Abstract
Protease Activated Receptor-1 (PAR-1) is a key player in melanoma metastasis with higher expression seen in metastatic melanoma cell lines and tissue specimens. cDNA microarray and Western blot analyses reveal that the gap junctional intracellular communication molecule, Connexin 43 (Cx-43), known to be involved in tumor cell diapedesis and attachment to endothelial cells, is significantly decreased after PAR-1 silencing in metastatic melanoma cell lines. Furthermore, Cx-43 promoter activity was significantly inhibited in PAR-1 silenced cells suggesting that PAR-1 regulates Cx-43 at the transcriptional level. Chromatin Immunoprecipitation studies found a reduction in the binding of SP-1 and AP-1 transcription factors to the promoter of Cx-43. Both transcription factors have previously been shown to be required for maximal Cx-43 promoter activity. These results were corroborated by mutating the AP-1 and SP-1 binding sites resulting in decreased Cx-43 promoter activity in PAR-1 positive cells. Moreover, as Cx-43 has been shown to facilitate arrest of circulating tumor cells at the vascular endothelium, melanoma cell attachment to endothelial cells was significantly decreased in PAR-1 silenced cells with this effect being abrogated after PAR-1 rescue. Herein, we report that upregulation of PAR-1 expression seen in melanoma progression, mediates high levels of Cx-43 expression. As both SP-1 and AP-1 transcription factors act as positive regulators of Cx-43, our data provide a novel mechanism for the regulation of Cx-43 expression by PAR-1. Indeed, Cx-43 expression was restored following PAR-1 rescue in PAR-1 silenced cells. Taken together, our data support the tumor promoting function of Connexin 43 in melanoma.
Keywords: Thrombin Receptor, Connexin 43, AP-1, SP-1, Melanoma, shRNA
Introduction
The thrombin receptor, Protease Activated Receptor-1 (PAR-1), is a transmembrane G-protein-coupled receptor (GPCR) that has been found to be involved in many types of cancers. PAR-1 can be activated by coagulation factor Xa, granzyme A, trypsin and matrix metalloprotease 1 (MMP-1) although thrombin is the most potent activator (1–4). PAR-1 activation leads to induction of G proteins that trigger various downstream molecules and signal transduction pathways such as Rho kinase, phosphoinositol-3-kinase (PI3-K) and mitogen-activated protein kinases (MAPK), which have been shown to be involved in cell growth, tumor promotion and carcinogenesis (5, 6).
PAR-1 overexpression has been found in various cancers, including those of breast, lung and prostate, among others (7–11). Our laboratory has found PAR-1 to be a key player in the progression of melanoma. PAR-1 is overexpressed in metastatic melanoma cell lines as compared to non-metastatic cell lines (12). Furthermore, melanoma tumors have increased PAR-1 expression as compared to dysplastic nevi (13, 14). Recently, we have demonstrated that silencing PAR-1 via lentiviral shRNA or through PAR-1 siRNA-DOPC delivery, significantly decreases both melanoma tumor growth and metastasis (7). Based on these results, we sought to determine via cDNA microarray studies, novel downstream gene targets regulated by PAR-1 that contribute to the metastatic phenotype of melanoma. This led us to identify Connexin 43 (Cx-43) as a target gene of PAR-1.
Increased Cx-43 expression has been observed in several cancers, including breast cancer, hepatocellular carcinoma and gliomas (15–18). Connexin gap junctions are intracellular membrane channels that form when six connexin subunits are arranged to form a pore. They align with complementary connexins on the plasma membrane of adjacent cells. This allows for small molecules (<1.2 kD) such as Ca2+, secondary messengers and metabolic products to pass between neighboring cells, a process known as gap junction intracellular communication (GJIC). This process appears to be key for maintaining tissue regulation, growth and proliferation (19, 20).
Unlike typical gap junctions, connexins are also considered membrane proteins with adhesive properties (21, 22). The attachment of tumor cells in transition from a primary site to a secondary organ site requires the attachment as well as the migration of tumor cells through the vascular endothelium, a process known as tumor cell diapedesis. It has been shown that immediately following adhesion to the endothelium, tumor cells establish gap junctional channels with the endothelial cells. Several studies have found that the communication between tumor cells and endothelial cells is mediated by connexins and is critical to tumor cell extravasation at the metastatic site (23–25). In fact, it has been reported that Connexin 43-mediated gap junctional communication enhances breast tumor cell diapedesis (24). Furthermore, decreased Cx-43 expression reduced adhesion of breast cancer cells to the pulmonary endothelium. Moreover, upregulation of Cx-43 was seen in tumor cell-endothelial cell contact areas both in vivo and in vitro (26).
