Abstract
The endoplasmic reticulum is responsible for much of a cell’s protein synthesis and folding, but it also has an important role in sensing cellular stress. Recently, it has been shown that the endoplasmic reticulum mediates a specific set of intracellular signalling pathways in response to the accumulation of unfolded or misfolded proteins, and these pathways are collectively known as the unfolded-protein response. New observations suggest that the unfolded-protein response can initiate inflammation, and the coupling of these responses in specialized cells and tissues is now thought to be fundamental in the pathogenesis of inflammatory diseases. The knowledge gained from this emerging field will aid in the development of therapies for modulating cellular stress and inflammation.
Inflammation is the first response of the immune system to infection or tissue injury, leading to protection of the human body against these insults. But prolonged or chronic inflammation is detrimental and has an important role in the development of diseases such as arthritis, Alzheimer’s disease, type 1 and type 2 diabetes and cardiovascular disease1–3.
An inflammatory response begins when cells of the immune system and/or cells involved in metabolic pathways sense pathogens, irritants and cellular damage, triggering the release of inflammatory substances, including cytokines, free radicals, hormones and other small molecules. These inflammatory substances further stimulate the cells that secreted them and target specialized cells in immune and metabolic pathways, thereby altering cellular physiology to contribute to wound healing and pathogen resistance1. However, there is epidemiological, clinical and experimental evidence that cellular stress (that is, impaired biological processes within the cell) and excessive inflammation are causally linked to various metabolic conditions, such as obesity, type 1 and type 2 diabetes and atherosclerosis2,3.
Through recent intensive efforts, knowledge of the cellular and molecular mechanisms that control the inflammatory response has rapidly grown. However, crucial questions about how an inflammatory response originates have yet to be answered. For example, how does a cell interpret the presence of extracellular insults or metabolic overload and start transmitting signals that trigger an inflammatory response? Does the signalling in stress responses and inflammatory responses stem from a common mechanism or from different mechanisms that subsequently become integrated?
Recently, a set of intracellular pathways that signal the presence of cellular stress was identified. These pathways are collectively known as the unfolded-protein response (UPR), and studies of the UPR have broadened the understanding of the mechanisms by which inflammation can be initiated. Here we describe the research that has defined the molecular and cellular underpinnings of UPR-associated inflammation and then discuss how the UPR is coupled to inflammation in health and disease.
ER stress and the UPR in mammals
The endoplasmic reticulum (ER) is a membranous network of branching tubules and flattened sacs that is present in all eukaryotic cells. It extends throughout the cytoplasm of the cell and is contiguous with the nuclear envelope. The ER is mainly recognized as a protein-folding factory, responsible for the biosynthesis, folding, assembly and modification of numerous soluble proteins and membrane proteins4. About one-third of newly synthesized proteins translocate to the lumen of the ER, where they are folded into the correct three-dimensional structures before being targeted to various cellular organelles or transported to the surface of the cell. The ER also functions as a dynamic calcium store, which responds to growth factors, hormones, and stimuli that perturb cellular energy levels, nutrient availability or redox status. The ER seems to be a key site where intracellular signals mediated by these factors are sensed, integrated and transmitted, allowing the coordination of downstream responses. Physiological states that increase the demand for protein folding, or stimuli that disrupt the reactions by which proteins fold, create an imbalance between the protein-folding load and the capacity of the ER, causing unfolded or misfolded proteins to accumulate in the ER lumen — a condition referred to as ER stress. To ensure the fidelity of protein folding and to prevent such an accumulation of unfolded or misfolded proteins, eukaryotic cells have evolved the UPR, which alters a cell’s transcriptional and translational programs to cope with stressful conditions and to resolve the protein-folding defect5,6.
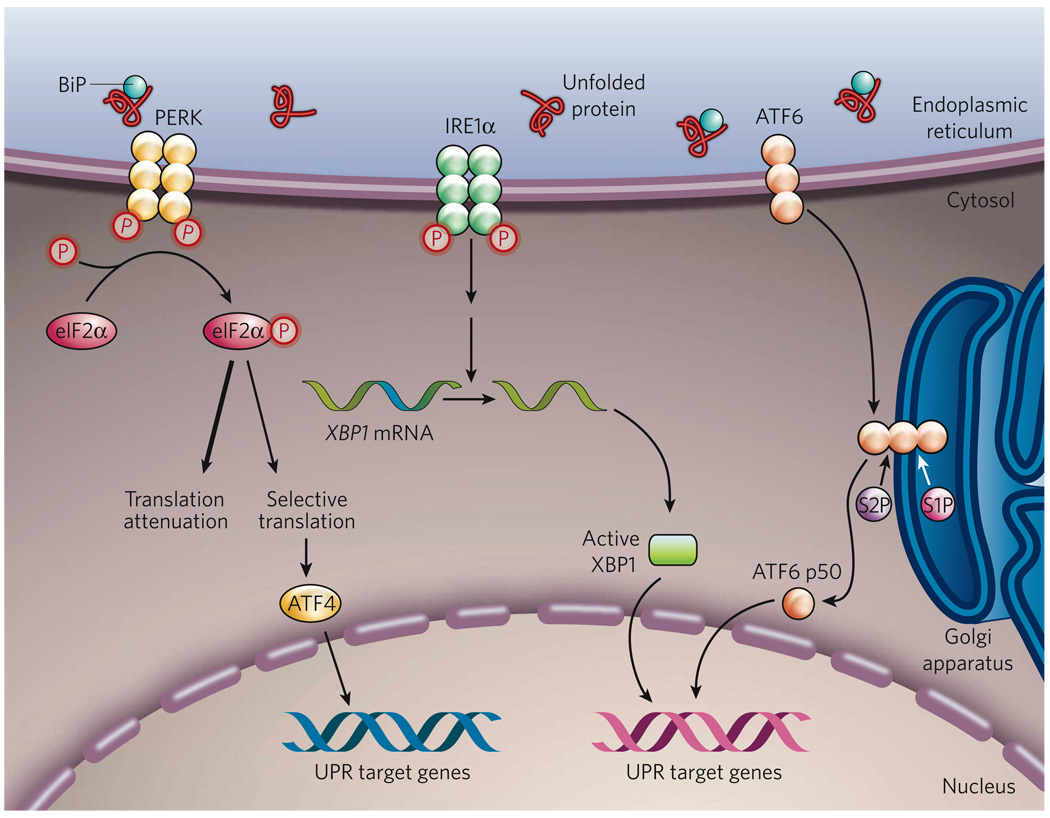
In mammalian cells, the main UPR signalling cascades are initiated by three ER-localized protein sensors: IRE1α (inositol-requiring 1α), PERK (double-stranded RNA-dependent protein kinase (PKR)-like ER kinase) and ATF6 (activating transcription factor 6)5,6. Each of these transmembrane proteins has an ER-luminal domain that senses unfolded proteins, a transmembrane domain by which it is targeted to the ER membrane, and a cytosolic domain that transmits signals to the transcriptional or translational apparatus. IRE1α has protein-kinase activity and site-specific endoribonuclease (RNase) activity (the functions of which are described later)7,8. PERK also has protein-kinase activity and functions to phosphorylate the α-subunit of eukaryotic translation-initiation factor 2α (eIF2α)9,10. ATF6 is a bZIP (basic region and leucine zipper)-domain-containing transcription factor belonging to the CREB (cyclic-AMP-responsive- element-binding protein) and ATF family of transcription factors11. In resting cells, all three ER-stress sensors are maintained in an inactive state through association with the abundant ER chaperone BiP (immunoglobulin-heavy-chain-binding protein; also known as HSPA5 and GRP78). It has been suggested that in conditions of ER stress, BiP is sequestered through binding to unfolded or misfolded polypeptide chains and/or unassembled multisubunit proteins, thereby leading to the release and, consequently, the activation of the ER-stress sensors12. Although this model of BiP sequestration by unfolded proteins is consistent with most experimental evidence, it is probably an oversimplification of the complex interactions between diverse signals that are necessary and/or sufficient to activate the UPR13 (Fig. 1).
