Abstract
Background
Few cohort studies have focused on risk factors for end-stage renal disease (ESRD). This investigation evaluated the prognostic value of several potential novel risk factors for ESRD after considering established risk factors.
Methods
We studied 177 570 individuals from a large integrated health care delivery system in northern California who volunteered for health checkups between June 1, 1964, and August 31, 1973. Initiation of ESRD treatment was ascertained using US Renal Data System registry data through December 31, 2000.
Results
A total of 842 cases of ESRD were observed during 5 275 957 person-years of follow-up. This comprehensive evaluation confirmed the importance of established risk factors, including the following: male sex, older age, proteinuria, diabetes mellitus, lower educational attainment, and African American race, as well as higher blood pressure, body mass index, and serum creatinine level. The 2 most potent risk factors were proteinuria and excess weight. For proteinuria, the adjusted hazard ratios (HRs) were 7.90 (95% confidence interval [CI], 5.35–11.67) for 3 to 4+ on urine dipstick, 3.59 (2.82–4.57) for 1 to 2+ on urine dipstick, and 2.37 (1.79–3.14) for trace vs negative on urine dipstick. For excess weight, the HRs were 4.39 (95% CI, 3.38–5.70) for class 2 to class 3 obesity, 3.11 (2.51–3.84) for class 1 obesity, and 1.65 (1.39–1.97) for overweight vs normal weight. Furthermore, several independent novel risk factors for ESRD were identified, including lower hemoglobin level (1.33 [1.08–1.63] for lowest vs highest quartile), higher serum uric acid level (2.14 [1.65–2.77] for highest vs lowest quartile), self-reported history of nocturia (1.36 [1.17–1.58]), and family history of kidney disease (HR, 1.40 [95% CI, 1.02–1.90]).
Conclusions
We confirmed the importance of established ESRD risk factors in this large cohort with broad sex and racial/ethnic representation. Lower hemoglobin level, higher serum uric acid level, self-reported history of nocturia, and family history of kidney disease are independent risk factors for ESRD.
Patients with end-stage renal disease (ESRD) experience high rates of morbidity and mortality. Incidence and prevalence counts of ESRD in the United States are expected to increase by 44% and 85%, respectively, from 2000 to 2015.1 Reducing the incidence of ESRD is widely recognized as a major public health goal. However, few longitudinal cohort studies have been conducted focusing on identifying risk factors for ESRD.
Prior studies2,3 have identified the following independent risk factors for ESRD: older age, proteinuria, diabetes mellitus (DM), elevated blood pressure, African American race, and elevated serum creatinine level (or decreased estimated glomerular filtration rate). More recent studies4,5 have established overweight and obesity as independent risk factors.
In addition to these established risk factors, many potential “novel” risk factors have been suggested, ranging from cigarette smoking to history of urolithiasis. Many of these novel risk factors have not been rigorously examined in the context of a longitudinal cohort study design. Compared with cross-sectional study or case-control study designs, the advantages of a cohort study include reducing recall and ascertainment biases and establishing temporal sequence, which is important for determining possible causality. The goal of this study was to evaluate the prognostic value of several potential novel risk factors for ESRD after considering established risk factors. Simultaneous assessment is important because some of the proposed novel risk factors (eg, high serum uric acid level or low hemoglobin level) may be highly correlated with established risk factors (eg, baseline kidney function).
Identification of novel risk factors that predict ESRD independent of traditional risk factors may help improve risk stratification and facilitate more focused preventive efforts among higher-risk subgroups. It may also uncover new potential therapeutic targets to combat the rising tide of ESRD.
METHODS
STUDY POPULATION
The study population is the large well-characterized cohort of Kaiser Permanente of Northern California members who participated in the Multiphasic Health Testing Services Program in Oakland and San Francisco.4 Kaiser Permanente of Northern California is a large integrated health care delivery system that cares for more than one-third of the insured population in the greater San Francisco Bay area. The Multiphasic Health Checkup was a voluntary health assessment offered at initial and yearly open enrollment periods. Details of the study population have been described previously.4,6 In the present analysis, we focused on the subset of subjects who were examined between June 1, 1964, and August 31, 1973 (N=177 570). We chose this period because subjects examined during this time underwent much more detailed assessment of comorbidities and laboratory testing than subjects examined in later visits.4,6 Institutional review boards at the collaborating institutions approved the study. Because the study was a secondary analysis of existing data, the need for obtaining informed consent was waived.
IDENTIFICATION OF TREATED ESRD AND DEATH
As described previously,4,6 we identified cases of ESRD treated with maintenance dialysis or renal transplantation by matching our cohort against the US Renal Data System ESRD treatment registry. We a priori chose ESRD from any etiology as the outcome of interest rather than specific causes of ESRD because of uncertainty about the accuracy of ascribed causes. For example, it is clear that hypertensive nephrosclerosis is often diagnosed without solid evidence that this is the underlying cause.7 Many patients with DM and chronic kidney disease may not have underlying diabetic glomerulosclerosis.8 Deaths were ascertained using the California Automated Mortality Linkage System.4,6 Occurrence of ESRD and death were assessed through December 31, 2000.
EVALUATION OF EXPOSURES AND STATISTICAL ANALYSIS
The Multiphasic Health Checkup database contains a broad array of potential risk factors measured using standard protocols previously described.4,6 All potential novel risk factors considered had less than 10% missing data, with the following 3 exceptions: self-reported history of kidney disease, self-reported history of kidney surgery, and family history of kidney disease. For these 3 dichotomous variables, missing values were coded as absent, which we believe would not introduce substantial bias given the rarity of these particular exposures. Novel risk factors were further divided into the following 4 groups: clinical, family history, occupational exposure, and behavioral (Table 1).
Table 1.