In melanoma, increased expression of Connexin 43 has been implicated in establishing a crucial link between melanoma cells and endothelial cells which enhances tumor metastasis (15, 17, 24–26). However, the exact mechanism by which Cx-43 is regulated in melanoma cells is unknown. Herein, we describe that decreased Cx-43 expression occurs through differential binding of AP-1 and SP-1 transcription factors to the Cx-43 promoter mediated by PAR-1. Furthermore, this results in a decrease in melanoma cell attachment to endothelial cells. This is the first report to identify PAR-1 as a regulator of Cx-43 expression, thus, adding an alternative mechanism by which PAR-1 contributes to the malignant phenotype of melanoma.
Materials and Methods
Cell lines and culture conditions
A375SM human melanoma cell line was maintained in Eagle’s MEM supplemented with 10% fetal bovine serum (FBS), as previously described (27). C8161 human melanoma cell line was maintained in DMEM-F12 supplemented with 5% FBS, as previously described (28). Human umbilical vein endothelial cells (HUVEC) were obtained from the American Type Culture Collection. HUVECs were plated on 0.5% gelatin-coated flasks and maintained in DMEM supplemented with 15% FBS and 10 ng/mL basic fibroblast growth factor as described previously (29). Human dermal microvessel endothelial cells (HDMEC) were purchased from PromoCell (Heidelberg, Germany) and maintained in Endothelial Cell Growth Medium (PromoCell).
Antibodies
ATAP2 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The PAR-1 antibody utilized for immunoprecipitation studies was purchased from Biodesign International (Saco, ME) The phycoerythrin (PE anti-mouse) antibody was purchased from Jackson ImmunoResearch (West Grove, PA). Connexin 43 antibody was purchased from BD Pharmingen (San Diego, CA). SP-1, c-Jun, c-Fos and IgG antibodies utilized for ChIP and Western blot assays were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase conjugated anti-mouse IgG antibodies for immunoprecipitation studies were purchased from GE Healthcare (Piscataway, NJ).
Lentiviral shRNA to PAR-1 and Connexin 43
PAR-1 shRNA (target sequence: AGATTAGTCTCCATCAATA), Connexin 43 shRNA (target sequence: CCATCTTCATCATCTTCAT) and a non-targeting shRNA (target sequence: TTCTCCGAACGTGTCACGT) were used with the lentiviral system developed and kindly provided to us by Didier Trono (Ecole Polytechnique Fédérale de Lausanne, Switzerland) as described previously (7).
Flow Cytometry
Flow cytometry was performed as previously described (7).
Western blot analysis
Cx-43 was detected in total cell extracts by 10% SDS-polyacrylamide gel electrophoresis as we previously described (7). SP-1 (1:1000) c-Jun (1:1000), and c-Fos (1:1000) were detected in nuclear extracts by utilizing the Nuclear Extraction Kit from Panomics as per manufacturers’ instructions.
cDNA microarray
Microarray analysis was performed by using a human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA). The microarrays were produced in the microarray core facility of Codon Bioscience (Houston, TX). Total RNA was isolated from NT shRNA and PAR-1 silenced cells with the Clontech Advantage RT-for-PCR Kit (Mountain View, CA) according to the manufacturer’s instructions. The data were analyzed using the Affymetrix program as previously described (30).