Figure 1. The mammalian UPR pathways.
In non-stressed cells (not shown), the ER chaperone BiP binds to the luminal domains of the ER-stress sensors IRE1α, PERK and ATF6, maintaining these proteins in an inactive state. During ER stress (shown), BiP preferentially binds to unfolded or misfolded proteins, thus driving the equilibrium of BiP binding away from IRE1α, PERK and ATF6. These three proteins are the initiators of the three main signalling cascades of the UPR. The release of BiP results in the activation of PERK, through PERK homodimerization and trans-autophosphorylation. Activated PERK then phosphorylates the translation-initiation factor eIF2α, reducing the overall frequency of messenger RNA translation initiation. However, selected mRNAs, such as ATF4 mRNA, are preferentially translated in the presence of phosphorylated eIF2α. ATF4 activates the transcription of UPR target genes encoding factors involved in amino-acid biosynthesis, the antioxidative-stress response and apoptosis. The release of BiP also allows IRE1α to dimerize, activating its protein-kinase activity (through autophosphorylation) and its endoribonuclease activity. IRE1α then removes a 26-base intron from XBP1 mRNA. The spliced XBP1 mRNA encodes a potent transcription factor that translocates to the nucleus, activating the expression of UPR target genes. The release of BiP from ATF6 allows ATF6 to translocate to the Golgi apparatus, where it is cleaved by the proteases S1P and S2P, yielding an active cytosolic ATF6 fragment (ATF6 p50). This fragment migrates to the nucleus, activating the transcription of UPR target genes. S1P, site-1 protease; S2P, site-2 protease; XBP1, X-box-binding protein 1.
The most immediate response to ER stress, following the release of BiP from PERK, is the homodimerization and trans-phosphorylation of PERK, allowing PERK to phosphorylate eIF2α. The phosphorylation of eIF2α inhibits the assembly of the 80S ribosome and, consequently, the synthesis of proteins. This pathway promotes cell survival by preventing the influx of additional nascent polypeptides into an already-saturated ER lumen. Indeed, inhibition of PERK-mediated eIF2α phosphorylation reduces cell survival in conditions of ER stress14. However, phosphorylation of eIF2α is required for the translation of certain messenger RNAs that contain regulatory sequences, such as the short open reading frames in the 5′-untranslated region of the mRNA encoding the transcription factor ATF4 (refs 15, 16). ATF4 can induce the expression of UPR target genes, which are involved in amino-acid biosynthesis and transport, the oxidative stress response, and ER-stress-induced apoptosis17.
In response to ER stress, IRE1α autophosphorylates, thereby activating its RNase activity. It then initiates the removal of a 26-base intron from mRNA encoding X-box-binding protein 1 (XBP1), resulting in a translational frameshift and translation of an XBP1 isoform with potent activity as a transcription factor (referred to here as active XBP1)5,6.
In parallel, when ATF6 is released from BiP, it translocates to the Golgi apparatus, where it is cleaved by the proteases site-1 protease (S1P) and S2P. This process results in the release of a functional (bZIP-containing) fragment of ATF6 into the cytosol. This fragment then migrates to the nucleus and activates transcription11,18. Notably, S1P and S2P also cleave ER-associated sterol-regulatory-element-binding proteins (SREBPs), which are required for cholesterol and fatty-acid biosynthesis18. Cleaved ATF6 and active XBP1 isoform function mainly in parallel pathways to induce the transcription of genes encoding ER chaperones and enzymes that promote protein folding, maturation, secretion and ER-associated protein degradation19,20. However, if the cell fails to resolve the protein-folding defect and restore homeostasis in the ER, the UPR will initiate apoptosis, to protect the organism by removing the stressed cells that produce misfolded or malfunctioning proteins5,6. ER-stress-induced apoptosis is mediated largely by CHOP, a transcription factor that is homologous to C/EBP (CCAAT/enhancer-binding protein) and is downstream of the PERK–eIF2α–ATF4 pathway and the ATF6 pathway in the UPR. Although deletion of the gene encoding CHOP is known to protect cells against ER-stress-induced apoptosis, the mechanism by which CHOP induces apoptosis remains obscure. CHOP has been shown, however, to induce the expression of numerous pro-apoptotic factors (including DR5, TRB3, BIM and GADD34), which promote protein synthesis and oxidative stress in stressed cells21–24.
Pathways that connect ER stress to inflammation
In addition to the UPR, other signalling pathways radiate from the ER to the mitochondria and nucleus, and possibly to other organelles. A growing body of evidence suggests that the signalling pathways in the UPR and inflammation are interconnected through various mechanisms, including the production of reactive oxygen species (ROS), the release of calcium from the ER, the activation of the transcription factor nuclear factor-κB (NF-κB) and the mitogen-activated protein kinase (MAPK) known as JNK (JUN N-terminal kinase), and the induction of the acute-phase response.
