Abstract
Bone morphogenetic protein (BMP) signals coordinate developmental patterning and have essential physiological roles in mature organisms. Here we describe the first known small-molecule inhibitor of BMP signaling—dorsomorphin, which we identified in a screen for compounds that perturb dorsoventral axis formation in zebrafish. We found that dorsomorphin selectively inhibits the BMP type I receptors ALK2, ALK3 and ALK6 and thus blocks BMP-mediated SMAD1/5/8 phosphorylation, target gene transcription and osteogenic differentiation. Using dorsomorphin, we examined the role of BMP signaling in iron homeostasis. In vitro, dorsomorphin inhibited BMP-, hemojuvelin- and interleukin 6–stimulated expression of the systemic iron regulator hepcidin, which suggests that BMP receptors regulate hepcidin induction by all of these stimuli. In vivo, systemic challenge with iron rapidly induced SMAD1/5/8 phosphorylation and hepcidin expression in the liver, whereas treatment with dorsomorphin blocked SMAD1/5/8 phosphorylation, normalized hepcidin expression and increased serum iron levels. These findings suggest an essential physiological role for hepatic BMP signaling in iron-hepcidin homeostasis.
Signals mediated by BMP ligands serve diverse roles throughout the life of vertebrates. During embryogenesis, the dorsoventral axis is established by BMP signaling gradients formed by the coordinated expression of ligands, receptors, co-receptors and soluble antagonists1–3. Excess BMP signaling causes ventralization (an expansion of ventral structures at the expense of dorsal structures) whereas diminished BMP signaling causes dorsalization (an expansion of dorsal structures at the expense of ventral structures)1,3,4. BMPs are key regulators of gastrulation, mesoderm induction, organogenesis and endochondral bone formation, and they regulate the fates of multipotent cell populations5. BMP signals also have critical roles in physiology and disease, and they are implicated in primary pulmonary hypertension, hereditary hemorrhagic telangiectasia syndrome, fibrodysplasia ossificans progressiva and juvenile polyposis syndrome6–8.
The BMP signaling family is a diverse subset of the transforming growth factor-β (TGF-β) superfamily9. Over 20 known BMP ligands are recognized by three distinct type II receptors (BMPRII, ActRIIa and ActRIIb) and at least three type I receptors (ALK2, ALK3 and ALK6). Dimeric ligands facilitate assembly of receptor heteromers, thereby allowing the constitutively active type II receptor serine/threonine kinases to phosphorylate type I receptor serine/threonine kinases. Activated type I receptors phosphorylate BMP-responsive SMAD effectors (SMAD1, SMAD5 and SMAD8) to facilitate nuclear translocation in complex with SMAD4, a co-SMAD that also facilitates TGF signaling. In addition, BMP signals can activate intracellular effectors such as mitogen-activated protein kinase (MAPK) p38 in a SMAD-independent manner10. Soluble BMP antagonists such as noggin, chordin, gremlin and follistatin limit BMP signaling by ligand sequestration. Co-receptors such as members of the repulsive guidance molecule (RGM) family (including RGMa, DRAGON (RGMb) and hemojuvelin (HJV, RGMc)) enhance the response to low levels of BMP ligands in the target tissues in which they are expressed11,12.
Recent work suggests a role for BMP signals in regulating expression of hepcidin, a peptide hormone and central regulator of systemic iron balance13–16. Hepcidin binds and promotes degradation of ferroportin, the sole iron exporter in vertebrates. Loss of ferroportin activity prevents mobilization of iron to the bloodstream from intracellular stores in enterocytes, macrophages and hepatocytes17. Hepatic hepcidin expression is responsive to states of iron deficiency or overload, inflammation, and hypoxia, but the mechanisms by which the liver senses these stimuli and regulates hepcidin expression remain unclear, especially because the promoter for the HAMP gene (the gene that encodes hepcidin) does not seem to have elements known to be modulated by iron levels. Mice conditionally deficient in SMAD4 fail to induce hepcidin following iron or interleukin 6 (IL-6) challenge18, which suggests that BMP and/or TGF-β pathways transduce signals mediated by iron or inflammation. As further evidence of a link between BMP signaling and iron metabolism, mutations in Hfe2 (which encodes the BMP co-receptor HJV) have been identified in juvenile hemochromatosis, a disease in which iron overload fails to trigger hepcidin expression7.
Pharmacological tools that selectively inhibit BMP signaling, but not TGF-β or other pathways, could help elucidate the roles of BMP signaling in diverse physiological processes, including iron homeostasis. Specific inhibitors of the BMP receptors have not yet been identified, perhaps because of the difficulty in targeting a set of BMP receptor kinases while avoiding hundreds of structurally similar kinases. Traditional high-throughput in vitro screens identify small molecules with activity against a kinase of interest, but both extensive counterscreening against other kinases and animal testing are required to confirm specificity. To circumvent these challenges, we sought an in vivo screening approach that would identify compounds that inhibit BMP signaling while selecting against those with nonspecific or undesirable biological effects. By screening a diverse chemical library for small molecules that dorsalize zebrafish embryos, we identified a compound that blocks BMP signaling by inhibiting type I receptors ALK2, ALK3 and ALK6. Although previously described as an inhibitor of AMP-activated protein kinase (compound C), we found that the compound elicits phenotypes reflecting a high degree of selectivity for BMP signaling in vivo. Using this selective BMP inhibitor, we report that BMP signaling is essential for iron homeostasis and that pharmacological BMP inhibition can be used to increase serum iron levels in vivo.
RESULTS
Dorsomorphin induces dorsalization in zebrafish embryos
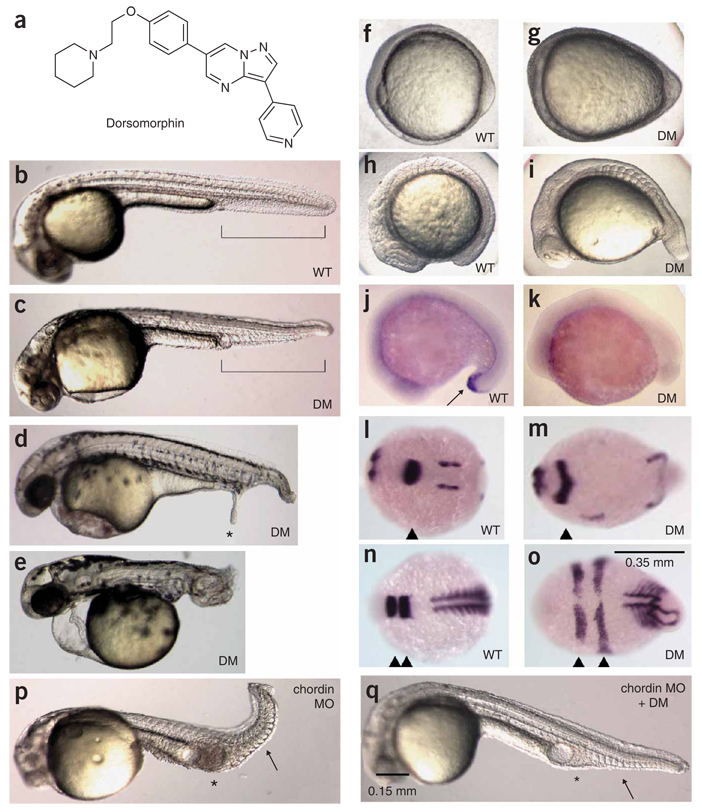
Small-molecule inhibitors of BMP signaling were sought using an in vivo screening assay based on the premise that BMP antagonists would dorsalize developing zebrafish embryos. Over 7,500 compounds were tested from our small-molecule collection, which consists of known bioactive molecules, US Food and Drug Administration–approved drugs and a commercial molecular diversity library. Zebrafish embryos were arrayed into 96-well plates, incubated with compounds starting 4 h post fertilization (h.p.f.) and assessed visually at 24 and 48 h.p.f. One compound, which we call dorsomorphin (1, Fig. 1a), produces substantial and reproducible dorsalization of zebrafish embryos. Dorsomorphin-treated embryos show expansion of structures derived from the dorsal pole of spherical embryos at the expense of structures derived from the ventral pole1,3,4 (Fig. 1b–e).