Reporter constructs and luciferase activity assays
The Connexin 43 promoter region (−360 to +150) was amplified from C8161 genomic DNA using the following primers: forward, 5′-GGGGTACCTGTAAGCTACTTTAAAAATTTTAGAC-3′; reverse, 5′-GAAGATCTCCTTGGAGGATGAAGTAAAATGAAAAG-3′. The fragment was digested with KpnI and BglII and ligated into the pGL3-basic vector (Promega, Madison, WI). Site-directed mutagenesis for SP-1 elements were created by di-nucleotide substitutions (underlined) in the SP-1 binding motif (GGGAGG to GTTAGG) as previously described (31):
SP1 (−100) 5′-GAAAGGCTGGGAGGAGGTAGTTAGGAGGCTGGTAAATGTG-3′
SP1 (−89) 5′-GGGCAAAGGGAAAGGCTGTTAGGAGGTAGGGAGGAGGCTG-3′
SP1 (−68) 5′-GGAGGGGCTAGAAGAAAGGTTAGGGCAAAGGGAAAGGCTG-3′
SP1 (−46) 5′-CAAGCCACTGACTCAACTGTTAGGAGGGGCTAGAAGAAAG-3′
The number in parenthesis indicates the position relative to the transcription initiation site. Site-directed mutagenesis for the AP-1 element was created by single base pair mutation (underlined) in the AP-1 binding motif (TGAGTCA to TGAGCCA) as previously described (32):
AP1 (−40) 5′-CCTCCTCCCAGTTGAGCCAGTGGCTTGAAACTTTTAAAAG-3′
Transient transfections were performed by using Lipofectin (Invitrogen) according to the manufacturer’s instructions. The dual luciferase construct was performed as previously described. (33).
Chromatin immunoprecipitation assay
ChIP assays were performed utilizing the ChIP-IT Express kit from Active Motif (Carlsbad, CA) according to the manufacturers’ protocol. A 345-bp fragment spanning the −170 to +175 region of the Cx-43 promoter was amplified by PCR using 10 μM of the following primer sequences: 5′-ACTTTATCCTGATCCCACTGCTGCT-3′ and 5′-GAAGTCACGCCAAGTGATTGAACTC-3′. The reaction mixture was carried out by an initial denaturation at 94°C for 2 minutes, followed by 37 cycles of denaturation at 94°C for 20 seconds, annealing at 50°C for 45 seconds, and extension at 72°C for 1 minute. A final elongation step was carried out at 72°C for 10 minutes. PCR products were analyzed on a 2% agarose gel containing ethidium bromide.
Attachment of melanoma cells to endothelial cells
Human Umbilical Vein Endothelial Cells (HUVEC) or Human Dermal Microvessel Endothelial Cells (HDMEC) were plated in 24 well plates and allowed to reach confluency. A thin overlay of 2% BSA was placed in each well and incubated for 10 hours at 37°C to prevent melanoma cell attachment to tissue culture plate. 5 × 104 A375SM or C8161 (NT, PAR-1 shRNA or Cx-43 shRNA) cells were added to each well and incubated overnight at 37°C. Wells were rinsed twice with PBS, fixed with methanol, and stained with crystal violet. For experiments using HDMECs, melanoma cells were identified by GFP. Five fields per well were counted and averaged. Results are presented as average number of cells adhered per field.
PAR-1 rescue experiments
PAR-1 constructs with an N-terminal prolactin signal peptide and flag tag (kindly provided by Shaun R. Coughlin, Univ. of California, San Francisco, CA) was combined with non-targetable PAR-1 coding region (7 base pair silent mutations that will not be recognized by PAR-1 shRNA). The resulting open reading frame insert was then ligated into the pLVX-DsRed-Monomer-C1 vector (Clontech. Mountain View, CA) replacing the red protein coding sequence of DsRed and the final rescue lentiviral vector was obtained. The recombinant lentivirus was produced as previously described (7).
Immunoprecipitation
C8161 cells were plated on 10cm plates and allowed to reach approximately 85% confluency. The wells were washed twice in PBS and lysed using passive lysis buffer (10mM Tris-HCl, 150mM NaCl, 1% Triton-X100, 5mM EDTA, 10% glycerol, pH 7.6). Total cell lysate (500 μg) was incubated with 1μg of anti-PAR1 (ATAP2) or IgG isotype control at 4°C overnight under rotation. 30μl of ProteinG Plus-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) were added to each sample and incubated at 4°C for 4 hours under rotation. Samples were collected by centrifugation at 7000 × g for 5 minutes at 4°C and washed twice with T-TBS and once with PBS. Proteins were eluted in 1×SDS gel loading buffer and subjected to 10% SDS-PAGE, blotted onto nitrocellulose membranes and probed with the anti-PAR-1 antibody (Biodesign International). Peroxidase-conjugated anti-mouse antibody (GE) was used as secondary antibody and visualized with an enzyme linked chemiluminescence kit (ECL, Amersham, Pittsburgh, PA) according to manufacturers’ instructions.
