Aggregation of amyloid β peptide (Aβ) in the brain is the primary element in the pathogenesis of Alzheimer’s disease (AD).1 Aβ is produced from amyloid precursor protein (APP), which is a type I transmembrane (TM) glycoprotein in neural and non-neural cells. APP is first cleaved on the β-site by β-secretase, and the extracellular domain of APP is dissociated from the remaining protein (APP-C99). γ-secretase then cleaves the γ-site (Gly38-Thr43), which is located on the TM domain of APP-C99. Finally, Aβ is released to the extracellular region. Because the γ-site contains several cleavage points (see supporting figure 1), Aβ of different chain lengths are observed. Of these, Aβ1-40 and Aβ1-42 are primary and secondary isoforms, respectively.
Structural information on Aβ and its aggregated forms has been accumulated by NMR spectroscopy and X-ray crystallography.2 A number of molecular dynamics (MD) simulations on the aggregation of Aβ in solution have also been performed.3 In contrast, little is known about the TM structures of APP/APP-C99. Since the amyloid accumulation depends on the chain length of Aβ, it is relevant to understand how Aβ is cleaved by γ-secretase and released from APP-C99. To address this key question, we have determined the monomer structure of APP-C99 fragment (Aβ1-55), which has two α-helical regions from His13 to Val18 and from Ala30 to Lys53 by replica-exchange molecular dynamics (REMD) simulations.4
APP/APP-C99 contains three Gly-XXX-Gly motifs in TM and juxtamembrane (JM) regions. The motif is known to promote dimerization of polypeptides via Cα-H…O hydrogen bonds between two segments in a membrane environment. A pairwise replacement of Gly (Gly29 and Gly33) with Leu (Leu29 and Leu33) in APP enhances the homo dimerization, but leads to a drastic reduction of Aβ1-40 and Aβ1-42 secretion.5 To resolve this apparent discrepancy, it would be useful to predict the homo-dimer conformation of APP/APP-C99 in the membrane. Understanding the homodimer conformation of APP/APP-C99 is essential for elucidating the last step in the formation of Aβ associated Alzheimer’s disease.
We performed REMD simulations of two APP fragments (Aβ23-55) in a membrane environment for both the wild type (WT) sequence and a mutant, in which Gly29 and Gly33 are replaced with Leu29 and Leu33. MMTSB toolsets with the CHARMM 19 EEF1.1 force field were used for the calculations6 (the simulation details are given in the supporting materials). The effects of solvent and membrane on the APP fragments were included implicitly in IMM1 model.7
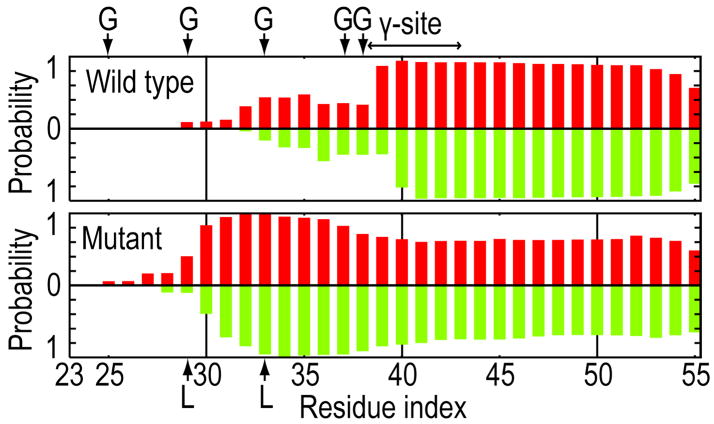
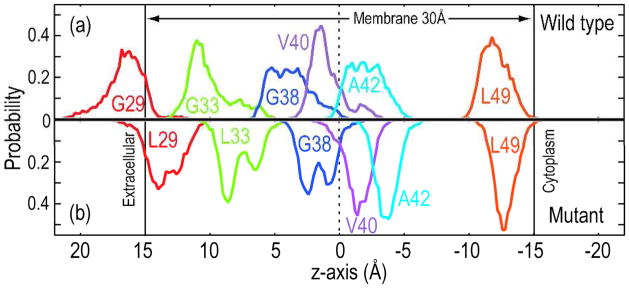
At 300 K, secondary and tertiary structures of the mutant APP fragments differ from those of the WT. In figure 1, we compare α-helicity of each residue in the WT with that in the mutant. The similarity of α-helicity in the top (colored in red) and bottom (in green) plots indicates that the REMD simulations were able to sample all the possible configurations of the APP fragments in the membrane. Marked contrasts in the α-helicity between the WT and the mutant are observed in residues 29–38. This region was observed to be unwound in the WT, whereas it formed an α-helix in the mutant. In figure 2, Leu29 in the mutant was located in the membrane, whereas Gly29 in the WT was in the extracellular region. The position of Leu49 was not altered by the mutation. Each mutant APP fragment was, therefore, more tilted (see supporting figure 3). As a result, the γ-site in the mutant was shifted toward the center of the membrane.