Oxidative protein folding and accumulation of ROS
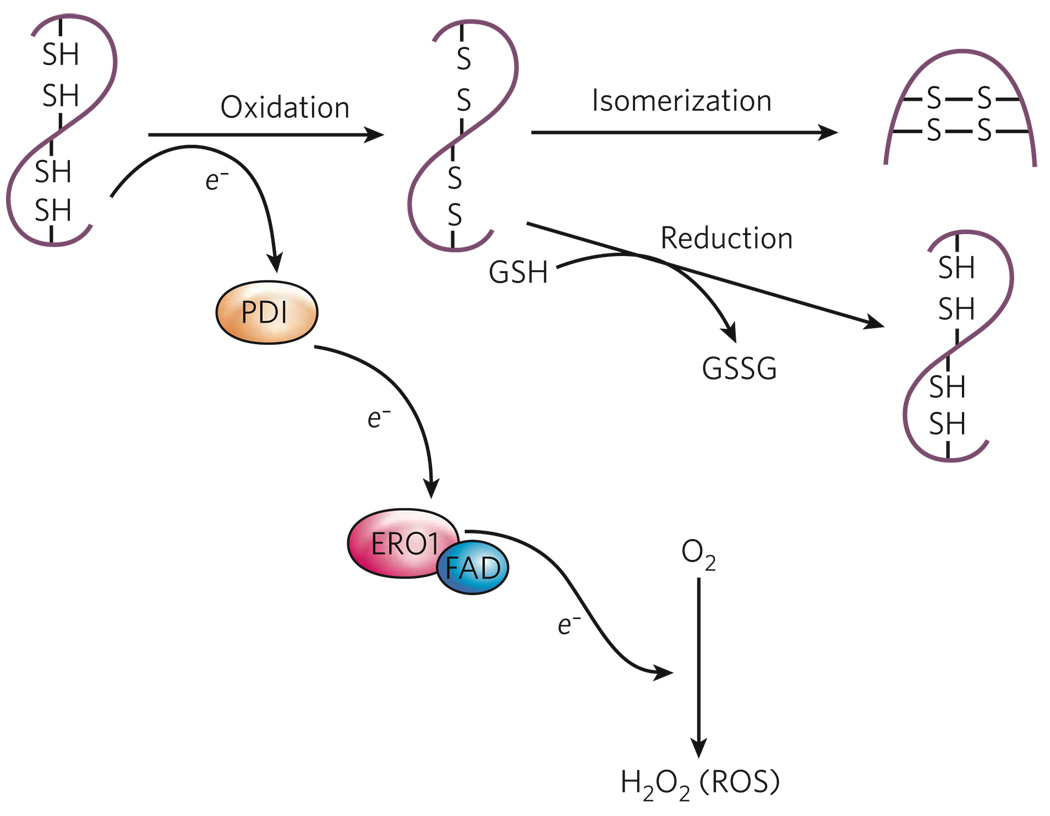
ROS are small molecules that are highly reactive as a result of the presence of unpaired electrons. ROS are important mediators of inflammation25, and recent findings have linked ER stress to the generation and accumulation of intracellular ROS, a state commonly referred to as oxidative stress. The folding of proteins into the correct conformations in the ER is an energy-consuming process, and oxidizing conditions are required for the formation of intramolecular and intermolecular disulphide bonds26. Electron transport during disulphide-bond formation is driven by a protein relay that involves two ER-resident enzymes: protein disulphide isomerase (PDI) and ER oxidoreductin 1 (ERO1)27 (Fig. 2). PDI directly accepts electrons, resulting in the oxidation of cysteine residues and the formation of disulphide bonds. ERO1 then uses a flavin-dependent reaction to transfer electrons from PDI to molecular oxygen, thereby oxidizing PDI. Although it provides a robust driving force for disulphide-bond formation, the use of molecular oxygen as the terminal electron recipient leads to the production of ROS27. Furthermore, additional oxidative stress can result from the depletion of reduced glutathione, because reduced glutathione is consumed in reactions that reduce unstable and improperly formed disulphide bonds28. Therefore, an increase in the protein-folding load in the ER can lead to the accumulation of ROS, which might initiate an inflammatory response.
Figure 2. Oxidative protein folding.
The formation of disulphide bonds in proteins in the ER is driven by the enzymes PDI and ERO1. ERO1 operates in association with the flavin FAD, which is synthesized in the cytosol but can readily enter the ER lumen. PDI accepts electrons (e−) from protein-folding substrates, thereby oxidizing the thiol (SH) groups in the protein’s cysteine residues and resulting in the formation of disulphide bonds. ERO1 uses an FAD-dependent reaction to transfer electrons from PDI to molecular oxygen (O2), resulting in the production of ROS in the form of hydrogen peroxide (H2O2). Reduced glutathione (GSH) can assist in disulphide-bond reduction, which occurs when there is a overload of proteins to fold or an accumulation of misfolded proteins, and results in the production of oxidized glutathione (GSSG). In addition, reduced PDI can mediate a reduction of mispaired thiol groups in oxidized protein-folding substrates, functioning as an isomerase. Because the activity of ERO1 is modulated by the amount of FAD in the ER, disulphide-bond formation is linked to the nutritional and/or metabolic status of the cell.
Importantly, cells have evolved mechanisms to limit the accumulation of ROS in response to ER stress. The PERK pathway of the UPR can activate an antioxidant program by preferentially translating mRNA encoding the bZIP-containing transcription factor ATF4 and by phosphorylating NRF2 (nuclear factor-erythroid-derived 2 (NF-E2)-related factor 2), another bZIP-containing transcription factor17,29. After PERK-mediated phosphorylation, NRF2 translocates to the nucleus and activates the transcription of a set of antioxidant and oxidant-detoxifying enzymes, including NAD(P)H–quinone oxidoreductase, haem oxygen-ase 1 and glutathione S-transferase30,31. In addition, NRF2 and ATF4 each induce the transcription of genes whose products maintain the cellular level of glutathione, the main redox buffer in the cell17,29,32. The overall antioxidant effect of the PERK pathway is supported by the finding that a potent ER-stress-inducing chemical, tunicamycin, induces only weak accumulation of ROS in wild-type cells, whereas this treatment induces a toxic accumulation of ROS in cells that lack PERK17,32.
ER-associated NF-κB activation and the PERK pathway
NF-κB is a key transcriptional regulator that has a central role in the onset of inflammation33. In the absence of inflammatory stimuli, NF-κB remains in an inactive state through binding to a member of the family of inhibitors of NF-κB (IκB), which are constitutively expressed. Activation of NF-κB is initiated by signal-induced phosphorylation of IκB, which is subsequently degraded. The degradation of IκB exposes a nuclear-localization signal in NF-κB, allowing NF-κB to translocate to the nucleus, where it induces the transcription of numerous inflammatory genes. An increase in the ER protein-folding load (for example, during viral infection) has been shown to result in the activation of NF-κB34,35. However, the details of the mechanism by which NF-κB is activated in these conditions are poorly understood. Experiments using calcium chelators and antioxidants indicate that, together, these signals contribute to the activation of NF-κB in response to ER stress36. Therefore, ER-associated NF-κB activation might result from the oxidative stress of excessive protein folding and/or from an ER-stress-mediated leakage of calcium into the cytosol37. In addition, in response to ER stress, the UPR can directly promote NF-κB activation through a PERK–eIF2α-mediated attenuation of translation. Because the half-life of IκB is much shorter than that of NF-κB, attenuating translation increases the ratio of NF-κB to IκB, thereby freeing NF-κB to translocate to the nucleus in response to ER stress38 (Fig. 3). This effect has been observed in cells treated with reagents that induce ER stress and in cells irradiated with ultraviolet light, both of which activate the PERK pathway of the UPR38,39.