Statistics
The Student’s t-test was used to evaluate the data. P values less than 0.05are considered statistically significant.
Results
Identification of Connexin 43 as a downstream target gene of PAR-1
Previously we have demonstrated that silencing PAR-1 in metastatic melanoma cells resulted in an inhibition of tumor growth and metastasis (7). PAR-1 has been shown to regulate the expression of pro-angiogenic molecules such as VEGF, PDGF, IL-8 and uPA thus contributing to melanoma growth and metastasis. To further investigate how PAR-1 contributes to the malignant phenotype, we sought here-in to identify other potential downstream PAR-1 target genes.
A375SM and C8161 metastatic melanoma cell lines are both high expressors of PAR-1 (8, 12). These cells were stably transduced with PAR-1 small hairpin RNA (shRNA) and Non-Targeting (NT) lentiviral constructs. The Non-Targeting construct has no known homology to any human gene. FACS analyses were used to determine the levels of PAR-1 expression on the cell surface. Figure 1A and B show a significant decrease in PAR-1 expression in both cell lines after transduction (7). These stably silenced PAR-1 melanoma cell lines were subjected to cDNA microarrays to determine downstream gene targets of PAR-1. Among the genes that were differentially expressed, Connexin 43 was found to be downregulated by almost six fold in PAR-1 silenced cells as compared to NT transduced cells. Furthermore, Western blot analyses revealed an 82% and 72% decrease of Cx-43 expression in both A375SM and C8161, respectively, after PAR-1 silencing (Figure 1C), thereby validating the results obtained from the cDNA microarrays.
Figure 1. Connexin 43 protein expression after PAR-1 silencing.
Flow cytometry analysis reveals decreased PAR-1 expression (less PE intensity) in (A) A375SM and (B) C8161 cells after transduction with PAR-1 shRNA as compared to NT shRNA transduced cells. Mouse IgG was utilized as an isotype control. As a negative control, only secondary PE antibody was utilized without adding PAR-1 antibody. C) Western blot of PAR-1 silenced A375SM and C8161 melanoma cell lines depicting about 82% and 72% decrease in Connexin 43 expression, respectively, as compared to Non-Targeting transduced cells. Actin is used as a loading control. The ratio of Connexin 43 to Actin was calculated using Image J software to determine band intensities.
Regulation of Cx-43 by PAR-1 at the transcriptional level
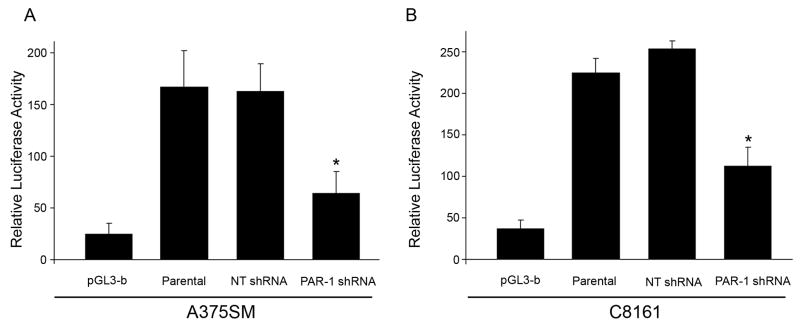
To further determine the mechanism by which PAR-1 regulates Cx-43, the Cx-43 promoter (−360 to +150) was cloned in front of the luciferase reporter gene. The luciferase activity driven by the Cx-43 promoter was significantly inhibited by 3 fold and 2.5 fold (p< 0.001) by PAR-1 silencing in A375SM and C8161 cell lines as compared to NT transduced cells, respectively (Figures 2A and B). These results indicate a possible transcriptional regulation of Cx-43 by PAR-1.
Figure 2. Connexin 43 promoter activity after PAR-1 silencing.