Figure 1.
α-helical content of each residue in the wild type (a) and the mutant (b) proteins at 300 K. The α-helical residue is defined with DSSP.8 Green and red lines represent the α-helical content for two fragments in WT and the mutant. The locations of Gly, mutated Leu, and the γ-site are explicitly shown.
Figure 2.
The distributions of the Cα positions of G29 and L29 (red), G33 and L33 (green), G38 (blue), V40 (purple), A42 (cyan), and L49 (orange) along the z-axis for the WT (a) and the mutant (b) proteins.
We also investigated the homo-dimer conformations of the WT and the mutant APP fragments at 300 K by principal component analysis (PCA)9 of the backbone atoms in the region from Gly29 to Thr43. By using the first and third principal components (PC1 and PC3), we obtained two major peaks for the mutant (PM1: 65.4%, PM2: 28.5%) and several other peaks for the WT fragments (see supporting figure 4). Because of the backbone flexibility observed in the WT, PCA could not classify major dimer structures. Instead, we used Cα-H…O hydrogen bonds between two fragments for these classifications as shown in supporting figure 5.
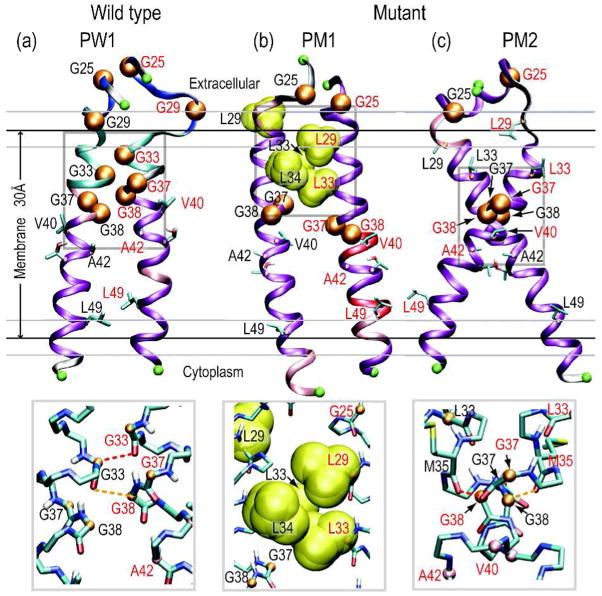
We observed three different types of the homo-dimer conformation of APP fragments in the membrane. The first type observed only in the WT, was stabilized by Cα-H…O hydrogen bonds between the two APP fragments. These bonds are the most characteristic interaction between two fragments that contain Gly-XXX-Gly motifs. Due to the three Gly-XXX-Gly motifs in the WT APP fragments, multiple Cα-H…O hydrogen bonds were observed. Of these, the hydrogen bonds involving Gly33 and Gly38 were essential for the dimerization of the WT APP fragments (figure 3a inset and supporting figure 6). This causes a partial unwinding of α-helix in the residues 29–38 as shown in figure 1. Solid-state NMR spectroscopy has also shown that the glycines in the Gly-XXX-Gly motif line at the dimer interface.10
Figure 3.
Structures of major dimer conformations of the WT (a), and the mutant (b and c). Gray boxes indicate specific dimerization sites, and are enlarged at the bottom. Red and orange dashes indicate strong and medium Cα-H…O hydrogen bonds, respectively.
In the second type of dimer conformation, hydrophobic residues intervened between two APP fragments. This conformation was observed mainly in the mutant (PM1 in figure 3), because the mutated Leu29 and Leu33 contributed significantly. In addition to the mutated residues, Ile31, Leu34, or Val36 intervened between two APP fragments. Therefore, this form was also observed in the WT as a minor conformation. In contrast, in the third type of dimer conformation two APP fragments crossed with each other at Gly38. This conformation is similar to the conformation of glycophorin A, which also has a Gly-XXX-Gly motif in the TM region.11 This conformation populated roughly 28.5% (in PM2) in the mutant, whereas it represented less than 1.0 % in the WT. The Gly-rich region of the TM and JM regions in the WT would be too flexible to take on this conformation.
