;3(2):153–160. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rassaf T, Kleinbongard P, Preik M, et al. Plasma nitrosothiols contribute to the systemic vasodilator effects of intravenously applied NO: experimental and clinical Study on the fate of NO in human blood. Circ Res. 2002 Sep 20;91(6):470–477. doi: 10.1161/01.res.0000035038.41739.cb. [DOI] [PubMed] [Google Scholar]
- 112.Fox-Robichaud A, Payne D, Hasan SU, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998 Jun 1;101(11):2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox-Robichaud A, Payne D, Kubes P. Inhaled NO reaches distal vasculatures to inhibit endothelium- but not leukocyte-dependent cell adhesion. Am J Physiol. 1999 Dec;277(6 Pt 1):L1224–L1231. doi: 10.1152/ajplung.1999.277.6.L1224. [DOI] [PubMed] [Google Scholar]
- 114.Kubes P, Payne D, Grisham MB, Jourd-Heuil D, Fox-Robichaud A. Inhaled NO impacts vascular but not extravascular compartments in postischemic peripheral organs. Am J Physiol. 1999 Aug;277(2 Pt 2):H676–H682. doi: 10.1152/ajpheart.1999.277.2.H676. [DOI] [PubMed] [Google Scholar]
- 115.Cannon RO, 3rd, Schechter AN, Panza JA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001 Jul;108(2):279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hataishi R, Rodrigues AC, Neilan TG, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006 Jul;291(1):H379–H384. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 117.Liu X, Huang Y, Pokreisz P, et al. Nitric oxide inhalation improves microvascular flow and decreases infarction size after myocardial ischemia and reperfusion. J Am Coll Cardiol. 2007 Aug 21;50(8):808–817. doi: 10.1016/j.jacc.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 118.Troncy E, Francoeur M, Salazkin I, et al. Extra-pulmonary effects of inhaled nitric oxide in swine with and without phenylephrine. Br J Anaesth. 1997 Nov;79(5):631–640. doi: 10.1093/bja/79.5.631. [DOI] [PubMed] [Google Scholar]
- 119.Bloomfield GL, Sweeney LB, Fisher BJ, et al. Delayed administration of inhaled nitric oxide preserves alveolar-capillary membrane integrity in porcine gram-negative sepsis. Arch Surg. 1997 Jan;132(1):65–75. doi: 10.1001/archsurg.1997.01430250067016. [DOI] [PubMed] [Google Scholar]
- 120.Bloomfield GL, Holloway S, Ridings PC, et al. Pretreatment with inhaled nitric oxide inhibits neutrophil migration and oxidative activity resulting in attenuated sepsis-induced acute lung injury. Crit Care Med. 1997 Apr;25(4):584–593. doi: 10.1097/00003246-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 121.Neviere R, Guery B, Mordon S, et al. Inhaled NO reduces leukocyte-endothelial cell interactions and myocardial dysfunction in endotoxemic rats. Am J Physiol Heart Circ Physiol. 2000 Jun;278(6):H1783–H1790. doi: 10.1152/ajpheart.2000.278.6.H1783. [DOI] [PubMed] [Google Scholar]
- 122.Barbotin-Larrieu F, Mazmanian M, Baudet B, et al. Prevention of ischemia-reperfusion lung injury by inhaled nitric oxide in neonatal piglets. J Appl Physiol. 1996 Mar;80(3):782–788. doi: 10.1152/jappl.1996.80.3.782. [DOI] [PubMed] [Google Scholar]
- 123.Fullerton DA, Eisenach JH, McIntyre RC, Jr, et al. Inhaled nitric oxide prevents pulmonary endothelial dysfunction after mesenteric ischemia-reperfusion. Am J Physiol. 1996 Aug;271(2 Pt 1):L326–L331. doi: 10.1152/ajplung.1996.271.2.L326. [DOI] [PubMed] [Google Scholar]
- 124.Neviere R, Mordon S, Marechal X, et al. Inhaled nitric oxide modulates leukocyte kinetics in the mesenteric venules of endotoxemic rats. Crit Care Med. 2000 Apr;28(4):1072–1076. doi: 10.1097/00003246-200004000-00027. [DOI] [PubMed] [Google Scholar]
- 125.Gries A, Bode C PeterK, et al. Inhaled nitric oxide inhibits human platelet aggregation, P-selectin expression, and fibrinogen binding in vitro and in vivo. Circulation. 1998 Apr 21;97(15):1481–1487. doi: 10.1161/01.cir.97.15.1481. [DOI] [PubMed] [Google Scholar]
- 126.Sato Y, Walley KR, Klut ME, et al. Nitric oxide reduces the sequestration of polymorphonuclear leukocytes in lung by changing deformability and CD18 expression. Am J Respir Crit Care Med. 1999 May;159(5 Pt 1):1469–1476. doi: 10.1164/ajrccm.159.5.9808063. [DOI] [PubMed] [Google Scholar]
- 127.Guidot DM, Repine MJ, Hybertson BM, Repine JE. Inhaled nitric oxide prevents neutrophil-mediated, oxygen radical-dependent leak in isolated rat lungs. Am J Physiol. 1995 Jul;269(1 Pt 1):L2–L5. doi: 10.1152/ajplung.1995.269.1.L2. [DOI] [PubMed] [Google Scholar]