The Connexin 43 promoter region (−360 to +150) was amplified from genomic DNA and cloned into the pGL3-basicfirefly luciferase vector. The luciferase activity driven by the Cx-43 promoter was significantly inhibited (p< 0.001) by PAR-1 silencing in both A) A375SM and B) C8161 cell lines as compared to NT transduced cells. The ratio of firefly luciferase activity to CMV-driven renilla luciferase activity was used to normalize for differences in transfection efficiency among samples. * P< 0.001.
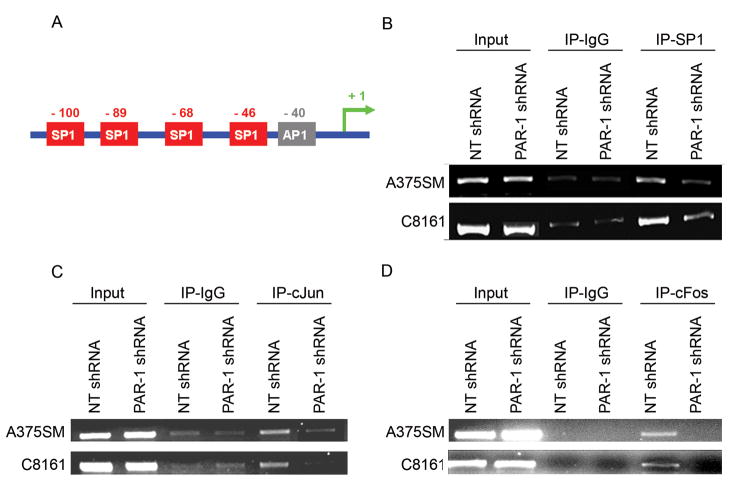
Promoter analyses revealed four SP-1 binding sites and one AP-1 (c-Fos, c-Jun) binding site within 100 bp of the transcriptional initiation site (34, 35) (Figure 3A). Previous studies have shown that both SP-1 and c-Jun are required for maximal Cx-43 promoter activity (35, 36). Western blot analyses were performed to determine whether expression levels of these transcription factors were affected by PAR-1 silencing. No differences in protein expression for c-Fos, c-Jun or SP-1 were seen between NT shRNA and PAR-1 shRNA transduced cells (data not shown). Therefore, we determined whether there was differential binding of these transcription factors to the Cx-43 promoter in PAR-1 shRNA transduced metastatic melanoma cell lines. Chromatin Immunoprecipation (ChIP) studies depict decreased binding of SP-1, c-Jun and c-Fos transcription factors to the promoter of Cx-43 in PAR-1 silenced cells as compared to NT transduced A375SM and C8161 cell lines (Figure 3B–D).
Figure 3. Differential binding of transcription factors to Connexin 43 promoter.
A) Cartoon depicting four SP-1 and one AP-1 (c-Fos, c-Jun) transcription factor binding sites within 100 base pairs of the transcriptional initiation site. Previous studies have also shown that both SP-1 and c-Jun are required for maximal Connexin 43 promoter activity. B) Chromatin Immunoprecipitation studies depict decreased binding of SP-1, C) c-Jun and D) c-Fos transcription factors to the promoter of Cx-43 in both PAR-1 silenced cell lines (A375SM and C8161) as compared to NT transduced cell lines. IgG antibodies were used as negative controls. Input DNA is used to determine equal amount of chromatin in each condition used.
To further establish the link between PAR-1 regulation of Cx-43 expression via SP-1 and AP-1, point mutations in the binding sites of SP-1 and AP-1 were made in the Cx-43 promoter. Site-directed mutagenesis for SP-1 elements were created by di-nucleotide substitutions (underlined) in the SP-1 binding motif (GGGAGG to GTTAGG) as previously described (31). Site-directed mutagenesis for the AP-1 element was created by single base pair mutation (underlined) in the AP-1 binding motif (TGAGTCA to TGAGCCA) as previously described (32). These mutants were cloned in front of the luciferase reporter gene. Figure 4 depicts significant decreases in luciferase expression driven by the Cx-43 promoter in the NT shRNA transduced cells in all mutated SP-1 sites and in the AP-1 site as compared to NT cells transfected with non-mutated Cx-43 promoter in A375SM (Figure 4A) and C8161 (Figure 4B) cell lines. The levels of Cx-43 promoter activity in the NT cells were comparable to PAR-1 silenced cells after mutating the binding sites in both cell lines. Moreover, the activity of the Cx-43 promoter in PAR-1 silenced cells (which already have decreased promoter activity levels) was further decreased in AP-1 and SP-1 mutations as compared to the PAR-1 shRNA cells transfected with the non-mutated Cx-43 promoter.