Figure 3. Proposed models for UPR-mediated JNK and NF-κB activation.
In response to ER stress, PERK mediates a general repression of mRNA translation by phosphorylating eIF2α. Because IκB has a shorter half-life than NF-κB, PERK-mediated translational attenuation shifts the ratio of IκB to NF-κB, thereby freeing NF-κB to translocate to the nucleus. In addition, in response to ER stress, the cytoplasmic domain of phosphorylated IRE1α can recruit tumour-necrosis factor-α (TNF-α)-receptor-associated factor 2 (TRAF2). The IRE1α–TRAF2 complex interacts with JNK and/or IκB kinase (IKK), activating these protein kinases. Activated JNK phosphorylates the transcription factor activator protein 1 (AP1). Activated IKK phosphorylates IκB, initiating the degradation of IκB and thereby leading to NF-κB activation. Activated NF-κB and AP1 then migrate to the nucleus, where they induce the transcription of genes involved in the inflammatory response.
IRE1α-mediated NF-κB and JNK activation
In mammals, IRE1α might be important for integrating ER-stress signal-ling with inflammatory-response signalling. This is thought to occur in the following manner. In response to ER stress, the autophosphorylation of IRE1α induces a conformational change in its cytosolic domain, which can then bind to the adaptor protein tumour-necrosis factor-α (TNF-α)-receptor-associated factor 2 (TRAF2)40. The IRE1α–TRAF2 complex can recruit IκB kinase (IKK), which phosphorylates IκB, leading to the degradation of IκB and the nuclear translocation of NF-κB41 (Fig. 3). Consistent with these observations, ER-stress-induced NF-κB activation and production of the inflammatory cytokine TNF-α are impaired in mouse embryonic fibroblasts that lack IRE1α41. The IRE1α–TRAF2 complex can also recruit the protein kinase JNK, leading to the activation of JNK. Activated JNK induces the expression of inflammatory genes by phosphorylating the transcription factor activator protein 1 (AP1)42. Given that JNK activation in response to ER stress is impaired in mouse embryonic fibroblasts that lack IRE1α, IRE1α might provide a link between ER stress and inflammation40. Taking these findings together, the formation of the IRE1α–TRAF2 complex seems to be crucial for activating both JNK and NF-κB in response to ER stress. Further studies will be needed to identify how ER-stress-induced signalling involving these two factors, JNK and NF-κB, might be integrated and/or synergize to regulate inflammation, metabolism, cell survival and apoptosis.
The acute-phase response
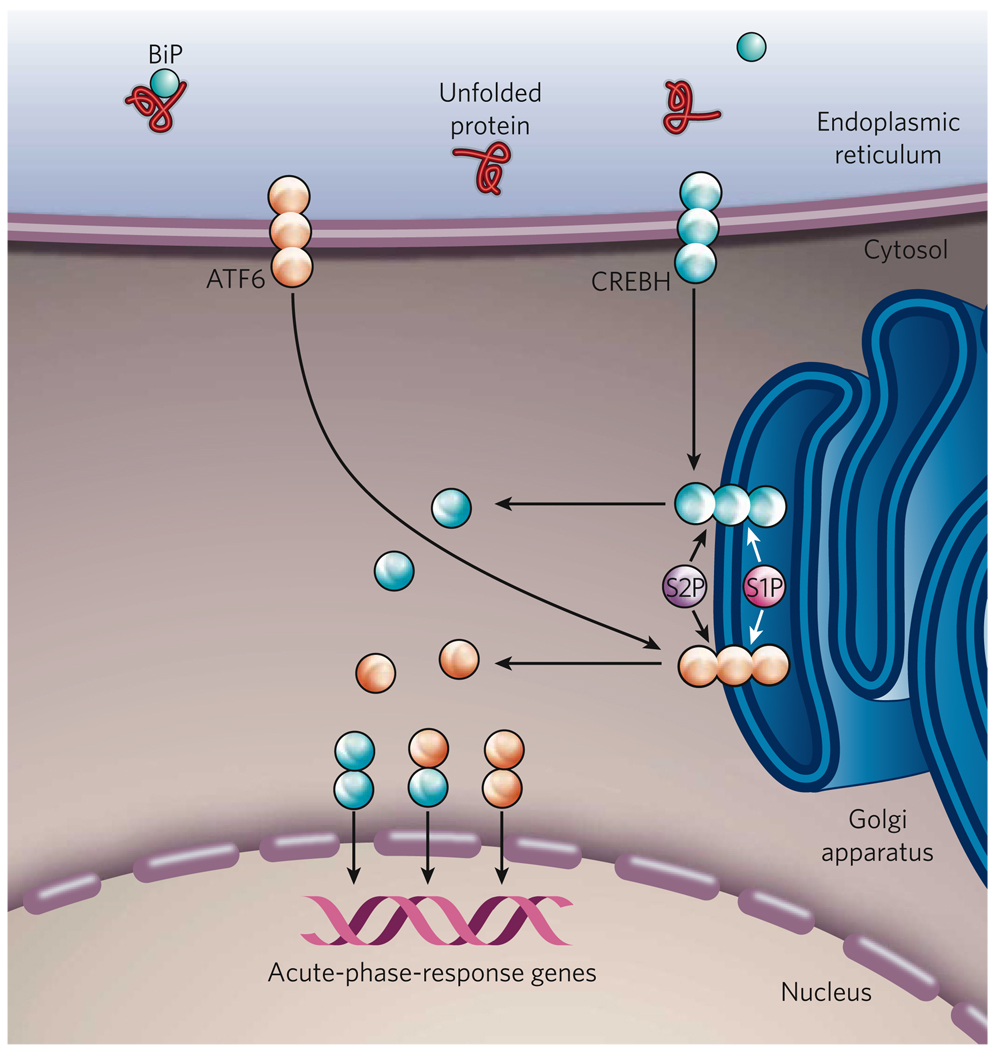
Regulated intramembrane proteolysis (RIP) is a process by which ER-resident bZIP-containing transcription factors (including SREBPs and ATF6) traffick from the ER to the Golgi apparatus, where they are cleaved, releasing functional isoforms18,43. Recently, CREBH, another RIP-regulated bZIP-containing transcription factor, was identified to mediate the acute-phase response in the liver44. CREBH is expressed mainly by hepatocytes, and its expression is highly induced by inflammatory cytokines, such as TNF-α, interleukin 1β (IL-1β) and IL-6. When ER stress occurs, CREBH is activated and mediates the acute-phase response in the liver44 (Fig. 4). CREBH is activated through translocation from the ER to the Golgi apparatus, where it is cleaved by S1P and S2P. An N-terminal fragment of CREBH is released into the cytosol, and this fragment translocates to the nucleus, where it can induce transcription. In the mouse liver, inflammatory cytokines and bacterial lipopolysaccharide (LPS) each induces ER stress and leads to such cleavage of CREBH44. However, CREBH does not induce the expression of genes involved in the UPR. Instead, it binds to a DNA-sequence motif in the promoter regions of a subset of acute-phase- response genes, including those encoding serum amyloid P component and C-reactive protein44. Further studies are required to elucidate how the trafficking of CREBH from the ER is regulated. In addition, targeted deletion of the gene encoding CREBH should identify the significance of this ER-stress signalling pathway in the inflammatory response.