Figure 1.
Dorsomorphin induces dorsalization in zebrafish embryos. (a) Structure of dorsomorphin. (b) Vehicle-treated WT zebrafish embryo 36 h.p.f. Ventral tail fin is highlighted in brackets. (c) Zebrafish embryo treated with 10 µM dorsomorphin (DM) at 6–8 h.p.f. and photographed at 36 h.p.f. (d) Zebrafish embryos treated with 10 µM dorsomorphin at 6 h.p.f. occasionally develop ectopic tails (*) at 48 h.p.f. (e) Embryos treated with 10 µM dorsomorphin at 1–2 h.p.f. show severe dorsalization at 48 h.p.f. Embryos b–e are shown on lateral view. (f,g) Bud stage embryos left untreated (f) or treated at 2 h.p.f. with dorsomorphin (g) are shown on ventral view. (h,i) 10-somite-stage embryos left untreated (h) or treated at 2 h.p.f. with dorsomorphin (i) are shown on lateral view. (j,k) In situ hybridization of 18-somite stage WT embryos (lateral view) reveals typical expression of the ventral marker eve1 (arrow) in the distal tail (j), whereas embryos treated with dorsomorphin (10 µM) at 1 h.p.f. do not (k). (l–o) In situ hybridization of 6-somite-stage (12 h.p.f.) embryos (dorsal view, anterior left). The mid-hindbrain boundary is marked by pax2a (l and m, arrowheads). Rhombomeres 3 and 5 are marked by egr2b (krox20) (n and o, arrowheads), and somitic mesoderm is marked by myod (n and o). (p,q) Embryos injected with 1 ng chordin morpholino were treated with vehicle (p) or 20 µM dorsomorphin at 2 h.p.f. (q) and photographed 36 h.p.f. Dorsomorphin treatment normalizes expanded intermediate cell mass (*) and ventral tail fin structures (arrow).
The extent of dorsalization induced by dorsomorphin varied as a function of dose and timing. When added at 6–8 h.p.f., dorsomorphin caused mild dorsalization manifested as the absence of the ventral tail fin (Fig. 1b,c), similar to that of noggin-overexpressing and lost-a-fin (ALK8 mutant) zebrafish2,3. Zebrafish treated with dorsomorphin at 4 h.p.f. occasionally had an ectopic tail appendage in addition to the absent ventral fin (Fig. 1d), which resembled transgenic zebrafish expressing a heat-shock-inducible dominant negative BMP type I receptor after the shield stage19. When dorsomorphin was added to embryos at 1–2 h.p.f., caudal and posterior structures of the tail derived from the embryonic ventral pole were more profoundly disrupted (Fig. 1e), which resembled fish with more severe BMP signaling defects2. The addition of compound at 2 h.p.f. led to altered gastrulation and somitogenesis similar to that observed in lost-a-fin fish2, with ovoid dorsalized morphology at the bud stage (Fig. 1f,g) and abnormal tailbud morphology at the 10-somite stage (Fig. 1h,i). Treatment of embryos with ≥5 µM dorsomorphin at ≤8 h.p.f. phenocopied with high penetrance the spectrum of dorsalization defects observed in zebrafish with defective BMP signaling (Supplementary Fig. 1 and Supplementary Table 1 online) but did not result in cyclopia, a phenotype associated with defective TGF-β signaling20.
Dorsomorphin is structurally identical to compound C, a molecule previously shown to antagonize AMP-activated kinase (AMPK) activity in vitro with an estimated functional Ki of 10 to 20 µM (ref. 21). To test whether AMPK inhibition contributes to the effects of dorsomorphin on dorsoventral axis formation, we tested structurally unrelated inhibitors of AMPK activity such as C75 (3) and Ara-A (2)22,23. These inhibitors did not dorsalize zebrafish embryos but instead caused nonspecific toxicity such as developmental delay and death that was not observed with dorsalizing concentrations of dorsomorphin (Supplementary Table 2 online). Moreover, knock-down of AMPK activity in zebrafish by morpholino injection did not cause dorsalization (Supplementary Fig. 2 online). Dorsomorphin is structurally related to a series of compounds that has been shown to inhibit the activity of the receptor tyrosine kinase KDR (ref. 24). At the doses of dorsomorphin used in our experiments, however, we did not observe any of the known effects of KDR inhibition on zebrafish vascular development, such as disrupted formation of intersomitic blood vessels25 (Fig. 1c–e). Therefore, dorsomorphin-induced dorsalization is not caused by AMPK or KDR inhibition.
Dorsomorphin expands dorsal marker expression
To confirm the impact of dorsomorphin on dorsoventral axis formation, the expression of dorsal and ventral markers were examined. Expression of the ventral marker eve1 at the 18-somite stage was reduced in dorsomorphin-treated embryos relative to controls (Fig. 1j,k), which is consistent with dorsalization2. At the 6-somite stage, pax2a (pax2.1) expression demarcates the optic stalk, mid-hindbrain boundary and otic vesicles26. Dorsomorphin treatment induced expansion of these dorsal tissues (Fig. 1l,m). Similarly, dorsomorphin induced lateral expansion of egr2b (krox20), a marker of rhombomeres 3 and 5, and myod, a marker of posterior myotomes (Fig. 1n,o). Thus, dorsomorphin induced changes in the expression of dorsal and ventral markers consistent with the effects of dorsalization seen in zebrafish BMP pathway mutants27.
Dorsomorphin reverses ventralization in chordin morphants
Because treatment with dorsomorphin mimics BMP antagonism in zebrafish, it was postulated that dorsomorphin might rescue ventralization caused by loss of the endogenous BMP antagonist chordin28,29. Zebrafish were injected with chordin morpholino (1 ng) at the 1-cell stage, which caused moderate ventralization with expansion of the ventral fin and the intermediate cell mass (ICM) (Fig. 1p)29. Dorsomorphin treatment of chordin morphants at 2 h.p.f. reduced the size of the ventral fin and ICM (Fig. 1q). Therefore, dorsomorphin treatment compensates for the loss of an endogenous BMP antagonist in zebrafish embryos, thus confirming its function as a BMP antagonist.