Figure 4. Connexin 43 promoter activity after SP-1 and AP-1 binding site mutations.
Four SP-1 binding sites as well as one AP-1 binding site were mutated in the Connexin 43 promoter. Site-directed mutageneses for SP-1 elements were created by di-nucleotide substitutions (underlined) in the SP-1 binding motif (GGGAGG to GTTAGG). Site-directed mutagenesis for the AP-1 element was created by single base pair mutation (underlined) in the AP-1 binding motif (TGAGTCA to TGAGCCA). These mutants were subsequently cloned into the pGL3-basic firefly luciferase vector. The luciferase activity driven by the Connexin 43 promoter was significantly decreased in both NT and PAR-1 shRNA cell lines after binding site mutations in A) A37SM and B) C8161 cell lines. The ratio of firefly luciferase activity to CMV-driven renilla luciferase activity was used to normalize for differences in transfection efficiency among samples. * P< 0.05, ** P< 0.001 when comparing mutated to non-mutated within each cell type. The numbers in parenthesis refer to the mutation site for each transcription factor.
Decreased Cx-43 expression after PAR-1 silencing affects melanoma cell attachment to endothelial cells
Previous studies have shown that Cx-43 have adhesive properties and can mediate cell-cell interactions (21, 22). Connexin 43 expression has also been shown to increase attachment of breast cancer cells to the pulmonary endothelium, thereby augmenting metastasis (23–26). Therefore, attachment assays were performed to determine if decreases in Cx-43 expression, caused by PAR-1 silencing, affects melanoma cell attachment to Human Umbilical Vein Endothelial Cells (HUVEC) or Human Dermal Microvessel Endothelial Cells (HDMEC). Results from the attachment assays reveal that there is less binding of PAR-1 silenced A375SM (P< 0.001) and C8161 (P< 0.001) cells to the endothelial cells when compared to both cell lines transduced with NT shRNA (Figures 5A). Furthermore, to determine if this decrease in attachment was specifically due to the decrease in Cx-43 expression after PAR-1 silencing, Connexin 43 was silenced via lentiviral shRNA in A375SM and C8161 cell lines (Figure 5B), both of which are high expressors of Connexin 43. Attachment assays again reveal less binding of Cx-43 silenced melanoma cells as compared to NT transduced cells (P< 0.001; Figure 5C) similar to the results obtained with the PAR-1 silenced cells.
Figure 5. Melanoma cell attachment to endothelial cells after PAR-1 and Connexin 43 silencing.
A) PAR-1 shRNA reveal significant decreases in A375SM and C8161 attachment to HUVEC as compared to NT shRNA transduced cells. * P< 0.001. B) Western blot depicting decreased Connexin 43 protein expression in cells transduced with Connexin 43 shRNA. These cells were subsequently used for attachment assays to determine if the effects of PAR-1 were due to Connexin 43. C) Silencing Connexin 43 reveals significant decreases in A375SM and C8161 attachment to HUVEC as compared to NT shRNA transduced cells. * P< 0.001.
As a further proof of principle, PAR-1 was rescued in PAR-1-silenced C8161 melanoma cell line (Figure 6A). This results in increased Connexin 43 protein levels similar to that of NT shRNA-transduced melanoma cells (Figure 6B). Re-expressing PAR-1 also reverts the decrease seen in melanoma cell attachment to endothelial cells after PAR-1 silencing (Figure 6C and D). Furthermore, to ascertain that functional gap junctions were indeed occurring between melanoma cells and endothelial cells, dye transfer assays were performed, thereby corroborating functional gap junction formation (Supplemental Figure 1)
Figure 6. Effects of re-expressing PAR-1 on Connexin 43 expression and activity.