Variables Examined as Risk Factors for ESRD and Associated Data Sources and Definitions
| Risk Factor | Source | Classification | ||
|---|---|---|---|---|
| Established Risk Factors | ||||
| Age at baseline examination, y | Self-report date of birth | ≤30 [Reference], 31–40, 41–50, 51–60, or >60 | ||
| Sex | Self-report | Male vs female [reference] | ||
| Race | Self-report | White [reference], African-American, Asian, or other | ||
| Blood pressure, mm Hg | Measured systolic/diastolic blood pressure | JNC 7 normal, <120/80 [reference]; prehypertension, 120–139/80–89; stage 1 hypertension, 140–159/90–99; or stage 2 hypertension, >160/100 | ||
| Serum creatinine, mg/dL | Laboratory test | <1.10 [Reference], 1.10–1.59, 1.60–2.00, or >2.00 | ||
| History of diabetes mellitus | Self-report of physician diagnosis | Yes vs no [reference] | ||
| BMI | Measured height and weight | WHO: underweight (BMI <18.5); normal (BMI 18.5–24.9) [reference]; overweight (BMI 25.0–29.9); class 1 obesity (BMI 30.0–34.9); or classes 2 and 3 obesity (BMI ≥35.0) | ||
| Proteinuria |
|
Dipstick urinalysis | Negative [reference], trace, 1–2+, or 3–4+ | |
| Hematuria | Negative [reference], small, moderate, or large | |||
| Glycosuria | Negative [reference], light, medium, or dark | |||
| Level of educational attainment | Self-report | No college, some college, or college graduate or higher [reference] | ||
| Calendar year of examination | Administrative records | Continuous variable | ||
|
| ||||
| Novel Risk Factors—Clinical | ||||
| History of: | ||||
| Coronary artery disease | Self-report of physician diagnosis of heart attack (coronary, angina, infarction) |
|
Yes vs no [reference] | |
| Stroke | Self-report of physician diagnosis | |||
| Gout | Self-report of physician diagnosis | |||
| Kidney disease | Self-report of physician diagnosis of “kidney disease (such as nephritis)” | |||
| Kidney or bladder stones | Self-report of physician diagnosis | |||
| Nocturia | Self-report of “always having to interrupt sleep to urinate” | |||
| Kidney surgery | Self-report of history of operation on kidney | |||
| Abnormal ECG | Self-report of physician report of abnormal ECG | |||
| Cancer | Self-report of physician diagnosis | |||
| Kidney or bladder infection | Self-report of physician diagnosis | |||
| Prostate surgery | Self-report of history of operation on prostate | |||
| Pain with urination | Self-report of symptom | |||
| Difficulty with urination | Self-report of symptom | |||
| Loss of bladder control | Self-report of symptom | |||
| Bloody or dark-colored urine | Self-report of symptom | |||
| >10-lb Involuntary weight loss over the past 3–6 mo | Self-report of symptom | |||
| Currently pregnant | Self-report | |||
| Left ventricular hypertrophy on ECG |
|
ECG tracing | ||
| Right ventricular hypertrophy on ECG | ||||
| Probable old infarction on ECG | ||||
| Probable new infarction on ECG | ||||
| Pulse rate quartile, range, min |
|
Measured | ||
| 4 | 84–200 | |||
| 3 | 75–83 | |||
| 2 | 68–74 | |||
| 1 | 40–67 [Reference] | |||
| Serum cholesterol concentration quartile, range, mg/dL |
|
Laboratory | ||
| 4 | 245–570 | |||
| 3 | 220–244 | |||
| 2 | 192–219 | |||
| 1 | 50–191 [Reference] | |||
| Hemoglobin level quartile for women; men, range, mg/dL | ||||
| 4 | 13.9–19.8; 15.7–21.8 [References] | |||
| 3 | 13.2–13.8; 15.0–15.6 | |||
| 2 | 12.4–13.1; 14.2–14.9 | |||
| 1 | 3.6–12.3; 4.6–14.1 | |||
| Serum uric acid level quartile, range, mg/dL | ||||
| 4 | 6.00–14.9 | |||
| 3 | 5.10–5.99 | |||
| 2 | 4.18–5.09 | |||
| 1 | 0.10–4.17 [Reference] | |||
| White blood cell count quartile, range, ×109/L | ||||
| 4 | 8.6–75.0 | |||
| 3 | 7.2–8.5 | |||
| 2 | 6.1–7.1 | |||
| 1 | 1.4–6.0 [Reference] | |||
|
| ||||
| Novel Risk Factors—Family History | ||||
| Family history of kidney disease | Self-report of kidney disease in first-degree relative | Yes vs no [reference] | ||
| Family history of kidney stone | Self-report of kidney stone in first-degree relative | |||
|
| ||||
| Novel Risk Factors—Occupational Exposure | ||||
| Occupational exposure to | ||||
| Lead or other metal fumes |
|
Self-report of having worked in a place with frequent or daily exposure | Yes vs no [reference] | |
| Asbestos, cement, or grain dust | ||||
| Ammonia, chlorine, ozone, or nitrous gas | ||||
| Chemicals, cleaning fluids, or solvents | ||||
| Engine exhaust fumes, >2 h/d | ||||
| Extreme heat | ||||
| Insect or plant spray | ||||
| Silica, sandblasting, grinding, or rock dust | ||||
| Plastic or resin fumes | ||||
| X-ray or radiation | ||||
| UV radiation | ||||
|
| ||||
| Novel Risk Factors—Behavioral | ||||
| Cigarette smoking status |
|
Self-report | Never [reference], current, or former | |
| Alcohol intake in the past year, drinks/d | Never [reference], 1–2, 3–5, or >5 | |||
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ECG, electrocardiogram; ESRD, end-stage renal disease; JNC 7, The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; WHO, World Health Organization.
SI conversion factors: To convert serum creatinine to micromoles per liter, multiply by 88.4; cholesterol to millimoles per liter, multiply by 0.0259; hemoglobulin to grams per liter, multiply by 10.0; pounds to kilograms, multiply by 0.45; serum uric acid to micromoles per liter, multiply by 59.485; white blood cell count to cells per microliter, divide by 0.001.