Dorsomorphin inhibits SMAD-dependent BMP signals
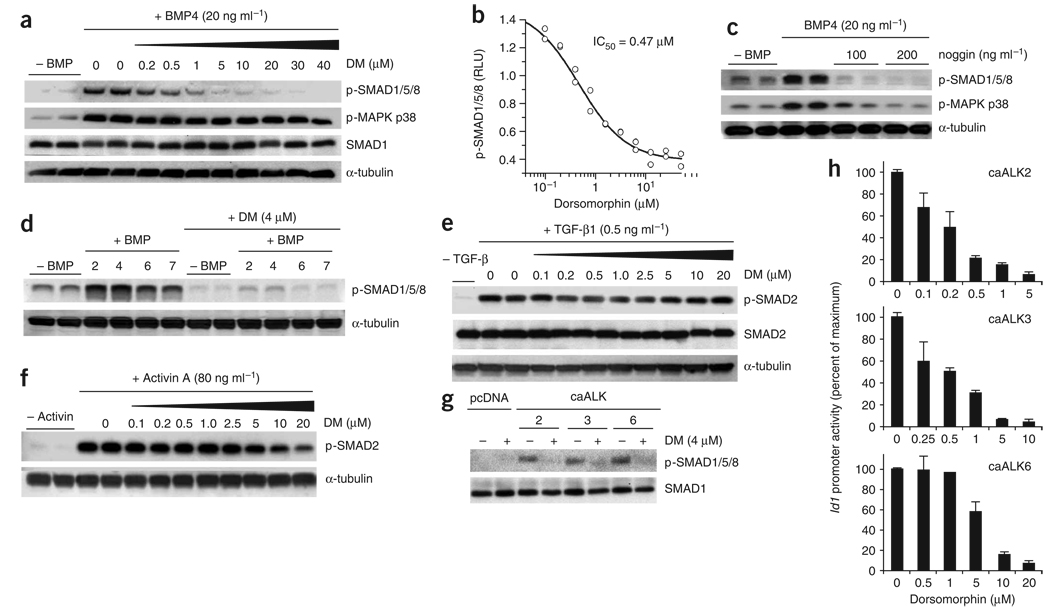
The effect of dorsomorphin on BMP signaling was tested in vitro. Cultured mouse pulmonary artery smooth muscle cells (PASMCs) express a complement of BMP receptors. In response to diverse BMP ligands, PASMCs show SMAD1/5/8 activation, MAPK p38 activation, and transcription of inhibitor of differentiation (Id) genes, a group of dominant negative–acting basic helix-loop-helix transcription factors that generally act to promote proliferation and inhibit differentiation30,31. Dorsomorphin inhibited BMP4-induced phosphorylation of BMP-responsive SMADs in a dose-dependent manner (half maximal inhibitory concentration (IC50) = 0.47 µM), without affecting MAPK p38 activation (Fig. 2a,b). The unique ability of dorsomorphin to functionally separate SMAD1/5/8 and MAPK p38 activation suggests that BMPs activate these effectors by distinct mechanisms. In contrast to dorsomorphin, noggin inhibited BMP4-induced phosphorylation of both SMAD1/5/8 and MAPK p38 (Fig. 2c), which is consistent with inhibition of SMAD-dependent and SMAD-independent signaling by sequestration of BMP ligands. Dorsomorphin (4 µM) inhibited the activity of structurally diverse BMPs, blocking BMP-responsive SMAD activation by BMP2, BMP4, BMP6 and BMP7 (Fig. 2d) and reducing phosphorylated SMAD levels in untreated cells to below basal levels. Dorsomorphin did not inhibit SMAD2 activation by TGF-β1 at concentrations equal to or greater than those that were inhibitory to BMP signaling, and it modestly inhibited SMAD2 activation by activin A only at concentrations ≥10 µM (Fig. 2e,f). These results are depicted quantitatively in Supplementary Figure 3a–e online.
Figure 2.
Dorsomorphin inhibits BMP-mediated activation of SMAD by inhibiting BMP type I receptor function. (a) Phosphorylation of SMAD1/5/8 and MAPK p38 in PASMCs detected by immunoblot after pretreatment with dorsomorphin for 30 min followed by treatment with BMP4 for 30 min. Equivalent protein loading was confirmed by detection of total SMAD1 and α-tubulin. (b) Hill plot of the inhibition of BMP4-stimulated SMAD1/5/8 phosphorylation by incubating PASMCs with dorsomorphin. RLU, relative light units. (c) Phosphorylation of SMAD1/5/8 and MAPK p38 in PASMCs after pretreatment with noggin for 30 min followed by treatment with BMP4 for 30 min. (d) Phosphorylation of SMAD1/5/8 in PASMCs after stimulation by BMP2, BMP4, BMP6 and BMP7 (10 ng ml−1) detected by immunoblot with and without pretreatment with dorsomorphin (4 µM) for 30 min. (e) SMAD2 phosphorylation in PASMCs treated with TGF-β1. Pretreatment with dorsomorphin (0.1–20 µM) did not inhibit TGF-β1–mediated activation of SMAD2 at 30 min. (f) SMAD2 phosphorylation in PASMCs treated with activin A. Dorsomorphin inhibited activin A–mediated activation of SMAD2 only modestly at concentrations ≥10 µM. (g) Levels of phosphorylated SMAD1/5/8 in cell extracts from BMPR-II–deficient PASMCs transiently transfected with constitutively active type I receptor cDNA (caALK2, caALK3 or caALK6), with or without coincubation with dorsomorphin (10 µM), as detected by immunoblot. pcDNA indicates plasmid-only control. (h) Transient transfection of BMPR-II–deficient PASMCs with caALK2, caALK3 or caALK6 resulted in 5- to 12-fold increases in Id1 promoter activity (BRE-Luc). Id1 promoter activity mediated by each of the constitutively active type I receptors was decreased by cotreatment with dorsomorphin in a dose-dependent manner (n = 3, results expressed as mean ± s.d.).
Dorsomorphin inhibits BMP type I receptor function
To test whether or not dorsomorphin inhibits the function of BMP type II receptors, we tested dorsomorphin in PASMCs lacking the BMP type II receptor (BMPR-II). BMPR-II–deficient cells use the activin type IIa receptor (ActR-IIa) instead of BMPR-II to activate the type I BMP receptors30. Dorsomorphin inhibited signaling by BMP2, BMP4, BMP6 and BMP7 in BMPR-II–deficient cells (Supplementary Fig. 3f), which demonstrates that dorsomorphin inhibits BMP signals in the presence or absence of BMPR-II. This finding suggested dorsomorphin might inhibit signaling of BMP type I receptors downstream of type II receptors. To test this hypothesis, cells were transfected with constitutively active (ca) forms of the BMP type I receptors ALK2, ALK3 and ALK6. Transfection with these constitutively active receptors increased the phosphorylation of SMAD1/5/8 in cell extracts—an effect that was inhibited by preincubation with dorsomorphin (Fig. 2g). Similarly, transfection with caALK2, caALK3 or caALK6 increased Id1 promoter (BRE-Luc) activity by 5- to 12-fold in the absence of ligand. Dorsomorphin inhibited the activity of caALK2, caALK3 and caALK6, with apparent selectivity for caALK2 and caALK3 over caALK6 (Fig. 2h). At similar concentrations, dorsomorphin did not inhibit the function of constitutively active ALK4, ALK5 or ALK7, the TGF-β and activin type I receptors (Supplementary Fig. 4 online). The ability of dorsomorphin to (i) block BMP-mediated SMAD1/5/8 phosphorylation and (ii) inhibit the ability of activated BMP type I receptors to phosphorylate SMAD1/5/8 and induce downstream transcription argues strongly that dorsomorphin inhibits BMP type I receptor kinase function.