A) Immunoprecipitation studies show an increase in PAR-1 expression in PAR-1-silenced C8161 melanoma cells after PAR-1 rescue similar to levels seen in NT transduced cells. B) Re-expressing PAR-1 in PAR-1-silenced C8161 shows an increase in Cx-43 expression similar to Cx-43 levels seen in NT transduced cells. C) PAR-1 shRNA revealed significant decrease in C8161 attachment to HDMECs as compared to NT transduced cells. Re-expressing PAR-1 in silenced cells significantly increased attachment to HDMECS as compared to PAR-1 silenced cells transduced with an empty vector control. * P< 0.001 D) Representative images showing differences in attachment of melanoma cells (green) to HDMECs (bright field).
In summary, our results demonstrate that silencing PAR-1 decreases Connexin 43 expression. This decrease is mediated via reduced binding of SP-1 and AP-1 transcription factors to the Cx-43 promoter after PAR-1 silencing. We further show that decreasing Cx-43, via both PAR-1 shRNA and Cx-43 shRNA, results in decreased binding of these cells to endothelial cells, thereby attesting to the important role PAR-1 plays in tumor cell diapedesis via regulation of Connexin 43.
Discussion
PAR-1 plays a major role in the progression of melanoma. We and others have shown that PAR-1 is over-expressed in metastatic melanoma cell lines and tumors as compared to non-metastatic cell lines and dysplastic nevi (8, 12–14). We, therefore, silenced PAR-1 by utilizing both shRNA as well as in vivo liposomal-delivery of siRNA and found a significant decrease in both tumor growth and metastasis, further establishing the central role PAR-1 plays in melanoma progression (7). Interestingly, the addition of thrombin or hirudin in our studies revealed no differences in the effects of PAR-1 activity on melanoma cells beyond that of basal levels probably due to activation of PAR-1 by means other than thrombin. We cannot exclude the possibility that activation of PAR-1 in our system could occur in an autocrine manner.
In order to fully understand how PAR-1 is involved in melanoma growth and metastasis, we utilized cDNA microarray profiling on PAR-1 silenced cells. Based on these results, we have found a link between PAR-1 expression and the gap junctional intracellular communication molecule, Connexin 43, in melanoma. This is the first time that Connexin 43 has been identified to be regulated by PAR-1in human melanoma.
The role of Connexin 43 in cancer is controversial. Studies have shown that in several cancers, Cx-43 acts as a tumor suppressor gene with loss of Cx-43 contributing to metastasis (37–39). Conversely, expression of Cx-43 has also been shown to increase tumor metastasis in breast cancer as well as in gliomas through increase attachment and communication with the vascular endothelium (15, 17, 21–26).
Previous studies using murine melanoma cells report increase coupling of melanoma cells expressing higher levels of Cx-43 to the vascular endothelial cells (23). In fact, the ability of tumor cells to metastasize appears to correlate with the ability of tumor cells to communicate with endothelial cells (24). Nevertheless, studies analyzing the early steps in melanoma progression found a decrease in Cx-43 in human melanoma cells (19, 40). Our findings, however, show high levels of Cx-43 protein expression in metastatic melanoma cell lines and that loss of PAR-1 expression results in the loss of Cx-43. Our finding does not support the role of Cx-43 acting as a tumor suppressor gene in malignant melanoma. Its expression level was high in the metastatic A375SM and C8161 cell lines, but was dramatically reduced in these cells transduced with PAR-1 shRNA. Our data suggest another possible mechanism by which PAR-1 contributes to invasion and metastasis in melanoma, namely by regulating Connexin 43.
After identifying Cx-43 as a possible gene target of PAR-1, we validated our cDNA microarray results to determine if there was indeed a decrease in Cx-43 protein levels. This validation is essential as cDNA microarrays only analyze the mRNA, thereby necessitating further studies to determine if actual protein levels are decreased. Therefore, Western blot analyses on both PAR-1 silenced metastatic melanoma cell lines were performed. These studies revealed a significant decrease in Cx-43 protein levels after PAR-1 silencing, thus confirming that Cx-43 was being regulated by PAR-1 at the protein level. We, therefore, sought to determine the mechanism by which PAR-1 was affecting Cx-43 expression. Promoter analyses revealed that the Cx-43 promoter activity was significantly inhibited by PAR-1 shRNA, suggesting that PAR-1 regulates Cx-43 expression at the transcriptional level.