We designed our analytic approach a priori to minimize the likelihood of false-positive associations because of the many novel risk factors examined. We screened each novel risk factor in a univariate Cox proportional hazards model to determine if it was associated with risk of ESRD. We used quartile cutoffs for continuous variables to avoid assumptions regarding the nature of the associations. Only risk factors that had univariate associations of P<.05 were further considered in subsequent multivariate models. There were no prespecified searches for interactions.
We then constructed 5 separate multivariate Cox proportional hazards models. The first model included only the following established risk factors: sex; DM; level of educational attainment; African American, Asian, or other race; overweight and obesity (body mass index); elevated blood pressure or serum creatinine level; older age (age at the Multiphasic Health Checkup); and urine dipstick levels of protein, glucose, and hemoglobin. The year when the examination took place was also included given our previous findings from this cohort.6 Next, we examined the prognostic value of each of the 4 groups of potential novel risk factors already described. Therefore, the second model included all the established risk factors and the novel clinical risk factors. The third model included the established risk factors plus the novel family history variables. The fourth model included the established risk factors plus the novel occupational exposures. The fifth model included the established risk factors plus the novel behavioral risk factors. Using a likelihood ratio statistic, we compared the predictive value of the first model with that of each of the second, third, fourth, and fifth models. Novel risk factors from the second to the fifth models were further considered only if the likelihood ratio statistic indicated that the entire category of novel risk factors contributed additional prognostic information over and above the established risk factors.
The final multivariate model included established risk factors and individual predictors chosen from the 4 novel risk factor groups that contributed additional information and had an individual multivariate association with ESRD. The cutoff value was P<.05.
RESULTS
Among 177 570 subjects followed up for a total of 5 275 957 person-years, there were 842 cases of treated ESRD identified. Among ESRD cases, the mean (SD) duration between the baseline examination and the development of ESRD was 24.5 (6.8) years (median, 25.7 years; interquartile range, 20.2–29.7 years). The baseline characteristics of subjects are given in Table 2.
Table 2.
Baseline Characteristics of Subjects Who Did and Did Not Develop End-Stage Renal Disease (ESRD) Based on Kaiser Permanente of Northern California Multiphasic Health Checkup, 1964–1973
| Characteristic | No ESRD (n=176 728) | ESRD (n=842) |
|---|---|---|
| Age, mean (SD), y | 40.7 (14.0) | 42.3 (10.3) |
| Time of examination, No. (%) | ||
| 1964–1966 | 72 011 (40.7) | 359 (42.6) |
| 1967–1969 | 47 479 (26.9) | 225 (26.7) |
| 1970–1973 | 57 238 (32.4) | 258 (30.6) |
| Female sex, No. (%) | 95 965 (54.3) | 344 (40.9) |
| Race, No. (%) | ||
| White | 135 765 (76.8) | 398 (47.3) |
| African American | 26 144 (14.8) | 361 (42.9) |
| Asian | 7430 (4.2) | 35 (4.2) |
| Other | 7361 (4.2) | 48 (5.7) |
| Unknown | 28 (0.02) | 0 |
| Blood pressure, mean (SD), mm Hg | ||
| Systolic | 130.7 (21.0) | 141.9 (23.5) |
| Diastolic | 77.0 (13.9) | 85.8 (15.3) |
| History of diabetes mellitus, No. (%) | 3997 (2.3) | 75 (8.9) |
| Educational attainment, No. (%) | ||
| No college | 86 706 (49.1) | 522 (62.0) |
| Some college | 50 194 (28.4) | 203 (24.1) |
| College graduate or higher | 30 502 (17.3) | 76 (9.0) |
| Unknown | 9326 (5.3) | 41 (4.9) |
| Body mass index, mean (SD)a | 24.7 (4.1) | 28.5 (5.7) |
| Serum creatinine level, mean (SD), mg/dL | 0.98 (0.27) | 1.09 (0.45) |
| Urine dipstick protein, No. (%) | ||
| Negative | 167 548 (94.8) | 678 (80.5) |
| Trace | 4570 (2.6) | 53 (6.3) |
| 1–2+ | 4131 (2.3) | 79 (9.4) |
| 3–4+ | 479 (0.3) | 32 (3.8) |
| Urine dipstick hemoglobin, No. (%) | ||
| Negative | 169 203 (95.7) | 791 (93.9) |
| Small | 5490 (3.1) | 31 (3.7) |
| Moderate | 1423 (0.8) | 13 (1.5) |
| Large | 612 (0.3) | 7 (0.8) |
| Urine dipstick glucose, No. (%) | ||
| Negative | 146 613 (83.0) | 643 (76.4) |
| Small | 11 638 (6.6) | 74 (8.8) |
| Medium | 10 831 (6.1) | 56 (6.7) |
| Dark | 7646 (4.3) | 69 (8.2) |
| Family history, No. (%) | ||
| Kidney disease | 6475 (3.7) | 43 (5.1) |
| Kidney stone | 5571 (3.2) | 23 (2.7) |
| Alcoholic drinks/d in past y, No. (%) | ||
| Never | 34 887 (19.7) | 186 (22.1) |
| 1–2 | 111 428 (63.1) | 508 (60.3) |
| 3–5 | 13 090 (7.4) | 68 (8.1) |
| >5 | 4193 (2.4) | 24 (2.9) |
| Unknown | 13 130 (7.4) | 56 (6.7) |
| Cigarette smoking history, No. (%) | ||
| Never | 65 998 (37.3) | 283 (33.6) |
| Former | 28 770 (16.3) | 131 (15.6) |
| Current | 67 401 (38.1) | 366 (43.5) |
| Unknown | 14 559 (8.2) | 62 (7.4) |
| History, No. (%) | ||
| Coronary artery disease | 3001 (1.7) | 13 (1.5) |
| Abnormal electrocardiogram | 7451 (4.2) | 37 (4.4) |