Dorsomorphin inhibits osteoblast differentiation
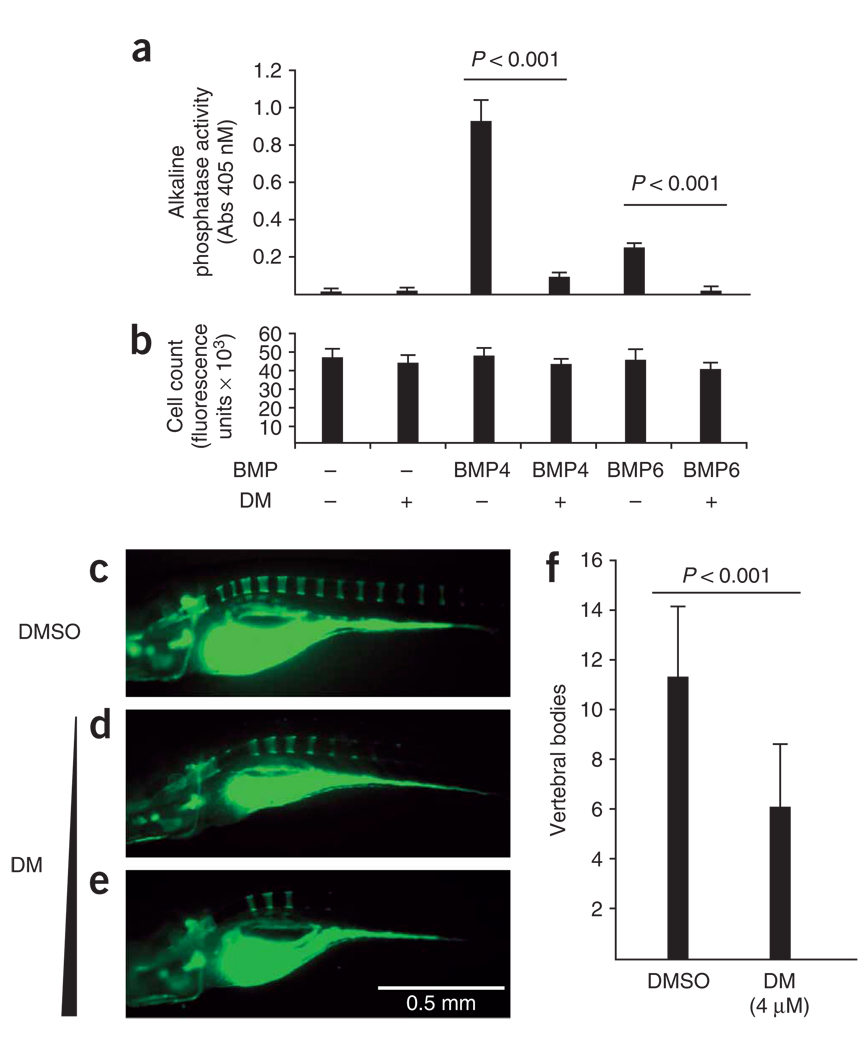
The ability of dorsomorphin to block BMP-mediated signaling and transcriptional activity suggested that dorsomorphin might block BMP-induced osteoblast differentiation. Alkaline phosphatase activity (a marker of osteogenic differentiation) in myofibroblast C2C12 cells was efficiently induced by BMP4 and was induced to a lesser extent by BMP6 after 5 d of culture (Fig. 3a). This activity was almost completely abolished by the addition of dorsomorphin at 4 µM, a concentration at which no significant effects on cell count (Fig. 3b) were observed. These findings suggest that, consistent with inhibition of BMP function, dorsomorphin inhibits BMP-induced osteogenic differentiation in multipotent mesenchyme-derived cells without cytotoxicity.
Figure 3.
Dorsomorphin inhibits osteogenic differentiation in vitro and bone mineralization in vivo. (a) Alkaline phosphatase activity as a marker of osteoblastic differentiation in C2C12 cells after treatment with BMP4 or BMP6 during 5 d of culture. Pretreatment with dorsomorphin (4 µM) inhibited BMP-mediated induction of alkaline phosphatase activity (n = 6 for each condition, results expressed as mean ± s.d.). (b) Treatment of C2C12 cells with dorsomorphin did not affect cell count as assayed using DNA-binding dye (CyQuant). (c–e) Visualization of calcified skeletal structures in zebrafish by calcein fluorescence staining at 10 d.p.f., left lateral view. DMSO-treated fish showed normal vertebral staining of 11–14 segments (c). Treatment of zebrafish at 24 h.p.f. with dorsomorphin (1–4 µM) resulted in viable fish at 10 d.p.f. without evidence of dorsalization. However, a decrease in vertebral segment and craniofacial bone calcification was observed with dorsomorphin-treated fish relative to vehicle-treated fish (d and e). (f) With dorsomorphin treatment at 4 µM, a 45% decrease (P < 0.001) in the number of mineralized vertebrae was observed (n = 19 for DMSO; n = 18 for dorsomorphin treated; results expressed as mean ± s.d.).
To test whether or not the effects of dorsomorphin on osteogenic differentiation in vitro could be extended to osteogenesis in vivo, we analyzed bone mineralization in zebrafish embryos. To circumvent dorsalization during early embryogenesis, zebrafish were exposed to dorsomorphin beginning 24 h.p.f.—that is, after establishment of the dorsoventral axis. 10 d post fertilization, calcein staining was performed to assess bone mineralization. Treatment with dorsomorphin resulted in inhibition of bone mineralization, with fewer calcein-stained vertebral segments relative to control larvae (Fig. 3c–f). Despite altered bone mineralization, fish treated with dorsomorphin otherwise appeared morphologically and functionally normal by brightfield microscopy (Supplementary Fig. 5 online).
Dorsomorphin inhibits hepcidin transcription
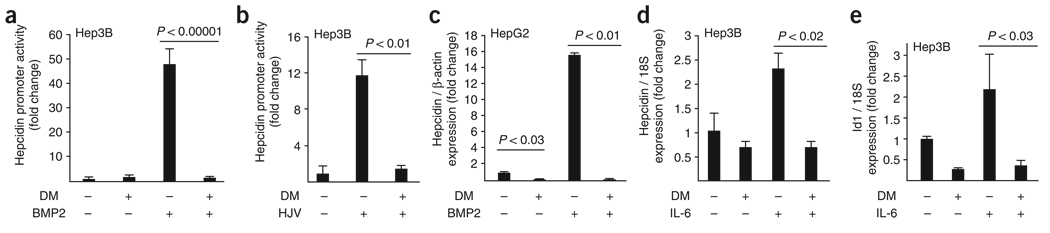
BMPs, HJV and IL-6 induce hepcidin expression in cultured hepatocytes11,32–34 and cultured hepatoma cells32,35. HJV functions as a BMP co-receptor, but it is unclear whether or not IL-6–induced hepcidin expression requires BMP signaling18. Treatment with BMP2 (Fig. 4a) or transfection with complementary DNA encoding HJV (Fig. 4b) induced transcriptional activity of the hepcidin promoter in hepatoma-derived Hep3B cells. BMP2- and HJV-induced hepcidin promoter activities were both effectively abrogated by the addition of dorsomorphin. HepG2 cells show higher basal hepcidin expression than Hep3B cells11. Treatment with dorsomorphin reduced basal hepcidin expression by over 50-fold in HepG2 cells and abolished the induction of hepcidin expression by BMP2 (Fig. 4c). In contrast to dorsomorphin, treatment of Hep3B cells with Ara-A at concentrations sufficient to inhibit AMPK (ref. 36) did not impair BMP-induced hepcidin mRNA expression, making it unlikely that dorsomorphin inhibited hepcidin expression via effects on AMPK (Supplementary Fig. 6 online). Treatment with IL-6 induced hepcidin and Id1 expression in Hep3B cells, whereas the addition of dorsomorphin blocked IL-6–mediated induction of both hepcidin and Id1 (Fig. 4d,e). These findings suggest that BMP-, HJV- and IL-6–mediated hepcidin expression are all regulated by signaling via BMP type I receptors.
Figure 4.