Figure 4. The ER-stress-induced acute-phase response.
When inflammatory cytokines, such as TNF-α, IL-1β and IL-6, are present in the extracellular environment, the gene encoding CREBH is transcribed (not shown). CREBH, similar to ATF6, is a bZIP-containing transcription factor that is localized to the ER membrane. CREBH, however, is mainly expressed by hepatocytes, whereas ATF6 is expressed by all cell types. In conditions of ER stress, such as those caused by inflammatory cytokines or the bacterial component lipopolysaccharide (LPS), CREBH translocates to the Golgi apparatus, where it is cleaved by the proteases S1P and S2P, releasing a cytosolic fragment. ER stress also activates the UPR sensor ATF6 by regulated intramembrane proteolysis. Activated CREBH and ATF6 can then form homodimers or heterodimers and migrate to the nucleus, where they activate the transcription of the genes encoding serum amyloid P component and C-reactive protein, which mediate the acute-phase response.
Factors at the crossroads of inflammation and ER stress
Accumulating evidence suggests that there is extensive cross-talk between the inflammatory response and the ER-stress response. Inflammation can be triggered by a chronic excess of metabolic factors (such as lipids, glucose and cytokines) and/or neurotransmitters. In many physiological or pathological settings, these stimuli can also elicit ER stress, which further disrupts metabolic functions, thereby causing more inflammation. Such vicious cycles could exacerbate inflammatory stress signalling (that is, the signalling pathways that integrate stress and inflammation), as well as metabolic deterioration, in specialized cells such as macrophages, β-cells (in the pancreas) and adipocytes. Moreover, intracellular calcium and free radicals (such as ROS and nitric oxide) are crucial for integrating inflammatory responses, metabolic responses and ER-stress responses, and dynamic signalling by these factors relies on there being functional interactions between the ER and mitochondria.
Calcium and free radicals
The oxidation state and concentration of calcium in the ER lumen crucially affect polypeptide folding, as well as chaperone function. The calcium concentration in the ER is many thousand-fold greater than that in the cytosol45. The calcium concentration in the ER is regulated by ATP-dependent uptake of calcium into the ER and receptor-mediated release of calcium from the ER. An accumulation of misfolded proteins in the ER can cause calcium to leak from the ER, possibly through inositol-trisphosphate receptors37. The calcium released from the ER is concentrated in the matrix of the mitochondria and causes depolarization of the inner mitochondrial membrane, disrupting electron transport and increasing ROS production46 (Fig. 5). Mitochondrial ROS can further increase calcium release from the ER by sensitizing ER calcium-release channels and causing protein misfolding. In addition, during oxidative protein folding in the ER, reducing equivalents are transferred from thiol groups in protein-folding substrates to molecular oxygen, thus producing membrane-permeable hydrogen peroxide (an ROS). Through this forward cycle, calcium release, ROS production and protein misfolding function together to activate calcium-dependent protein kinases, as well as JNK and NF-κB, leading to inflammatory responses and even cell death47.
Figure 5. The role of calcium and ROS in the UPR and inflammation.
Protein folding is an oxidative process that generates ROS. ROS can target chaperones (not shown) and ER-based calcium (Ca2+) channels, leading to the release of calcium from the ER into the cytosol and ER-stress signalling. Calcium released from the ER is concentrated in the inner matrix of the mitochondria, where it disrupts the electron-transport chain, thereby leading to the production of more ROS. These mitochondrially produced ROS can further exacerbate calcium release from the ER, resulting in the accumulation of ROS to a toxic level. Furthermore, perturbation of ER calcium homeostasis can disrupt the protein-folding process in the ER, which, in turn, causes ER stress, induces the UPR and generates more ROS.
In addition to ROS, reactive nitrogen species also contribute to inflammation and ER stress. Nitric oxide is a highly reactive, uncharged, membrane-permeable molecule that functions as a signal in many regulatory processes, such as blood-vessel dilation, immune responses and neurotransmission. Nitric oxide can react with superoxide to form peroxynitrite, and with thiols and metal centres in proteins to form nitrosyl adducts48. It has also been shown to modify the active site of PDI, thereby interfering with disulphide-bond formation and resulting in the accumulation of misfolded proteins in the ER49. In addition, excessive production of nitric oxide can alter the oxidative state and calcium concentration in the ER and disrupt the electron-transport chain, causing ER stress and ROS production50,51.
Metabolic factors
Several reports indicate that inflammatory cytokines can cause ER stress and therefore activate the UPR. For example, TNF-α causes ER stress, activating PERK, IRE1α and ATF6 in fibrosarcoma cells52. In addition, TNF-α, IL-1β and/or IL-6 can induce ER stress in hepatocytes, leading to the activation of CREBH, which then mediates an acute-phase response44. And the presence of the T-cell-derived cytokine interferon-γ (IFN-γ) has been associated with PERK activation and ER-stress-induced apoptosis in oligodendrocytes (cells that produce large amounts of myelin in the nervous system)53. Although the mechanism by which cytokines induce ER stress is not completely understood, experimental evidence supports the idea that cytokines trigger the release of calcium from the ER and the accumulation of ROS, which interfere with protein folding and mitochondrial metabolism52,53.
In addition to cytokines, excessive amounts of metabolic factors, such as cholesterol, non-esterified fatty acids, glucose, homocysteine and neurotransmitters, can also induce both the ER-stress response and the inflammatory response in a variety of cell types54–58. There is evidence to support the idea that the presence of large amounts of these metabolic factors can stimulate the release of calcium from the ER, the production of free radicals, and ER stress. But the molecular links between metabolic-factor excess, ER stress and inflammation are not well defined.