| Stroke | 839 (0.5) | 7 (0.8) |
| Cancer | 11 847 (6.7) | 56 (6.7) |
| Gout | 3146 (1.8) | 23 (2.7) |
| Kidney disease | 3612 (2.0) | 35 (4.2) |
| Kidney or bladder stone | 4364 (2.5) | 36 (4.3) |
| Kidney or bladder infection | 26 198 (14.8) | 116 (13.8) |
| Kidney surgery | 1298 (0.7) | 15 (1.8) |
| Prostate surgery | 1137 (0.6) | 7 (0.8) |
| Nocturia, self-reported | 40 753 (23.1) | 289 (34.3) |
| Pain with urination | 32 161 (18.2) | 144 (17.1) |
| Difficulty with urination | 16 535 (9.4) | 75 (8.9) |
| Loss of bladder control | 18 108 (10.2) | 84 (10.0) |
| History, No. (%) | ||
| Bloody or dark-colored urine | 7438 (4.2) | 37 (4.4) |
| >10-lb Involuntary weight loss over past 3–6 mo | 6240 (3.5) | 28 (3.3) |
| Currently pregnant, among women only, No. (%) | 1035 (1.1) | 1 (0.3) |
| Electrocardiogram findings, No. (%) | ||
| Left ventricular hypertrophy | 1055 (0.6) | 9 (1.1) |
| Right ventricular hypertrophy | 41 (0.02) | 0 |
| Probable old infarction | 635 (0.4) | 1 (0.1) |
| Probable new infarction | 11 (0.006) | 0 |
| Pulse rate, mean (SD), beats/min | 76.8 (12.9) | 79.0 (13.3) |
| Serum cholesterol concentration, mean (SD), mg/dL | 221.1 (43.7) | 226.5 (45.8) |
| Hemoglobin level, mean (SD), g/dL | 14.0 (1.5) | 14.0 (1.6) |
| Serum uric acid level, mean (SD), mg/dL | 5.2 (1.4) | 5.9 (1.6) |
| White blood cell count, mean (SD), ×109/L | 7.5 (1.9) | 7.5 (2.0) |
| Occupational exposure, No. (%) | ||
| Lead or other metal fumes | 8188 (4.6) | 66 (7.8) |
| Asbestos, cement, or grain dust | 6949 (3.9) | 54 (6.4) |
| Ammonia, chlorine, ozone, or nitrous gas | 10 810 (6.1) | 83 (9.9) |
| Chemicals, cleaning fluids, or solvents | 26 930 (15.2) | 184 (21.9) |
| Engine exhaust fumes, >2 h/d | 12 100 (6.8) | 101 (12.0) |
| Extreme heat | 8181 (4.6) | 63 (7.5) |
| Insect or plant spray | 7012 (4.0) | 55 (6.5) |
| Silica, sandblasting, grinding, or rock dust | 7107 (4.0) | 48 (5.7) |
| Plastic or resin fume | 5536 (3.1) | 31 (3.7) |
| X-ray or radiation | 6252 (3.5) | 33 (3.9) |
| UV radiation | 1638 (0.9) | 13 (1.5) |
SI conversion factors: To convert cholesterol concentration to millimoles per liter, multiply by 0.0259; hemoglobin level to grams per liter, multiply by 10.0; pounds to kilograms, multiply by 0.45; serum creatinine level to micromoles per liter, multiply by 88.4; serum uric acid level to micromoles per liter, multiply by 59.485; white blood cell count to cells per microliter, divide by 0.001.
Calculated as weight in kilograms divided by height in meters squared.
As summarized in Table 3, potential novel risk factors that were associated on univariate analysis with the development of ESRD included the following: history of stroke, gout, kidney disease, kidney or bladder stones, nocturia, and kidney surgery; left ventricular hypertrophy on electrocardiogram; higher serum cholesterol, lower hemoglobin, and higher uric acid levels; family history of kidney disease; selected occupational exposures (to lead or other metal fumes; asbestos, cement or grain dust; ammonia, chlorine, ozone or nitrous gas; chemicals, cleaning fluid or solvents; engine exhaust fumes [>2 h/d]; extreme heat; insect or plant spray; silica, sandblasting, grinding or rock dust); current smoking; and lack of consumption of alcohol (compared with consumption of 1–2 alcoholic drinks a day).
Table 3.
Novel Risk Factors Associated With End-Stage Renal Disease on Univariate Survival Analysis
| Novel Risk Factor | Hazard Ratio (95% Confidence Interval) |
|---|---|
| Clinicala | |
| History of stroke | 2.15 (1.02–4.52) |
| History of gout | 1.80 (1.19–2.73) |
| History of kidney disease | 2.02 (1.44–2.84) |
| History of kidney or bladder stone | 1.91 (1.36–2.66) |
| History of nocturia, self-reported | 1.87 (1.62–2.16) |
| History of kidney surgery | 2.78 (1.67–4.63) |
| Left ventricular hypertrophy on electrocardiogram | 2.14 (1.11–4.12) |
| Serum cholesterol concentration quartile | |
| 4 | 1.55 (1.28–1.88) |
| 3 | 1.09 (0.89–1.33) |
| 2 | 1.09 (0.89–1.34) |
| 1 | 1 [Reference] |
| Hemoglobin level quartile | |
| 4 | 1 [Reference] |
| 3 | 0.98 (0.80–1.21) |
| 2 | 1.13 (0.93–1.37) |
| 1 | 1.31 (1.08–1.58) |
| Serum uric acid level quartile | |
| 4 | 4.33 (3.46–5.41) |
| 3 | 2.40 (1.89–3.05) |
| 2 | 1.76 (1.37–2.26) |
| 1 | 1 [Reference] |
| Family History | |
| Family history of kidney disease | 1.47 (1.08–1.99) |
| Occupational Exposure | |
| Lead or other metal fumes | 1.85 (1.44–2.38) |
| Asbestos, cement, or grain dust | 1.77 (1.34–2.33) |
| Ammonia, chlorine, ozone, or nitrous gas | 1.71 (1.37–2.15) |
| Chemicals, cleaning fluids, or solvents | 1.61 (1.37–1.90) |
| Engine exhaust fumes, >2 h/d | 2.00 (1.62–2.46) |
| Extreme heat | 1.73 (1.34–2.24) |
| Insect or plant spray | 1.78 (1.36–2.34) |
| Silica, sandblasting, grinding, or rock dust | 1.53 (1.15–2.05) |
| Behavioral | |
| Cigarette smoking history | |
| Never | 1 [Reference] |
| Former | 1.12 (0.91–1.38) |
| Current | 1.28 (1.10–1.50) |
| Alcoholic drinks/d in past y | |
| Never | 1 [Reference] |
| 1–2 | 0.83 (0.70–0.98) |
| 3–5 | 1.03 (0.78–1.36) |
| >5 | 1.19 (0.78–1.82) |
Quartile 4 is highest, and quartile 1 is lowest.