Dorsomorphin inhibits BMP- and HJV-induced hepcidin expression in cultured hepatoma-derived cells. (a) Treatment of Hep3B cells with BMP2 (25 ng ml−1) increased hepcidin promoter activity nearly 50-fold compared with untreated cells, as measured by the hepcidin luciferase reporter assay (Hep-Luc). Cotreatment with dorsomorphin (10 µM) abrogated the induction of hepcidin promoter activity by BMP2 (triplicate measurements, results expressed as mean ± s.d.). (b) Transfection of Hep3B cells with HJV increased hepcidin promoter activity 12-fold. Treatment with dorsomorphin blocked the HJV-mediated increase in hepcidin promoter activity (triplicate measurements, results expressed as mean ± s.d.). (c) Levels of hepcidin mRNA were measured in HepG2 cells by qRT-PCR. Treatment with dorsomorphin (10 µM) reduced basal hepcidin expression by 50-fold. Treatment with BMP2 increased hepcidin by 16-fold over the basal level. Treatment with dorsomorphin (10 µM) in combination with BMP2 reduced the levels of hepcidin mRNA to below the basal level (triplicate measurements, results are expressed as mean ± s.d.) (d–e) In Hep3B cells, levels of hepcidin (d) and Id1 (e) mRNA measured by qRT-PCR were increased in response to IL-6 (100 ng ml−1) treatment for 6 h. Treatment with dorsomorphin (4 µM) alone or in combination with IL-6 reduced the levels of hepcidin and Id1 mRNA to below basal levels, with no effect on expression of the control gene (18S rRNA) (triplicate measurements, results are expressed as mean ± s.d.).
Dorsomorphin inhibits iron-mediated SMAD activation
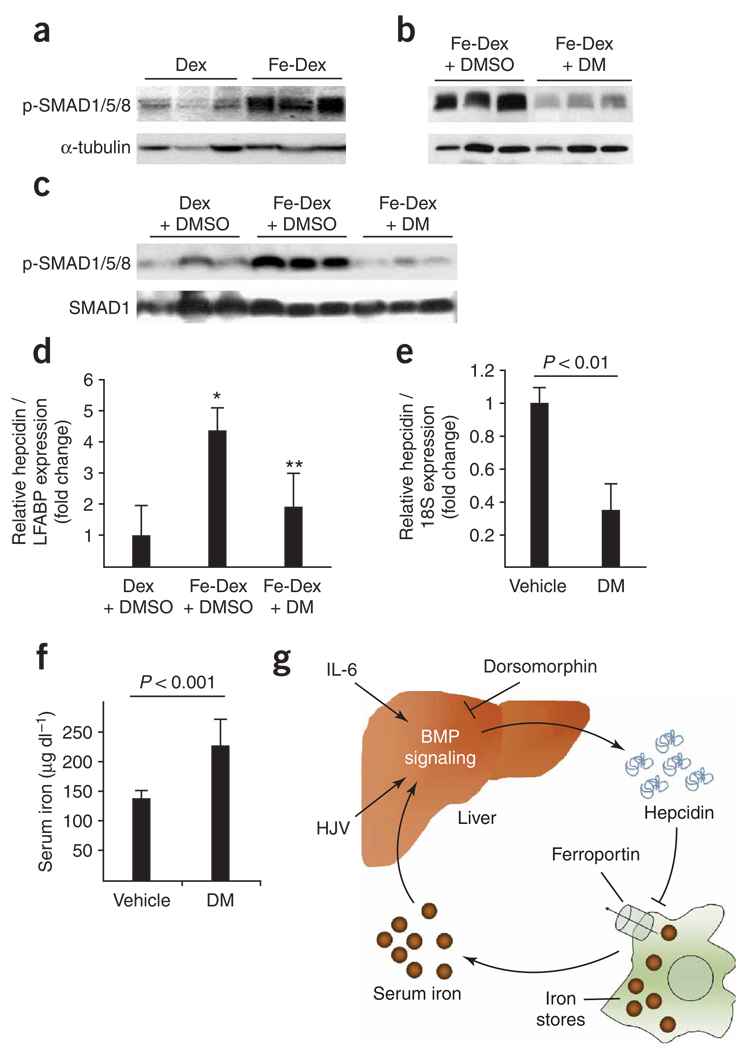
In vivo, iron induces the hepatic expression of hepcidin to adaptively curtail excess ferroportin-mediated iron transport14,17, but it is unclear whether or not BMP signaling is involved in this feedback process. To ascertain the potential role of BMP signaling in iron feedback regulation of hepcidin, the effect of systemic iron challenge on hepatic SMAD1/5/8 activation was examined. Intraperitoneal injection of iron-dextran in adult zebrafish led to a nearly three-fold increase in SMAD1/5/8 phosphorylation in liver extracts within 1 h, relative to dextran-injected controls (Fig. 5a). When zebrafish were coinjected with iron-dextran and dorsomorphin, SMAD1/5/8 phosphorylation decreased by nearly three-fold relative to fish injected with iron-dextran and vehicle (Fig. 5b). Similarly in mice, intravenous injection of iron-dextran and vehicle led to a more than three-fold increase in SMAD1/5/8 phosphorylation in liver extracts after 1 h, relative to mice injected with dextran and vehicle (Fig. 5c). Coinjection of dorsomorphin in mice abolished the iron-mediated increase in hepatic SMAD1/5/8 phosphorylation. These observations in two species demonstrate that hepatic SMAD1/5/8 activation is rapidly induced by iron and is effectively inhibited by dorsomorphin.
Figure 5.
Dorsomorphin inhibits iron-mediated BMP-responsive SMAD activation and expression of hepcidin. (a) SMAD1/5/8 phosphorylation (normalized to α-tubulin levels) in adult zebrafish liver extracts 1 h after intraperitoneal injection of dextran (Dex) or iron-dextran (Fe-Dex) (n = 3 for each group, P < 0.001, t-test). (b) SMAD1/5/8 phosphorylation in zebrafish livers injected with iron-dextran and vehicle (Fe-Dex + DMSO) or coinjected with iron-dextran and dorsomorphin at 23 µg g−1 (Fe-Dex + DM) (n = 3 for each group, P < 0.03, t-test). (c) SMAD1/5/8 phosphorylation in liver extracts from mice 1 h after intravenous injection with dextran and vehicle (Dex + DMSO), iron-dextran and vehicle (Fe-Dex + DMSO) or iron-dextran and dorsomorphin (Fe-Dex + DM) (P = 0.01, Fe-Dex versus Dex; P < 0.0003, Fe-Dex + DM versus Fe-Dex; t-test). (d) Hepatic hepcidin mRNA levels (normalized to liver fatty acid binding protein mRNA levels) from zebrafish injected intraperitoneally with dextran and vehicle (Dex + DMSO), iron-dextran and vehicle (Fe-Dex + DMSO), or iron-dextran with 23 µg g−1 dorsomorphin (Fe-Dex + DM, n = 4 for each group, *P < 0.01, **P < 0.02, ANOVA). (e) Hepatic hepcidin mRNA levels in C57BL/6 mice 6 h after a single tail vein injection of vehicle or dorsomorphin (10 mg kg−1) (n = 6 for each group, P < 0.01, t-test). (f) Serum iron levels in mice 24 h after the first of two intraperitoneal injections of dorsomorphin (10 mg kg−1) 12 h apart (n = 8 WT, n = 7 dorsomorphin, P < 0.001, t-test). Results in d–f are expressed as mean ± s.d. Panels are representative of two (c–e) or three (a,b,f) independent experiments each. (g) Proposed model for the role of BMP signaling in iron homeostasis. See discussion for details.