The UPR and inflammation in health and disease
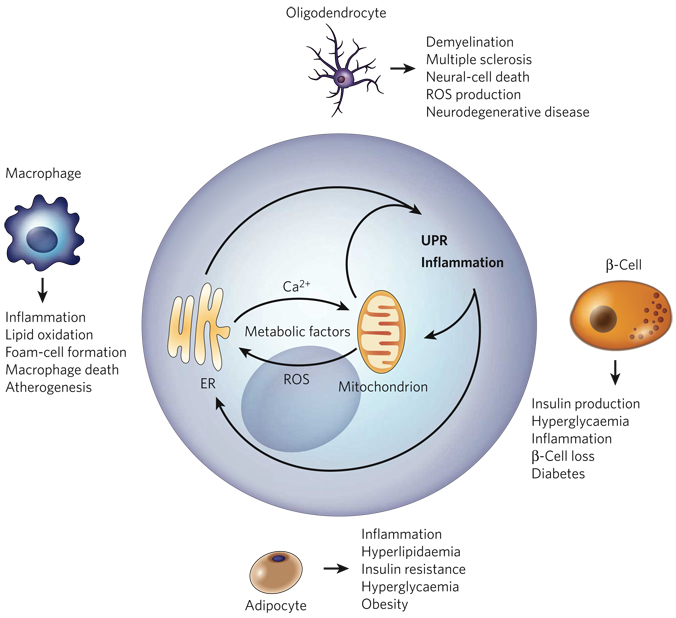
The cross-talk between the UPR and inflammation is exemplified in cell types that have metabolic or immune functions. These cell types include hepatocytes, β-cells, adipocytes, macrophages and oligodendrocytes. Because these specialized cell types require the trafficking of large amounts of ‘cargo’ through the ER, they are extremely sensitive to alterations in metabolism and/or ER homeostasis. Metabolic conditions such as lipid accumulation, increased glucose levels or excessive amounts of cytokines can trigger calcium release from the ER and ROS production in these cells, leading to ER stress and inflammation (Fig. 6). A wealth of evidence from in vitro studies suggests that pathological conditions that interfere with ER homeostasis and/or mitochondrial metabolism result in chronic activation of the UPR and in inflammation. Recent observations indicate that the molecular link between ER-stress responses and inflammatory responses might be mediated by activation of two signal-ling molecules involved in inflammatory responses, JNK and NF-κB. The coupling of the UPR and inflammation in specialized cells and tissues might be fundamental to the pathogenesis of metabolic, neurodegenerative and infectious diseases. In this section, we describe some of the compelling evidence that prolonged activation of the UPR and inflammation are integrated and ‘conspire’ in the pathogenesis of disease.
Figure 6. The ‘ER-stress–inflammation’ loop in specialized cells.
In specialized cells that secrete large amounts of protein — such as macrophages, adipocytes, β-cells and oligodendrocytes — the UPR and inflammatory-response signalling can be triggered by a chronic excess of extracellular and/or intracellular metabolic factors, such as lipids, glucose, cytokines, hormones, non-esterified fatty acids and neurotransmitters. More specifically, such metabolic factors stimulate protein synthesis, calcium signalling and ROS production by targeting the mitochondria and the ER in these cells (Fig. 5). The increased protein-folding demand and the signalling involving calcium and ROS induce the UPR and inflammatory-response signalling, leading to the transcription of genes whose products mount a broader inflammatory response. An excess of metabolic factors can further boost the UPR and inflammation, contributing to impaired lipid and glucose metabolism, insulin resistance and apoptosis. This forward ER-stress– inflammation loop could also further promote inflammatory stress signalling and contribute to the metabolic deterioration that is associated with atherosclerosis, obesity, type 2 diabetes and neurodegenerative diseases, depending on the cell type involved.
Obesity and type 2 diabetes
The ER centrally controls cellular metabolism by regulating protein synthesis and secretion, as well as triglyceride and cholesterol biosynthesis. Metabolic conditions such as insulin resistance and reduced glucose utilization are associated with the development of metabolic syndrome, and these processes are regulated by numerous mechanisms, including the UPR, JNK activation, NF-κB activation and apoptosis2,59. Obesity and type 2 diabetes, whether caused by lifestyle factors or genetic deficiency, result in conditions that increase the demand on the ER. This is particularly clear in the liver, adipose tissue and pancreas, where changes in tissue architecture, increases in protein synthesis, and perturbations in cellular energy fluxes occur2. Indeed, ER dysfunction has been linked to increased JNK activity, NF-κB activation and insulin resistance40,41,60,61.
In normal conditions, activated insulin receptors phosphorylate tyrosine residues on proximal signalling molecules, such as insulin-receptor substrate 1 (IRS1), that transmit the effects of insulin by interacting with other cytosolic molecules. Insulin resistance can result from JNK-mediated phosphorylation of serine residues in IRS1, which inhibits the phosphorylation of IRS1 on tyrosine residues62–64. In the liver and adipose tissues of obese animals, PERK and IRE1α, and their downstream effectors, have been found to be activated60. Because activated IRE1α can recruit TRAF2 and trigger JNK activation when ER stress occurs40, it has been proposed that IRE1α links ER stress and JNK-mediated serine phosphorylation of IRS1, causing peripheral insulin resistance. Consistent with this hypothesis, when mice that lacked one allele of Xbp1 (Xbp1+/− mice) were fed a high-fat diet, the liver and adipose tissues showed increased activation of PERK, IRE1α and JNK, and dysregulated phosphorylation of IRS1, coupled with insulin resistance60. It is possible that reduced signalling through XBP1 compromises protein folding and thereby causes insulin resistance, although further studies are required to validate this hypothesis. In addition, because both IRE1α and PERK activation can lead to NF-κB activation (through IKK activation and translation attenuation, respectively; Fig. 3), further studies are required to elucidate the significance of IRE1α and PERK in coordinating the activation of JNK and NF-κB, as well as the impact of this coordinated activation on the insulin resistance and inflammation that are associated with obesity and type 2 diabetes.
Atherosclerosis
Atherosclerosis, the leading cause of cardiovascular disease, is an inflammatory disease in which immune mechanisms interact with metabolic risk factors, causing lesions to develop in the arterial vasculature. Cholesterol deposition by macrophages, inflammation and cell death are crucial contributors to the formation and progression of these lesions, resulting in the acute occlusion of blood vessels65. Recent evidence suggests that the UPR and inflammation underlie the development of atherosclerotic lesions.
The accumulation of free cholesterol in the ER membranes of macrophages causes calcium release, UPR activation and CHOP-induced apoptosis54. This loading of macrophages with free cholesterol activates NF-κB and the MAPKs p38, extracellular-signal-regulated kinase 1 (ERK1) and ERK2, and JNK, thereby inducing the expression of genes encoding inflammatory cytokines (including TNF-α and IL-6)66. In these conditions, JNK and NF-κB activation might be mediated, in part, through PERK and IRE1α66. Interestingly, CHOP, which is mainly produced by way of the PERK pathway of the UPR, is required for IL-6 production and for full activation of ERK1 and ERK2 in response to loading with free cholesterol. The connections between the UPR, ER-stress- induced apoptosis and inflammation might help to explain the link between free-cholesterol accumulation and inflammation in the vulnerability of lesions to rupture in advanced atherosclerosis. In addition to the free-cholesterol loading of macrophages, oxidized lipids (such as oxidized low-density lipoprotein and its bioreactive component, oxidized 1-palmitoyl-2-arachidonyl-sn-3-glycero-phosphorylcholine) can result in ER stress and UPR activation in human aortic endothelial cells67. The UPR is also activated in human atherosclerotic lesions, where oxidized phospholipids have accumulated. Furthermore, in vitro studies have shown that the ER-stress-induced transcription factors ATF4 and XBP1 are required for the production of the inflammatory cytokine IL-6 and the chemokines IL-8 (also known as CXC-chemokine ligand 8 (CXCL8)), CC-chemokine ligand 2 (CCL2) and CXCL3 by human aortic endothelial cells in the basal state and on accumulation of oxidized lipids67. Together, these studies suggest that UPR signalling is an important mediator of vascular inflammation and possibly of the endothelial-cell dysfunction that is observed in atherosclerosis.