Based on differences in the likelihood ratio statistic between the base model that included only established risk factors, independent information was contributed by the novel clinical risk factors (P<.001) and by family history of kidney disease (P=.02). Therefore, occupational novel risk factors (P=.14) and behavioral novel risk factors (P=.11) were not considered further. Of note, the same conclusion about types of risk factors to include in our final model was reached when history of coronary artery disease was forced into the novel clinical risk factors model9,10 (P=.17) or when pack-years of smoking were used in alternate analysis of behavioral novel risk factors (P=.08).
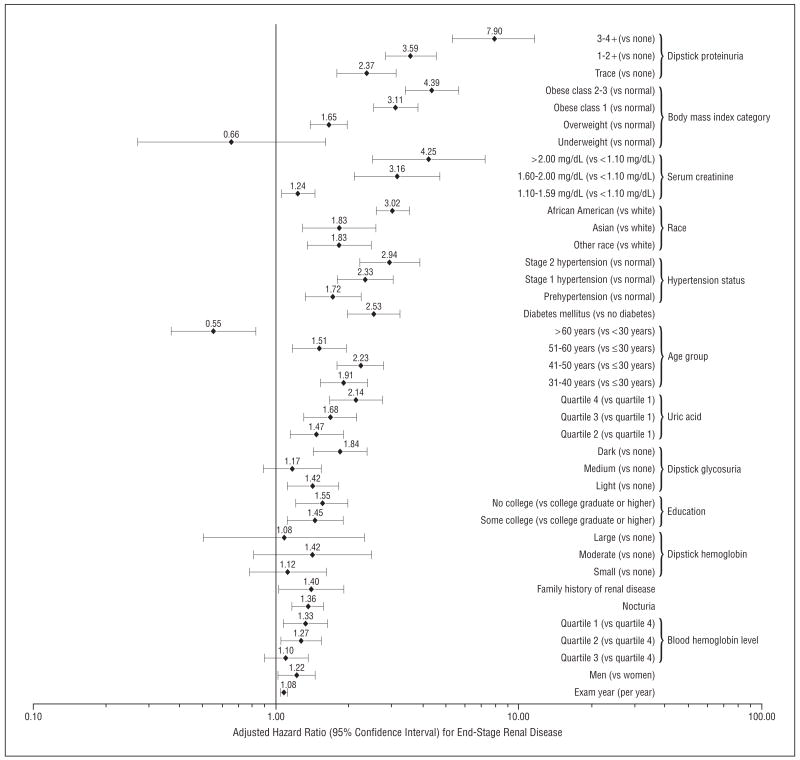
Among the novel clinical risk factors, only hemoglobin level, history of nocturia, and serum uric acid level demonstrated P<.05 in multivariate analyses; therefore these, along with family history of kidney disease, were included in the final model. The results of the final multivariate model are shown in the Figure. Persons in the highest quartile of serum uric acid level had a more than 2-fold higher adjusted risk of developing ESRD compared with those in the lowest quartile (adjusted hazard ratio [HR], 2.14; 95% confidence interval [CI], 1.65–2.77). Independent associations with ESRD were also observed for lower hemoglobin level (HR, 1.33; 95% CI, 1.08–1.63 for lowest vs highest quartile), self-reported history of nocturia (1.36; 1.17–1.58), and family history of kidney disease (1.40; 1.02–1.90).
Figure.
Adjusted relative risks among independent risk factors for end-stage renal disease (adjusted for all factors shown). To convert serum creatinine level to micromoles per liter, multiply by 88.4.
The importance of established risk factors was confirmed in this comprehensive evaluation (Figure). These included proteinuria (HR, 7.90 [95% CI, 5.35–11.67] for 3 to 4+ on urine dipstick, 3.59 [2.82–4.57] for 1 to 2+, and 2.37 [1.79–3.14] for trace vs negative on urine dip-stick); excess weight (HR, 4.39 [95% CI, 3.38–5.70] for class 2 and 3 obesity, 3.11 [2.51–3.84] for class 1 obesity, and 1.65 [1.39–1.97] for overweight vs normal weight); elevated serum creatinine (HR, 4.25 [95% CI, 2.49–7.25] for a level of >2.00 mg/dL, 3.16 [2.11–4.73] for a level of 1.60–2.00 mg/dL, and 1.24 [1.05–1.45] for a level of 1.10–1.59 mg/dL vs a level of <1.10 mg/dL); African American race (HR, 3.02 [95% CI, 2.58–3.54] vs white race); higher blood pressure (HR, 2.94 [95% CI, 2.21–3.92] for stage 2 hypertension, 2.33 [1.78–3.05] for stage 1 hypertension, and 1.72 [1.32–2.24] for prehypertension vs normal); DM (HR, 2.53 [95% CI, 1.97–3.25] presence vs absence); older age (HR, 0.55 [95% CI, 0.37–0.83] for age >60 years, 1.51 [1.17–1.96] for age 51–60 years, 2.23 [1.79–2.79] for age 41–50 years, and 1.91 [1.52–2.38] for age 31–40 years vs age ≤30 years); lower educational attainment (HR, 1.55 [95% CI, 1.21–2.00] for no college and 1.45 [1.11–1.90] for some college vs college graduate or higher); and male sex (HR, 1.22 [95% CI, 1.02–1.45]).