Dorsomorphin inhibits iron-induced hepcidin transcription
Although systemic iron challenge is known to induce hepcidin expression in vivo, the responsible mechanisms are incompletely understood given that the hepcidin promoter lacks recognizable transcriptional elements that are known to respond to changes in iron levels. The observation that iron challenge causes hepatic SMAD1/5/8 activation led to the hypothesis that BMP signaling mediates hepcidin expression in response to iron. Adult zebrafish normally express relatively low levels of hepcidin mRNA. After 3 h, fish treated with iron-dextran and vehicle showed a four-fold increase in hepatic hepcidin mRNA levels relative to fish injected with dextran and vehicle (Fig. 5d). Cotreatment of fish with dorsomorphin inhibited the increase in hepcidin mRNA levels seen with iron-dextran injection, which suggests that iron induces hepcidin expression via a BMP-dependent mechanism.
Dorsomorphin induces hyperferremia in mice
Wild-type (WT) C57BL/6 adult mice that are fed a standard iron-replete diet express high levels of hepcidin15, which is presumably induced by abundant provision of iron. The ability of dorsomorphin to block iron-induced hepcidin expression in zebrafish led to the hypothesis that dorsomorphin could inhibit basal hepcidin expression and increase serum iron levels in iron-replete mice. 6 h after dorsomorphin was administered intravenously, hepatic hepcidin mRNA levels were reduced to one-third of that of vehicle-injected mice (P < 0.01) (Fig. 5e). Alterations in hepcidin levels affect serum iron concentrations within 24 h via the altered mobilization of intracellular iron by ferroportin33. Administration of dorsomorphin over 24 h led to a 60% increase in total serum iron concentrations (Fig. 5f). Dorsomorphin treatment is therefore effective in reducing basal levels of hepcidin expression and increasing serum iron concentrations in adult mice.
DISCUSSION
Using the new approach of screening for stereotyped deviations in embryonic dorsoventral patterning, we have identified a molecule that selectively inhibits BMP signaling. This small-molecule inhibitor recapitulates with high specificity and low toxicity the phenotypic spectrum of embryos with genetic defects in the BMP signaling pathway. The functional specificity of dorsomorphin is demonstrated in vivo by its ability to compensate for the loss of the BMP antagonist chordin during dorsoventral axis formation and by its ability later in development to inhibit axial skeleton mineralization in the absence of other observable defects. Functional selectivity for BMP signaling is further supported by in vitro data demonstrating that dorsomorphin preferentially inhibits BMP signaling over TGF-β or activin signaling.
Despite the apparent selectivity of dorsomorphin for BMP signaling, it is important to consider the possibility that dorsomorphin, like all known kinase inhibitors, may affect other kinase targets, particularly at higher doses. Although dorsomorphin is structurally identical to a molecule previously shown to antagonize AMPK activity21, AMPK inhibition does not seem to be responsible for the effects of dorsomorphin on dorsoventral axis formation or hepcidin regulation, because neither pharmacological nor morpholino-mediated inactivation of AMPK reproduces these effects. Nevertheless, when using dorsomorphin, the possibility of confounding effects from secondary targets needs to be considered, and the lowest effective concentrations should be used to maximize selectivity for BMP signaling.
Previously, in vivo inhibition of BMP signaling in experimental therapy has been accomplished by administration of soluble receptor extracellular domains or ligand-specific neutralizing antibodies37, or by gene transfer of endogenous antagonists such as noggin or follistatin38,39. Because all of these strategies for inhibiting BMP signaling function by sequestering BMP ligands, they do not offer the possibility of differentiating between SMAD-dependent and SMAD-independent BMP signaling mechanisms. Dorsomorphin inhibits SMAD activation without compromising the ability of BMPs to activate MAPK p38 in PASMCs, which suggests that this function may be independent of SMAD activation. It is possible that adaptor proteins that transduce MAPK signals from BMP receptors40 do not require type I receptor kinase function. Dorsomorphin might help to elucidate the mechanisms by which SMAD-independent BMP signals are transduced, as well as the functional significance of such signals.
A small-molecule inhibitor provides great advantages in that it permits dissecting the function of BMP signals with exquisite temporal control while bypassing early embryonic lethality. Dorsomorphin inhibits osteogenic differentiation of myofibroblasts in vitro and alters bone mineralization in zebrafish, which is consistent with inhibition of BMP signals critical for osteoblast commitment. In a similar fashion, time- and dose-controlled use of dorsomorphin may allow characterization of BMP functions throughout early and late development and in formation of multiple organ systems including the heart, kidneys and vasculature. Because BMPs also regulate regeneration of mature tissues and stem cell differentiation41,42, modulating BMP signaling might also prove useful for the manipulation of progenitor cells.
Beyond development and cell fate determination, a potential role for BMP signaling in iron metabolism has recently emerged. The identification of Hfe2 gene mutations in juvenile hemochromatosis, which is characterized by low hepcidin levels and excessive iron accumulation, and the recognition that Hfe2 encodes the BMP co-receptor HJV, both suggest that BMPs have a role in iron regulation7,11. The observation that BMPs and HJV induce hepatocytes to express hepcidin, which in turn inactivates ferroportin, provides a mechanism by which BMP signals may modify systemic iron metabolism. Recently it was reported that holotransferrin inhibits shedding of HJV from hepatoma cells, which reveals a potential mechanism by which iron might modulate BMP signaling43. Here we provide the first experimental evidence that systemic iron administration rapidly activates BMP-responsive SMADs in the liver, and that iron-mediated hepcidin expression requires BMP signaling. The present data suggest that IL-6–induced hepcidin expression also requires intact BMP signaling in hepatocytes35. The functional importance of BMP signaling in iron regulation is supported by the ability of dorsomorphin to inhibit hepcidin expression and raise serum iron levels in vivo. Taken together, these findings demonstrate an essential role of BMP signaling in iron- and inflammation-mediated hepcidin expression (Fig. 5g).
Whereas impaired hepcidin expression leads to iron overload, excessive hepcidin expression leads to hypoferremia and anemia. Anemia of chronic disease, a condition affecting almost half of all chronically ill people, is thought to be caused by maladaptive overexpression of hepcidin induced by chronic inflammation33,34,44. Dorsomorphin’s ability to block hepatic BMP-responsive SMAD activation, inhibit hepcidin expression and increase serum iron concentrations strongly suggests that BMP type I receptor inhibition may be an effective therapeutic approach for reversing states of elevated hepcidin levels of diverse etiologies. Therefore, in addition to being a useful tool for fundamental studies of BMP signaling, dorsomorphin may lead to new therapies for treating anemia of chronic disease.
Until now, traditional methods for identifying small-molecule inhibitors have not yielded a specific BMP pathway inhibitor, perhaps because of the homology between BMP receptor kinases and other kinases. Kinase inhibitor discovery has been an area of intense interest, in part because of the therapeutic potential of kinase inhibitors in cancer chemotherapy. Despite this interest, identifying specific kinase inhibitors remains arduous, requiring coordinated selection for activity toward the target and selection against activity toward related kinases that might interfere with the desired effect. In this report, a rapid in vivo screening approach facilitated the identification of an inhibitor of the target pathway while excluding many nonspecific compounds with confounding toxicity. We screened for small molecules that could phenocopy genetic mutations in the BMP pathway without phenocopying genetic mutations in the TGF-β pathway. The selectivity of the resulting molecule for BMP over TGF-β signaling supports the notion that organism-based screening using well-defined phenotypes can identify previously elusive inhibitors of important target pathways.