Neurodegenerative diseases
Most acute and chronic neurodegenerative diseases involve inflammation, although the source of the inflammatory response is poorly characterized68. These diseases, including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis and diseases that result from the expansion of a polyglutamine repeat, are associated with protein aggregation and are characterized by abnormal neuronal physiology and neural-cell death69. Recent studies suggest that the protein aggregates associated with these diseases might inhibit the proteasome, thereby preventing ER-associated protein degradation (ERAD) and leading to the accumulation of unfolded proteins in the ER70,71. However, there is limited evidence from studies of animal models or humans to support the idea that the pathology associated with these diseases results from defects in ERAD that cause ER stress. Intriguingly, mutations in genes that have functions linked to ERAD and/or mitochondrial function can cause Parkinson’s disease in humans72. In addition, deletion of the gene encoding the pro-apoptotic UPR-induced transcription factor CHOP was reported to protect against apoptosis in a neurotoxin-induced mouse model of Parkinson’s disease73. However, in a mouse model, brain-specific deletion of the gene encoding XBP1, a transcriptional activator of genes whose products are involved in ERAD, did not affect the development of prion disease (a family of neurodegenerative diseases caused by prion-protein misfolding)74. Although the mechanisms underlying neurodegenerative diseases are still under investigation, it is clear that alterations in protein folding, calcium signals, redox homeostasis and inflammation are prominent features69,75,76.
Multiple sclerosis is a neurodegenerative disease that is marked by demyelination, oligodendrocyte loss and T-cell activation associated with IFN-γ production53,77. In animal models of multiple sclerosis, treatment with IFN-γ was found to induce ER stress in actively myelinating oligodendrocytes, leading to apoptosis of the oligodendrocytes and abnormalities in neuron myelination78. By contrast, treatment with IFN-γ has been shown to activate the PERK pathway of the UPR, protecting mature oligodendrocytes against immune-mediated damage79. It has been proposed that these divergent responses to IFN-γ depend on the rate of protein synthesis by the oligodendrocytes79. In an oligodendrocyte that is actively producing myelin, the increase in protein production stimulated by IFN-γ could convert an adaptive (able to be adjusted), moderate level of ER stress to a destructive, apoptosis-inducing, ER-stress response. By contrast, mature oligodendrocytes produce less protein, so an increase in protein production might not result in such a destructive ER-stress response79,80. Thus, regulating the balance between the rates of protein production and the inflammatory stress responses (which integrate inflammation and ER-stress signalling) in oligodendrocytes might be a crucial factor in the development of demyelinating diseases.
Therapeutic potential and future directions
Considerable progress has now been made towards understanding the signalling pathways that integrate the UPR and inflammation and the physiological significance of this connection. Recently, researchers have focused on designing effective therapeutics for inflammatory diseases by modulating the UPR and inflammatory response. However, manipulating the interface between these fundamental biological responses for therapeutic purposes, without causing severe side effects, is a formidable challenge. Because many mediators of cellular stress and inflammation are regulated simultaneously, it is unlikely that a single response that integrates inflammation and ER-stress signalling is responsible for the pathogenesis of a particular disease. Given this complexity, an effective approach would be to seek to re-establish functional homeostasis by modifying integrated biological outcomes rather than targeting single pathways.
Recent studies suggest that preserving or restoring ER function might be therapeutic. Small molecules that are classified as chemical chaperones can facilitate protein folding and protect against ER stress, thus relieving disease symptoms in animal models. For example, in insulin-resistant obese mice, the chemical chaperones 4-phenylbutyric acid and taurine-conjugated ursodeoxycholic acid were found both to reduce the phosphorylation of PERK and IRE1α significantly and to improve glucose tolerance and insulin sensitivity61. In addition, another chemical chaperone, the resveratrol tetramer vaticanol B, has been shown to inhibit both the UPR and the inflammatory response by reducing the protein-folding load and maintaining ER-membrane integrity, preventing ER-stress-induced apoptosis81. In addition to chemical chaperones, salubrinal, a phosphatase inhibitor, might have therapeutic benefit. Salubrinal can protect cells against ER-stress-induced apoptosis by selectively inhibiting the dephosphorylation of eIF2α such that further protein synthesis and accumulation in the ER is inhibited82.
Future studies will need to address the many open questions about the physiological significance of the various ER-stress signalling pathways in mediating inflammatory responses. The knowledge gained by such studies will improve the overall understanding of how inflammatory diseases develop and indicate how they might be treated with pharmacological interventions that modulate ER stress and inflammation.
Acknowledgments
We thank J. Mitchell for her efforts in preparing the manuscript. We apologize to those whose work could not be cited because of space limitations. K.Z. is supported by a grant from the American Heart Association (0635423Z). R.J.K. is supported by grants from the National Institutes of Health (DK042394, HL052173 and HL057346) and is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
References
- 1.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nature Rev. Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 5.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 6.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 7.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74:743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 8.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 11.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 13.Kohno K. How transmembrane proteins sense endoplasmic reticulum stress. Antioxid.Redox Signal. 2007;9:2295–2303. doi: 10.1089/ars.2007.1819. [DOI] [PubMed] [Google Scholar]
- 14.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 15.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaman I, et al. The zipper model of translational control: a small upstream ORF is the switch that controls structural remodeling of an mRNA leader. Cell. 2003;113:519–531. doi: 10.1016/s0092-8674(03)00345-3. [DOI] [PubMed] [Google Scholar]
- 17.Harding HP, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 18.Ye J, et al. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell. 2000;6:1355–1364. doi: 10.1016/s1097-2765(00)00133-7. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J. Biol. Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 23.Puthalakath H, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 24. Song B, Scheuner D, Ron D, Pennathur S, Kaufman R. Genetic deletion of C/EBP homologous protein CHOP reduces oxidative stress, improves β cell function, and prevents diabetes. J. Clin. Invest. doi: 10.1172/JCI34587. (in the press). This report describes how the ER-stress-induced pro-apoptotic factor CHOP is involved in oxidative stress and β-cell death.