Results were unchanged in sensitivity analyses when we added a linear term for serum creatinine level to the model to minimize residual confounding within categories of serum creatinine level. Results were also unchanged when estimated glomerular filtration rate was used instead of serum creatinine level to measure renal function.
COMMENT
This study contributes to our epidemiologic knowledge of ESRD by identifying, through a rigorous screening process, 4 risk factors for ESRD that seem to be independent of previously established prognostic factors. These include lower hemoglobin level, elevated serum uric acid level, self-reported history of nocturia, and family history of kidney disease.
Lower hemoglobin level has been found to be a risk factor in some11 but not other12 prior studies. Among patients with type 2 DM and overt nephropathy, Mohanram et al11 noted that a hemoglobin reading less than 13.8 g/dL (to convert hemoglobin to grams per liter, multiply by 10.0) was associated with increasing risk of ESRD even after adjusting for concurrent kidney function. The pathophysiologic mechanism underlying the association between lower hemoglobin level and risk of ESRD is unclear. Tissue hypoxia seems unlikely with such modest declines in hemoglobin level, but it is possible that more severe anemia developed in the years following the baseline examination. It is also possible that this observation may be explained by residual confounding; for example, inflammation can lower hemoglobin level, or variations in hemoglobin level may reflect differences in underlying glomerular filtration rate not captured by serum creatinine concentration level. Nevertheless, markers of disease may still be useful clinically for providing prognostic information.
Although the concept of “gouty nephropathy” was discredited in the past, there has been resurgent interest in the pathophysiologic role of uric acid in cardiac and renal disease via detrimental actions such as stimulation of vascular smooth muscle cell proliferation and induction of endothelial dysfunction. Animal studies have shown that hyperuricemia accelerates renal disease progression in the remnant kidney model via a mechanism linked to high systemic blood pressure and cyclooxygenase 2–mediated thromboxane-induced vascular disease.13 Iseki et al14 reported that men and women with hyperuricemia in a screened Japanese cohort were more likely to develop ESRD. However, after adjusting for blood pressure and serum creatinine level in that study, hyperuricemia was no longer an independent predictor for men but remained so for women (for serum uric acid level ≥6.0 mg/dL [to convert serum uric acid level to micromoles per liter, multiply by 59.485]). Our results extend this novel observation to a larger, more diverse cohort and suggest that greater consideration should be given to randomized controlled trials to examine whether lowering the serum uric acid level would be an effective renoprotective strategy.15
It is interesting that nocturia (defined herein as self-report of “always having to interrupt sleep to urinate”) emerged as an independent risk factor for ESRD because it is a widely held clinical belief that nocturnal polyuria is an early sign of chronic kidney disease due to decreased urinary concentrating ability,16 although some data suggest that increased salt, not water excretion, is more important.16 Therefore, nocturia may reflect subtle early renal disease not captured by serum creatinine level or urine dipstick analysis. We also cannot exclude the possibility that nocturia reflects undiagnosed DM. An alternative hypothesis is that nocturia reflects a high volume of ingested fluid that is detrimental (especially among patients with existing kidney disease), as high urine volume increases intratubular volume and pressure and these stretch forces induce fibrosis.17 Practically speaking, our data lend no support to the notion that a high volume of water intake should be recommended in clinical practice as being beneficial to kidney function.18
Our finding regarding family history of kidney disease (defined herein as self-report of kidney disease in a first-degree relative) is in agreement with previous studies that demonstrated the clustering of kidney disease within families19 and the heritability of traits such as glomerular filtration rate and albuminuria.20 These data further underscore the need to study the role of genetic influences on kidney disease beyond monogenic disorders such as polycystic kidney disease or Alport syndrome. These results also support the public health strategy of using family history of kidney disease to identify persons at increased risk to undergo screening for kidney disease.21
IMPLICATIONS
Our findings have several important implications. Our study confirmed the importance of established ESRD risk factors in this large diverse cohort with broad sex and racial/ethnic representation. As summarized in the Figure, independent risk for ESRD includes the following: male sex,22 older age,12 higher blood pressure,10,23 higher body mass index,4 DM (and more glycosuria),24 higher serum creatinine level,25 proteinuria26 (but not hematuria27), African American race,28 and lower educational attainment (and presumed lower socioeconomic status).29
The nonstepwise association in risk of ESRD with age (after adjusting for renal function and other comorbid conditions) is likely due to competing causes of mortality among older participants. The absence of a statistically significant association with smoking, alcohol intake, hematuria on dipstick analysis, and serum cholesterol concentration is consistent with prior literature showing that these are at best only weakly associated with the development of ESRD.2,3
The Figure shows the relative importance of these risk factors. The critical importance of proteinuria is highlighted by the fact that, on average, even trace proteinuria on urine dipstick is associated with a more than 2-fold increase in risk of ESRD. The strong association between excess weight and risk of ESRD is most concerning given the epidemic of overweight and obesity in the United States and other countries. It is notable that such a strong association was seen even after adjusting for concurrently determined blood pressure, proteinuria level, and DM status. Being African American (or belonging to other racial minority groups) remained a pronounced risk factor for ESRD even after controlling for extensive baseline explanatory variables. Consistent with what we previously concluded using a different analytic approach,30 the association between elevated blood pressure and ESRD does not seem to be due to confounding by clinically evident baseline kidney disease. We agree with prior commentators12,31 who stressed that data such as these would allow better identification of patients with heightened risk of ESRD for early screening to implement timely preventive interventions.