METHODS
Screening for small molecules that perturb dorsoventral axis in zebrafish embryos
All zebrafish experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. Pairs of WT zebrafish were mated, and newly fertilized eggs were arrayed in triplicate in 96-well plates containing E3 buffer (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4). At 4 h.p.f., compounds were added to wells at 5–10 µM. Embryos were incubated at 28.5 °C, and gross morphology of embryos was examined at 12, 24 and 48 h.p.f. for dorsalization or ventralization of embryonic axis, as previously described2. In total, 7,570 compounds were screened, including synthetic screening compounds (Chembridge Corporation, 5,580 small molecules) and known bioactive compounds (Microsource Discovery Systems, 1,840 small molecules, and Sigma-Aldrich, 150 small molecules). For follow-up studies, dorsomorphin (compound C, 6-[4-(2-piperidin-1-yl-ethoxy)phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a]pyrimidine) and C75 were purchased from EMD Biosciences, and Ara-A (adenine 9-β-d-arabinofurano-side) was purchased from Sigma.
Wholemount zebrafish in situ hybridization
In situ hybridization was performed as previously described45. Zebrafish egr2b, pax2a and myod probes were produced as previously described46.
Cell culture
WT and BMPR-II–deficient PASMCs were isolated as previously described30 and cultured in RPMI medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS). C2C12 cells (American Type Culture Collection) were cultured in DMEM (Invitrogen) supplemented with glutamine and 10% FBS. HepG2 cells and Hep3B cells (ATCC) were cultured in minimal essential alpha medium with l-glutamine (α-MEM, Invitrogen) containing 10% FBS. For studies of SMAD activation in vitro, PASMCs were incubated in 0.5% FCS RPMI for 24 h, followed by preincubation with drug compounds, noggin or vehicle for 30 min, followed by the addition of recombinant BMP2, BMP4, BMP6, BMP7, TGF-β1 or activin A (R&D Systems) for 30 min.
Immunoblot analysis of BMP and TGF-β–responsive SMAD phosphorylation
Cell extracts or tissues were mechanically homogenized in SDS-lysis buffer (62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 50 mM DTT, 0.01% bromophenol blue), separated by SDS-PAGE, immunoblotted with anti-phospho-SMAD1/5/8, anti-phospho-SMAD2 (Cell Signaling) or anti-α-tubulin (Upstate/Millipore) antibodies, and visualized using ECL Plus (GE Healthcare). Levels of immunoreactive proteins were quantitated by ImageQuant on a Storm phosphorimager (GE Healthcare).
Alkaline phosphatase activity
C2C12 cells were seeded into 96-well plates at 2,000 cells per well in DMEM supplemented with 2% FBS. Wells were treated in quadruplicate with BMP ligands and dorsomorphin or vehicle. Cells were harvested after 5 d in culture with 50 µl Tris buffered saline, 1% Triton X-100. Lysates were added to p-nitro-phenylphosphate reagent in 96-well plates (Sigma) for 1 h, and alkaline phosphatase activity expressed as absorbance at 405 nM. Cell viability and quantity were measured by Cell-titer Glo (Promega) and binding of nuclear dye CyQuant (Invitrogen), respectively, using replicate wells treated identically to those used for alkaline phosphatase measurements.
Zebrafish bone mineralization
WT zebrafish embryos were raised in E3 buffer containing phenylthiourea. At 1 day post fertilization (d.p.f.), embryos were treated with dorsomorphin (1–4 µM) or DMSO vehicle. At 5 d.p.f. and onward, larvae were fed for 1 h every other day. Following each feeding, residual food was washed out and medium was replaced with E3 containing dorsomorphin or vehicle. At 10 d.p.f., larvae were immersed in 0.2% calcein (Sigma) for 30 min. Embryos were washed repeatedly in E3 buffer for 3 h to remove unbound calcein and anesthetized with tricaine. Calcified skeletal structures were visualized by green fluorescence, and the number of vertebral bodies were counted.
BMP-responsive element and hepcidin promoter luciferase reporter assays
Mouse PASMCs grown to 50% confluence in 6-well plates were transiently transfected with 0.3 µg Id1 promoter luciferase reporter construct (BRE-Luc)47 in combination with 0.6 µg of plasmids expressing constitutively active forms of BMP type I receptors (caALK2, caALK3 or caALK6)48 using Fugene6 (Roche). To assess activin and TGF-β type I receptor function, PASMCs were transiently transfected with 0.3 µg PAI-1 promoter luciferase reporter construct (CAGA-Luc49) in combination with 0.6 µg of plasmids expressing constitutively active forms of type I receptors (caALK4, caALK5 and caALK7)50. For both reporter plasmids, 0.2 µg of pRL-TK Renilla luciferase (Promega) was used to control for transfection efficiency. PASMCs were incubated with dorsomorphin (4–10 µM) or vehicle starting 1 h after transfection. Cell extracts were harvested, and relative promoter activity was quantitated by the ratio of firefly to Renilla luciferase activity using the dual luciferase assay kit (Promega). HepG2 or Hep3B cells were transiently transfected with 2.5 µg hepcidin promoter luciferase reporter11 in combination with 0.25 µg pRL-TK to control for transfection efficiency, with or without 20 ng cDNA encoding FLAG-tagged human HJV. 2 d after transfection, HepG2 and Hep3B cells were incubated in 1% FBS α-MEM for 6 h, treated with dorsomorphin (10 µM) or vehicle for 30 min, and then incubated for 16 h in the presence or absence of BMP2 (25 ng ml−1). Hepcidin promoter activity was measured by luciferase assay as described above. See Acknowledgments for sources of luciferase reporter constructs and caALKs.
Iron-dextran injections
Adult fish were anesthetized with tricaine and injected with 10 µl of iron-dextran solution (100 mg ml−1, average dextranMW= 5,000, Sigma) into the abdominal cavity with dorsomorphin (23 µg g−1) or vehicle (DMSO). Control fish were injected with 10 µl of dextran (average MW = 5,000, Sigma). Fish were revived in water. 1 h after injection, fish were anesthetized on ice, and livers were collected into 200 µl SDS-lysis buffer and homogenized mechanically. 15 µl of protein extract was fractionated by SDS-PAGE and immunoblotted, as described above. 3 h after injection, total RNA was extracted from mechanically homogenized zebrafish livers using Trizol reagent (Invitrogen).
All mouse experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. 12-week-old C57BL/6 mice raised on a standard diet (Isopro RMH 3000, Prolab) were injected via the tail vein with 0.2 g kg−1 of dextran (average MW = 5,000, Sigma) or 0.2 g kg−1 of iron-dextran USP (DexFerrum, American Regent Laboratories). Dextran was injected with vehicle only, whereas iron-dextran was injected with either vehicle or dorsomorphin (10 mg kg−1). 1 h after injection, mice were killed and liver segments were collected in 500 µl of SDS-lysis buffer and mechanically homogenized. 20 µl of liver extracts were resolved by SDS-PAGE and immunoblotted. Total RNA was harvested using Trizol from mechanically homogenized mouse livers (6 h after injection with a single intraperitoneal dose of dorsomorphin (10 mg kg−1) or DMSO).
Quantitative hepcidin RT-PCR
Hep3B or HepG2 cells were incubated in α-MEM with 1% fetal calf serum for 6 h in the presence or absence of dorsomorphin (10 µM) with or without BMP2 (25 ng ml−1) or human IL-6 (R&D Biosystems, 100 ng ml−1), and RNA was extracted with Trizol. Quantitative RT-PCR was performed for mRNA from Id1 or hepcidin, as previously described11,30, and normalized to 18S rRNA or β-actin mRNA levels.