- 25.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem. Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 26.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu BP, Weissman JS. The FAD- and O2-dependent reaction cycle of Ero1-mediated oxidative protein folding in the endoplasmic reticulum. Mol. Cell. 2002;10:983–994. doi: 10.1016/s1097-2765(02)00696-2. [DOI] [PubMed] [Google Scholar]
- 28. Cuozzo JW, Kaiser CA. Competition between glutathione and protein thiols for disulphide-bond formation. Nature Cell Biol. 1999;1:130–135. doi: 10.1038/11047.. References 27 and 28 provide insights into how protein folding in the ER leads to the production of ROS.
- 29.Cullinan SB, et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathers J, et al. Antioxidant and cytoprotective responses to redox stress. Biochem. Soc. Symp. 2004;71:157–176. doi: 10.1042/bss0710157. [DOI] [PubMed] [Google Scholar]
- 31.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab. Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 32.Cullinan SB, Diehl JA. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 33.Rius J, et al. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pahl HL, Baeuerle PA. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J. Virol. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer M, et al. Hepatitis B virus transactivator MHBst: activation of NF-κB, selective inhibition by antioxidants and integral membrane localization. EMBO J. 1992;11:2991–3001. doi: 10.1002/j.1460-2075.1992.tb05369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pahl HL, Baeuerle PA. Activation of NF-κB by ER stress requires both Ca2+and reactive oxygen intermediates as messengers. FEBS Lett. 1996;392:129–136. doi: 10.1016/0014-5793(96)00800-9. [DOI] [PubMed] [Google Scholar]
- 37. Deniaud A, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638.. This paper shows that protein misfolding in the ER causes calcium to leak into the cytosol, resulting in the outer membrane of mitochondria becoming more permeable.
- 38.Deng J, et al. Translational repression mediates activation of nuclear factor κB by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu S, et al. Ultraviolet light activates NFκB through translational inhibition of IκBα synthesis. J. Biol. Chem. 2004;279:34898–34902. doi: 10.1074/jbc.M405616200.. References 38 and 39 show that NF-κB is activated by the PERK pathway of the UPR.
- 40. Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664.. This paper shows how ER stress activates JNK by way of IRE1α.
- 41.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor a links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-κB activation and down-regulation of TRAF2 expression. Mol. Cell. Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 43.Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- 44. Zhang K, et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040.. This study identifies CREBH, an ER-stress-inducible transcription factor that can mediate the acute-phase response.
- 45.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 46.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 47.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 48.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 49.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 50.Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc. Natl Acad. Sci. USA. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu W, Liu L, Charles IG, Moncada S. Nitric oxide induces coupling of mitochondrial signalling with the endoplasmic reticulum stress response. Nature Cell Biol. 2004;6:1129–1134. doi: 10.1038/ncb1188. [DOI] [PubMed] [Google Scholar]
- 52.Xue X, et al. Tumor necrosis factor α (TNFα) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFα. J. Biol. Chem. 2005;280:33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 53.Lin W, Harding HP, Ron D, Popko B. Endoplasmic reticulum stress modulates the response of myelinating oligodendrocytes to the immune cytokine interferon-γ. J. Cell Biol. 2005;169:603–612. doi: 10.1083/jcb.200502086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng B, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 55.Maedler K, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kharroubi I, et al. Free fatty acids and cytokines induce pancreatic β-cell apoptosis by different mechanisms: role of nuclear factor-κB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 57.Zhou J, et al. Association of multiple cellular stress pathways with accelerated atherosclerosis in hyperhomocysteinemic apolipoprotein E-deficient mice. Circulation. 2004;110:207–213. doi: 10.1161/01.CIR.0000134487.51510.97. [DOI] [PubMed] [Google Scholar]
- 58.Yamamuro A, Yoshioka Y, Ogita K, Maeda S. Involvement of endoplasmic reticulum stress on the cell death induced by 6-hydroxydopamine in human neuroblastoma SH-SY5Y cells. Neurochem. Res. 2006;31:657–664. doi: 10.1007/s11064-006-9062-6. [DOI] [PubMed] [Google Scholar]
- 59.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 61. Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294.. This paper shows that decreasing ER stress improves insulin sensitivity in mice with type 2 diabetes.
- 62.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 63.Aguirre V, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 64.Tuncman G, et al. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc. Natl Acad. Sci. USA. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Williams KJ, Tabas I. Atherosclerosis and inflammation. Science. 2002;297:521–522. doi: 10.1126/science.297.5581.521. [DOI] [PubMed] [Google Scholar]
- 66. Li Y, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-α and interleukin-6: model of NF-κB- and MAP kinase-dependent inflammation in advanced atherosclerosis. J. Biol. Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200.. This paper describes how ER-stress signalling and inflammatory-response signalling are integrated in cholesterol-loaded macrophages.
- 67.Gargalovic PS, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 68.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 70.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 71.Nishitoh H, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson’s disease. Antioxid. Redox Signal. 2007;9:553–561. doi: 10.1089/ars.2006.1524. [DOI] [PubMed] [Google Scholar]
- 73.Silva RM, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J. Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hetz C, et al. Unfolded protein response transcription factor XBP-1 does not influence prion replication or pathogenesis. Proc. Natl Acad. Sci. USA. 2008;105:757–762. doi: 10.1073/pnas.0711094105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paschen W, Aufenberg C, Hotop S, Mengesdorf T. Transient cerebral ischemia activates processing of xbp1 messenger RNA indicative of endoplasmic reticulum stress. J. Cereb. Blood Flow Metab. 2003;23:449–461. doi: 10.1097/01.WCB.0000054216.21675.AC. [DOI] [PubMed] [Google Scholar]
- 76.DeLegge MH, Smoke A. Neurodegeneration and inflammation. Nutr. Clin. Pract. 2008;23:35–41. doi: 10.1177/011542650802300135. [DOI] [PubMed] [Google Scholar]
- 77.Frohman EM, Racke MK, Raine CS. Multiple sclerosis — the plaque and its pathogenesis. N. Engl. J. Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 78.Lin W, et al. Interferon-γ inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 79. Lin W, et al. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J. Clin. Invest. 2007;117:448–456. doi: 10.1172/JCI29571.. This paper shows that IFN-γ can have a detrimental role or a protective role, mediated by the UPR, depending on the stage of multiple sclerosis.
- 80.Lees JR, Cross AH. A little stress is good: IFN-γ, demyelination, and multiple sclerosis. J. Clin. Invest. 2007;117:297–299. doi: 10.1172/JCI31254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabata Y, et al. Vaticanol B, a resveratrol tetramer, regulates endoplasmic reticulum stress and inflammation. Am. J. Physiol. Cell. Physiol. 2007;293:C411–C418. doi: 10.1152/ajpcell.00095.2007. [DOI] [PubMed] [Google Scholar]
- 82.Boyce M, et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]