Our study also contributes toward the goal envisioned by some researchers to develop a summary equation to predict the likelihood of developing ESRD. For example, Taal and Brenner32 proposed the use of a “renal risk score” similar to the Framingham coronary heart disease risk score.
Another area in which these data could be applicable is in the selection of potential kidney donors. Quantitative estimates about risk of ESRD for living donors would seem essential to defensible donor selection practices. This need for reliable data regarding long-term risk is particularly urgent given the current trend toward accepting live kidney donors with “isolated medical abnormalities.”33 Our findings could be used in conjunction with data regarding changes in blood pressure, urinary protein level, and glomerular filtration rate after kidney donation to provide more accurate informed consent of kidney donors.34,35 Of course, corroborating studies should be conducted among kidney donors.
Finally, the identification of novel modifiable risk factors such as hyperuricemia opens the door to potentially new interventions beyond blood pressure control and blockade of the renin-angiotensin-aldosterone system to reduce the risk of ESRD.
STRENGTHS AND LIMITATIONS
The strengths of this study include the long duration of follow-up, the unbiased ascertainment of ESRD, and the large size of the study cohort, which was diverse in terms of age, sex, and race/ethnicity. We were able to count actual treated ESRD cases and did not rely on surrogate outcomes such as slope of glomerular filtration rate loss. It cannot be assumed that the risk factors and the strengths of associations are identical, as most patients who experience progressive loss of kidney function do not develop ESRD but rather die of competing causes such as cardiovascular disease.36 Our study also differs from published studies37,38 that included treated ESRD along with “deaths attributed to kidney disease” as a composite outcome because patients who died and were not offered or declined renal replacement therapy are likely to be a special subset of subjects, and risk factors for these 2 outcomes may be different.
Our database not only captured the established risk factors for ESRD but also included a large array of potential novel risk factors. We systematically evaluated all of the potential novel risk factors and adopted a conservative analytic strategy to reduce chance findings. This study contributes to the literature by identifying lower hemoglobin level, elevated serum uric acid level, self-reported history of nocturia, and family history of kidney disease as independent novel risk factors for ESRD. We were also able to compare the relative strengths of associations of established and novel risk factors for ESRD in a comprehensive analysis within the same population. The availability of serum creatinine level and dip-stick urinalysis findings at baseline in all subjects represents an advancement over several prior studies.23,24,28,39
Limitations of this study include the fact that exposures were only assessed once. Misclassification (eg, of blood pressure category) would generally tend to bias our estimates toward the null, so the actual underlying associations may be stronger than those observed herein. In the intervening years between baseline assessment and development of ESRD, some subjects may have developed incident disease (eg, DM). Treatment of kidney disease has also changed over time, although we believe that this would not likely affect the relative associations seen between, for example, high serum uric acid level and risk of ESRD. Although many patient characteristics were assessed as potential predictors of ESRD, we were unable to assess variables such as illicit drug use, use of analgesic medications, or circulating inflammatory markers. Because our study was conducted among insured members of a northern California integrated health care delivery system, our results may not be completely generalizable to other populations.
In summary, this study contributes new information to our understanding of risk factors for ESRD. By studying women and men of different races/ethnicities, we quantified the magnitude of association of established risk factors with ESRD in a large and diverse cohort. We further established that lower hemoglobin level, elevated serum uric acid level, self-reported history of nocturia, and family history of kidney disease are independent novel risk factors for ESRD.
Acknowledgments
Funding/Support: This study was funded by grants HL71074 and DK61520 from the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
Role of the Sponsor: The National Institutes of Health had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclaimer: Some of the data reported herein were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Hsu, Iribarren, and Go. Acquisition of data: Iribarren, Darbinian, and Go. Analysis and interpretation of data: Hsu, Iribarren, McCulloch, Darbinian, and Go. Drafting of the manuscript: Hsu, Iribarren, and Go. Critical revision of the manuscript for important intellectual content: Hsu, Iribarren, McCulloch, Darbinian, and Go. Statistical analysis: Hsu and McCulloch. Obtained funding: Hsu and Iribarren. Administrative, technical, and material support: Iribarren, Darbinian, and Go. Study supervision: Iribarren and Go.