In mouse and zebrafish experiments, quantitative RT-PCR was performed on cDNA generated from equal quantities of RNA extracted from livers of treated animals. Triplicate or quadruplicate experiments were performed as indicated. Expression levels of mRNA from zebrafish and mouse hepcidin were measured as previously described14,18 and normalized to liver fatty acid binding protein mRNA (zebrafish) and 18S rRNA (mouse) levels, respectively.
Serum iron measurements
Whole blood was collected from mice by cardiac puncture into Microtainer serum separator tubes (BD Scientific), and serum was isolated according to the manufacturer’s instructions. Serum iron levels were measured by colorimetric assay using the Iron/UIBC kit (Thermo Fisher Scientific).
Statistical analysis
The statistical significance of compared measurements was measured using the Student’s two-tailed t-test or one-way ANOVA with Bonferroni correction. Hill plot analysis of dorsomorphin inhibition of SMAD1/5/8 phosphorylation was performed using GraFit (Erithacus Software).
ACKNOWLEDGMENTS
We are grateful to H. Beppu, C. MacRae and I. Drummond for feedback and advice and to A. Graveline for technical assistance. We thank P. ten Dijke (Leiden University Medical Center) for the BRE-Luc and the CAGA-Luc, and we thank K. Miyazono (University of Tokyo) for the caALK2, caALK3, caALK4, caALK5, caALK6 and caALK7. This work was supported by US National Institutes of Health grants HL079943 (P.B.Y.), HL081535 (C.C.H.), DK075846 (J.L.B.), DK071837 (H.Y.L.), HL074352 (K.D.B.), HL079267 (R.T.P.) and CA118498 (R.T.P.). This work was also supported by a Pulmonary Hypertension Association Mentored Clinical Scientist Award (P.B.Y.) and a grant from the GlaxoSmithKline Research & Education Foundation for Cardiovascular Disease (P.B.Y.).
Footnotes
Note: Supplementary information and chemical compound information is available on the Nature Chemical Biology website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions
References
- 1.Nguyen VH, et al. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev. Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- 2.Mullins MC, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 3.Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Dev. Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- 4.Mintzer KA, et al. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 5.Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- 6.Waite KA, Eng C. From developmental disorder to heritable cancer: it’s all in the BMP/TGF-beta family. Nat. Rev. Genet. 2003;4:763–773. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 7.Papanikolaou G, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat. Genet. 2004;36:77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 8.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat. Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 9.Sebald W, Nickel J, Zhang JL, Mueller TD. Molecular recognition in bone morphogenetic protein (BMP)/receptor interaction. Biol. Chem. 2004;385:697–710. doi: 10.1515/BC.2004.086. [DOI] [PubMed] [Google Scholar]
- 10.Nohe A, Keating E, Knaus P, Petersen NO. Signal transduction of bone morphogenetic protein receptors. Cell. Signal. 2004;16:291–299. doi: 10.1016/j.cellsig.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Babitt JL, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat. Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 12.Babitt JL, et al. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J. Biol. Chem. 2005;280:29820–29827. doi: 10.1074/jbc.M503511200. [DOI] [PubMed] [Google Scholar]
- 13.Pigeon C, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 14.Fraenkel PG, Traver D, Donovan A, Zahrieh D, Zon LI. Ferroportin1 is required for normal iron cycling in zebrafish. J. Clin. Invest. 2005;115:1532–1541. doi: 10.1172/JCI23780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolas G, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc. Natl. Acad. Sci. USA. 2002;99:4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicolas G, et al. Constitutive hepcidin expression prevents iron overload in a mouse model of hemochromatosis. Nat. Genet. 2003;34:97–101. doi: 10.1038/ng1150. [DOI] [PubMed] [Google Scholar]
- 17.Nemeth E, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 18.Wang RH, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- 20.Sampath K, et al. Induction of the zebrafish ventral brain and floorplate requires cyclops/nodal signalling. Nature. 1998;395:185–189. doi: 10.1038/26020. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim EK, et al. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J. Biol. Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- 23.Yoon MJ, et al. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 24.Fraley ME, et al. Synthesis and initial SAR studies of 3,6-disubstituted pyrazolo[1,5-a] pyrimidines: a new class of KDR kinase inhibitors. Bioorg. Med. Chem. Lett. 2002;12:2767–2770. doi: 10.1016/s0960-894x(02)00525-5. [DOI] [PubMed] [Google Scholar]
- 25.Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr. Biol. 2002;12:1405–1412. doi: 10.1016/s0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- 26.Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res. C Embryo. Today. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- 27.Little SC, Mullins MC. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development. 2004;131:5825–5835. doi: 10.1242/dev.01464. [DOI] [PubMed] [Google Scholar]
- 28.Hammerschmidt M, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- 29.Leung AY, et al. Characterization of expanded intermediate cell mass in zebrafish chordin morphant embryos. Dev. Biol. 2005;277:235–254. doi: 10.1016/j.ydbio.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Yu PB, Beppu H, Kawai N, Li E, Bloch KD. Bone morphogenetic protein (BMP) type II receptor deletion reveals BMP ligand-specific gain of signaling in pulmonary artery smooth muscle cells. J. Biol. Chem. 2005;280:24443–24450. doi: 10.1074/jbc.M502825200. [DOI] [PubMed] [Google Scholar]
- 31.Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci. STKE. 2002;2002:PE40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- 32.Truksa J, Peng H, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc. Natl. Acad. Sci. USA. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee P, Peng H, Gelbart T, Beutler E. The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta 2-microglobulindeficient hepatocytes. Proc. Natl. Acad. Sci. USA. 2004 101;:9263–9265. doi: 10.1073/pnas.0403108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babitt JL, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J. Clin. Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen J, et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev. Biol. 2006;291:227–238. doi: 10.1016/j.ydbio.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 37.Sugimoto H, et al. BMP-7 functions as a novel hormone to facilitate liver regeneration. FASEB J. 2007;21:256–264. doi: 10.1096/fj.06-6837com. [DOI] [PubMed] [Google Scholar]
- 38.Feeley BT, et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006;38:154–166. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Takabe K, et al. Adenovirus-mediated overexpression of follistatin enlarges intact liver of adult rats. Hepatology. 2003;38:1107–1115. doi: 10.1053/jhep.2003.50483. [DOI] [PubMed] [Google Scholar]
- 40.Kimura N, Matsuo R, Shibuya H, Nakashima K, Taga T. BMP2-induced apoptosis is mediated by activation of the TAK1-p38 kinase pathway that is negatively regulated by Smad6. J. Biol. Chem. 2000;275:17647–17652. doi: 10.1074/jbc.M908622199. [DOI] [PubMed] [Google Scholar]
- 41.Qi X, et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc. Natl. Acad. Sci. USA. 2004;101:6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu RH, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 43.Zhang AS, et al. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J. Biol. Chem. 2007;282:12547–12556. doi: 10.1074/jbc.M608788200. [DOI] [PubMed] [Google Scholar]
- 44.Weiss G, Goodnough LT. Anemia of chronic disease. N. Engl. J. Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 45.Westerfield M. The Zebrafish Book. Eugene, Oregon, USA: University of Oregon Press; 1993. pp. 1–385. [Google Scholar]
- 46.Weinberg ES, et al. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- 47.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 48.Fujii M, et al. Roles of bone morphogenetic protein type I receptors and Smad proteins in osteoblast and chondroblast differentiation. Mol. Biol. Cell. 1999;10:3801–3813. doi: 10.1091/mbc.10.11.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennler S, et al. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu A, et al. Identification of receptors and Smad proteins involved in activin signalling in a human epidermal keratinocyte cell line. Genes Cells. 1998;3:125–134. doi: 10.1046/j.1365-2443.1998.00174.x. [DOI] [PubMed] [Google Scholar]