- 128.Kubes P, Kurose I, Granger DN. NO donors prevent integrin-induced leukocyte adhesion but not P-selectin-dependent rolling in postischemic venules. Am J Physiol. 1994 Sep;267(3 Pt 2):H931–H937. doi: 10.1152/ajpheart.1994.267.3.H931. [DOI] [PubMed] [Google Scholar]
- 129.Walley KR, McDonald TE, Higashimoto Y, Hayashi S. Modulation of proinflammatory cytokines by nitric oxide in murine acute lung injury. Am J Respir Crit Care Med. 1999 Aug;160(2):698–704. doi: 10.1164/ajrccm.160.2.9809081. [DOI] [PubMed] [Google Scholar]
- 130.Kang JL, Park W, Pack IS, et al. Inhaled nitric oxide attenuates acute lung injury via inhibition of nuclear factor-kappa B and inflammation. J Appl Physiol. 2002 Feb;92(2):795–801. doi: 10.1152/japplphysiol.00202.2001. [DOI] [PubMed] [Google Scholar]
- 131.Taylor RW, Zimmerman JL, Dellinger RP, et al. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. Jama. 2004 Apr 7;291(13):1603–1609. doi: 10.1001/jama.291.13.1603. [DOI] [PubMed] [Google Scholar]
- 132.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999 Sep;25(9):911–919. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 133.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007 Oct;35(10):2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 134.Fink MP. Bench-to-bedside review: Cytopathic hypoxia. Crit Care. 2002 Dec;6(6):491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hotchkiss RS, Karl IE. Reevaluation of the role of cellular hypoxia and bioenergetic failure in sepsis. Jama. 1992 Mar 18;267(11):1503–1510. [PubMed] [Google Scholar]
- 136.Watts JA, Kline JA. Bench to bedside: the role of mitochondrial medicine in the pathogenesis and treatment of cellular injury. Acad Emerg Med. 2003 Sep;10(9):985–997. doi: 10.1111/j.1553-2712.2003.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 137.Cinel I, Dellinger RP. Advances in pathogenesis and management of sepsis. Curr Opin Infect Dis. 2007 Aug;20(4):345–352. doi: 10.1097/QCO.0b013e32818be70a. [DOI] [PubMed] [Google Scholar]
- 138.Singer M, De Santis V, Vitale D, Jeffcoate W. Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet. 2004 Aug 7–13;364(9433):545–548. doi: 10.1016/S0140-6736(04)16815-3. [DOI] [PubMed] [Google Scholar]
- 139.Vincent JL. Endpoints in sepsis trials: more than just 28-day mortality? Crit Care Med. 2004 May;32(5 Suppl):S209–S213. doi: 10.1097/01.ccm.0000126124.41743.86. [DOI] [PubMed] [Google Scholar]
- 140.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama. 2001 Oct 10;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 141.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996 Jul;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 142.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003 Apr 17;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 143.Marshall JC, Vincent JL, Guyatt G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005 Aug;33(8):1708–1716. doi: 10.1097/01.ccm.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 144.Angus DC. Caring for the critically ill patient: challenges and opportunities. Jama. 2007 Jul 25;298(4):456–458. doi: 10.1001/jama.298.4.456. [DOI] [PubMed] [Google Scholar]
- 145.Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. Jama. 1993 Dec 8;270(22):2699–2707. [PubMed] [Google Scholar]
- 146.Kern JW, Shoemaker WC. Meta-analysis of hemodynamic optimization in high-risk patients. Crit Care Med. 2002 Aug;30(8):1686–1692. doi: 10.1097/00003246-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 147.Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988 Dec;94(6):1176–1186. doi: 10.1378/chest.94.6.1176. [DOI] [PubMed] [Google Scholar]
- 148.Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995 Oct 19;333(16):1025–1032. doi: 10.1056/NEJM199510193331601. [DOI] [PubMed] [Google Scholar]
- 149.Heyland DK, Cook DJ, King D, Kernerman P, Brun-Buisson C. Maximizing oxygen delivery in critically ill patients: a methodologic appraisal of the evidence. Crit Care Med. 1996 Mar;24(3):517–524. doi: 10.1097/00003246-199603000-00025. [DOI] [PubMed] [Google Scholar]
- 150.Boerma EC, Mathura KR, van der Voort PH, Spronk PE, Ince C. Quantifying bedside-derived imaging of microcirculatory abnormalities in septic patients: a prospective validation study. Crit Care. 2005;9(6):R601–R606. doi: 10.1186/cc3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.