How do the conformational differences between the WT and the mutant affect the secretion of Aβ or the cleavage by γ-secretase? As shown in figure 2, the γ-site (Gly38-Thr43) in the mutant is shifted downward about 3 Å along the bilayer normal. In addition, the conformational flexibility of the γ-site might be increased in the mutant, because of the lack of inter-fragment Cα-H…O hydrogen bonds at Gly38. These changes likely induce mismatched interactions between the γ-site of APP-C99 and the active site of γ-secretase, which would reduce the secretion of Aβ1-42 or Aβ1-42.5
In summary, we have predicted the APP (Aβ23-55) fragment dimer structures of the WT and a mutant protein using REMD simulations and found drastic changes of dimer structures by the mutation.5 The results are in good agreement with the existing experimental data5,10 and provide fundamental insight into the initial steps in the amyloid formation.
Supplementary Material
Acknowledgments
This research was supported in part by a Grant for Scientific Research on a Priority Areas ‘Membrane Interface’ (to YS) and the Development and Use of the Next-Generation Supercomputer Project of the Ministry of Education Culture, Sports, Science and Technology (MEXT), and by CREST & BIRD, Japan Science and Technology Agency (JST) (to YS). DT and JES are thankful for the support of a grant from the national institute of health (RO1 GM076688-05). We thank the RIKEN Super Combined Cluster (RSCC) for providing computational resources.
Footnotes
Supporting Information Available: The supporting methods and figures are available free of charge via the Internet at (http://pubs.acs.org/paragonplus/submission/jacsat/).
References
- 1.See for instance, Haass C, Selkoe DJ. Nature Reviews Molecular Cell Biology. 2007;8:101–112. doi: 10.1038/nrm2101.
- 2.See for instance, Petkova AT, Yau WM, Tycko R. Biochemistry. 2006;45:498–512. doi: 10.1021/bi051952q.Nelson R, Eisenberg D. Curr Opin Struc Biol. 2006;16:260–265. doi: 10.1016/j.sbi.2006.03.007.
- 3.There are number of papers. See for ex., Tarus B, Straub JE, Thirumalai D. J Am Chem Soc. 2006;128:16159–16168. doi: 10.1021/ja064872y.Tarus B, Straub JE, Thirumalai D. J Mol Biol. 2005;345:1141–1156. doi: 10.1016/j.jmb.2004.11.022.Massi F, Peng JW, Lee JP, Straub JE. Biophys J. 2001;80:31–44. doi: 10.1016/S0006-3495(01)75993-0.
- 4.(a) Sugita Y, Okamoto Y. Chem Phys Lett. 1999;314:141–151. [Google Scholar]; (b) Miyashita N, Straub JE, Thirumalai D. to be published. [Google Scholar]
- 5.Kienlen-Campard P, Tasiaux B, Hees JV, Li M, Huysseune S, Sato T, Fei JZ, Aimoto S, Courtoy PJ, Smith SO, Constantinescu SN, Octave JN. J Biol Chem. 2008;283:7733–7744. doi: 10.1074/jbc.M707142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Feig M, Karanicolas J, Brooks CL. J Mol Graphics and Modeling. 2004;22:377–395. doi: 10.1016/j.jmgm.2003.12.005. [DOI] [PubMed] [Google Scholar]; (b) MacKerell AD, Jr, et al. J Phys Chem B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- 7.Lazaridis T. Proteins. 2003;52:176–192. doi: 10.1002/prot.10410. [DOI] [PubMed] [Google Scholar]
- 8.Kabsch W, Sander C. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 9.(a) Kitao A, Go N. Curr Opin Struc Biol. 1999;9:164–169. doi: 10.1016/S0959-440X(99)80023-2. [DOI] [PubMed] [Google Scholar]; (b) García AE. Phys Rev Lett. 1992;68:2696–2699. doi: 10.1103/PhysRevLett.68.2696. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Tang T, Reubins G, Fei JZ, Fujimoto T, Kienlen-Campard P, Constantinescu SN, Octave JN, Aimoto S, Smith SO. Proc Natl Acad Sci USA. 2009;106:1421–1426. doi: 10.1073/pnas.0812261106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) MacKenzie RA, Prestegard JH, Engelman DM. Science. 1997;276:131–133. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]; (b) Senes A, Ubarretxena-Belandia I, Engelman DM. Proc Natl Acad Sci USA. 2001;98:9056–9061. doi: 10.1073/pnas.161280798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.