References
- 1.Gilbertson DT, Liu J, Xue JL, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015 [published correction appears in J Am Soc Nephrol. 2006;17(2):591] J Am Soc Nephrol. 2005;16(12):3736–3741. doi: 10.1681/ASN.2005010112. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg ME, Hsu CY. Chronic kidney disease and progression. NephSAP. 2004;3(6):304–308. [Google Scholar]
- 3.Hsu CY, Schieppati A. Chronic kidney disease and progression. NephSAP. 2006;5(3):156–160. [Google Scholar]
- 4.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds K, Gu D, Muntner P, et al. Body mass index and risk of ESRD in China. Am J Kidney Dis. 2007;50(5):754–764. doi: 10.1053/j.ajkd.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Hsu CY, Go AS, McCulloch CE, Darbinian J, Iribarren C. Exploring secular trends in the likelihood of receiving treatment for end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(1):81–88. doi: 10.2215/CJN.01950606. [DOI] [PubMed] [Google Scholar]
- 7.Schlessinger SD, Tankersley MR, Curtis JJ. Clinical documentation of end-stage renal disease due to hypertension. Am J Kidney Dis. 1994;23(5):655–660. doi: 10.1016/s0272-6386(12)70275-5. [DOI] [PubMed] [Google Scholar]
- 8.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289(24):3273–3277. doi: 10.1001/jama.289.24.3273. [DOI] [PubMed] [Google Scholar]
- 9.Iseki K, Wakugami K, Maehara A, Tozawa M, Muratani H, Fukiyama K. Evidence for high incidence of end-stage renal disease in patients after stroke and acute myocardial infarction at age 60 or younger. Am J Kidney Dis. 2001;38(6):1235–1239. doi: 10.1053/ajkd.2001.29219. [DOI] [PubMed] [Google Scholar]
- 10.Perry HM, Jr, Miller JP, Fornoff JR, et al. Early predictors of 15-year end-stage renal disease in hypertensive patients. Hypertension. 1995;25(4 pt 1):587–594. doi: 10.1161/01.hyp.25.4.587. [DOI] [PubMed] [Google Scholar]
- 11.Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int. 2004;66(3):1131–1138. doi: 10.1111/j.1523-1755.2004.00863.x. [DOI] [PubMed] [Google Scholar]
- 12.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17(5):1444–1452. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 13.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 14.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44(4):642–650. [PubMed] [Google Scholar]
- 15.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Motokawa M, Miyagi S, et al. Polynocturia in chronic kidney disease is related to natriuresis rather than to water diuresis. Nephrol Dial Transplant. 2006;21(8):2172–2177. doi: 10.1093/ndt/gfl165. [DOI] [PubMed] [Google Scholar]
- 17.Hebert LA, Greene T, Levey A, Falkenhain ME, Klahr S. High urine volume and low urine osmolality are risk factors for faster progression of renal disease. Am J Kidney Dis. 2003;41(5):962–971. doi: 10.1016/s0272-6386(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 18.Wenzel UO, Hebert LA, Stahl RA, Krenz I. My doctor said I should drink a lot! recommendations for fluid intake in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1(2):344–346. doi: 10.2215/CJN.01140905. [DOI] [PubMed] [Google Scholar]
- 19.Freedman BI, Soucie JM, McClellan WM. Family history of end-stage renal disease among incident dialysis patients. J Am Soc Nephrol. 1997;8(12):1942–1945. doi: 10.1681/ASN.V8121942. [DOI] [PubMed] [Google Scholar]
- 20.Langefeld CD, Beck SR, Bowden DW, Rich SS, Wagenknecht LE, Freedman BI. Heritability of GFR and albuminuria in Caucasians with type 2 diabetes mellitus. Am J Kidney Dis. 2004;43(5):796–800. doi: 10.1053/j.ajkd.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation. Kidney Early Evaluation Program: KEEP Annual Data Report 2006. Am J Kidney Dis. 2007;49(3 suppl 3):S1–S160. [Google Scholar]
- 22. [Accessed April 1, 2008];US Renal Data System 2007 annual data report. http://www.usrds.org/2007/pdf/02_incid_prev_07.pdf.
- 23.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 24.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT: Multiple Risk Factor Intervention Trial. JAMA. 1997;278(23):2069–2074. [PubMed] [Google Scholar]
- 25.Iseki K, Ikemiya Y, Fukiyama K. Risk factors of end-stage renal disease and serum creatinine in a community-based mass screening. Kidney Int. 1997;51(3):850–854. doi: 10.1038/ki.1997.119. [DOI] [PubMed] [Google Scholar]
- 26.Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 2003;63(4):1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 27.Halbesma N, Kuiken DS, Brantsma AH, et al. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol. 2006;17(9):2582–2590. doi: 10.1681/ASN.2005121352. [DOI] [PubMed] [Google Scholar]
- 28.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men: 16-year MRFIT findings. JAMA. 1997;277(16):1293–1298. [PubMed] [Google Scholar]
- 29.Fored CM, Ejerblad E, Fryzek JP, et al. Socio-economic status and chronic renal failure: a population-based case-control study in Sweden. Nephrol Dial Transplant. 2003;18(1):82–88. doi: 10.1093/ndt/18.1.82. [DOI] [PubMed] [Google Scholar]
- 30.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C. Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med. 2005;165(8):923–928. doi: 10.1001/archinte.165.8.923. [DOI] [PubMed] [Google Scholar]
- 31.Gansevoort RT, Bakker SJ, de Jong PE. Early detection of progressive chronic kidney disease: is it feasible? J Am Soc Nephrol. 2006;17(5):1218–1220. doi: 10.1681/ASN.2006030247. [DOI] [PubMed] [Google Scholar]
- 32.Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70(10):1694–1705. doi: 10.1038/sj.ki.5001794. [DOI] [PubMed] [Google Scholar]
- 33.Karpinski M, Knoll G, Cohn A, Yang R, Garg A, Storsley L. The impact of accepting living kidney donors with mild hypertension or proteinuria on transplantation rates. Am J Kidney Dis. 2006;47(2):317–323. doi: 10.1053/j.ajkd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Boudville N, Prasad GV, Knoll G, et al. Donor Nephrectomy Outcomes Research (DONOR) Network. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med. 2006;145(3):185–196. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 35.Garg AX, Muirhead N, Knoll G, et al. Donor Nephrectomy Outcomes Research (DONOR) Network. Proteinuria and reduced kidney function in living kidney donors: a systematic review, meta-analysis, and meta-regression. Kidney Int. 2006;70(10):1801–1810. doi: 10.1038/sj.ki.5001819. [DOI] [PubMed] [Google Scholar]
- 36.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 37.Erlinger TP, Tarver-Carr ME, Powe NR, et al. Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am J Kidney Dis. 2003;42(2):256–263. doi: 10.1016/s0272-6386(03)00650-4. [DOI] [PubMed] [Google Scholar]
- 38.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington county, Maryland. J Am Soc Nephrol. 2003;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds K, Gu D, Munter P, et al. A population-based prospective study of blood pressure and risk for end-stage renal disease in China. J Am Soc Nephrol. 2007;18(6):1928–1935. doi: 10.1681/ASN.2006111199. [DOI] [PubMed] [Google Scholar]