Based on these results, we determined whether PAR-1 was affecting the binding of transcription factors that were previously shown to be required for maximal promoter activity; AP-1 and SP-1 (35, 36). Although PAR-1 did not affect the expression of these transcription factors, it did affect binding of SP-1, c-Jun and c-Fos to the promoter of Cx-43. As both transcription factors (SP-1 and AP-1) act as positive regulators of Cx-43, our data provide a novel mechanism for the regulation of Cx-43 expression by PAR-1.
The link between PAR-1 affecting binding of AP-1 and SP-1 to the Cx-43 promoter was further strengthened by performing mutation analyses on these transcription factor binding sites and determining the effects on Cx-43 promoter activity after PAR-1 silencing. With mutations in both SP-1 and AP-1 sites, there is significantly less PAR-1 induction of the Cx-43 promoter as seen in the NT transduced cells (PAR-1 positive) after the transcription factor binding sites were mutated. Furthermore, the promoter activity in PAR-1 silenced cells is further decreased after promoter mutagenesis. This allowed us to conclude that the mechanism for the regulation of Cx-43 by PAR-1 occurs through differential binding of AP-1 and SP-1 to the promoter of Cx-43.
Previous studies have found that increased Connexin 43 levels have an effect on tumor cell attachment to cells including endothelial cells (21–26). These studies support our findings showing that with decreased Cx-43 expression, via PAR-1 shRNA, there was a significant decrease in melanoma cell attachment to endothelial cells. To ascertain that the changes seen in attachment of PAR-1 silenced cells to endothelial cells was truly a result of decreased Cx-43 expression, lentiviral Cx-43 shRNA was utilized to silence Cx-43 in A375SM and C8161 cell lines. As with PAR-1 silenced cells, silencing of Cx-43 also caused a reduction in binding of melanoma cells to HUVEC. Further proof that PAR-1 was regulating attachment of melanoma cells to endothelial cells via Cx-43 was obtained when PAR-1 was re-expressed in PAR-1-silenced C8161 melanoma cells. Rescuing PAR-1 resulted in an increase in Cx-43 expression. This also resulted in an increase of melanoma cell attachment to endothelial cells as compared to PAR-1 silenced cells or control. HDMECs were utilized in these experiments to illustrate that these effects on attachments were also seen in microvessel endothelial cells and was therefore, not an artifact of using HUVECs. Finally, to illustrate that Cx-43 gap junctions are present between melanoma cells and microvessel endothelial cells, dye transfer assays were utilized to show functional gap junction formations (Supplemental Figure 1). We therefore concluded that attachment of melanoma cells to endothelial cells was in part due to the expression of Connexin 43.
PAR-1 regulation of Connexin 43 in melanoma cells might have direct impacts on melanoma metastasis. The ability of tumor cells to metastasize appears to correlate with the ability of tumor cells to communicate with endothelial cells (24). The metastatic cascade is complex and involves expression and silencing of a myriad of genes. It has been argued that Cx-43 is lost in the early phases of melanoma progression in which melanocytes, but not melanoma cells, were able to communicate with keratinocytes through connexins (19, 40). However, these findings did not include studies on melanoma cells en route to the metastatic organ. Once melanoma cells have reached the vasculature, they must arrest and extravasate through the vascular endothelium in the metastatic organ. In this process (tumor cell diapedesis), Connexin 43 plays an important role in melanoma progression. Previous studies have found this increase in Cx-43 is not only crucial for communication between tumor cells and endothelial cells, but also plays a role in tumor cell adherence and diapedesis (21, 22). Studies have also shown the importance of Cx-43 in enhancing angiogenesis in vivo (41, 42) which correlate with our previous findings of decreased blood vessel formation and angiogenesis after treating tumor-bearing mice with liposomal delivered PAR-1 siRNA (7).
Our data indicate that Cx-43 is not a tumor suppressor gene in melanoma. Rather, it functions to enhance attachment and diapedesis of circulating melanoma cells to the vascular endothelium. Moreover, upregulation of Cx-43 allow for the establishment of intracellular communication between the tumor microenvironment and the metastatic tumor cells allowing for the passage of ions and second messengers which further enhances the metastatic process. Herein, we report that upregulation of PAR-1 expression seen in melanoma progression, mediates high levels of Cx-43 expression through increased binding of SP-1 and AP-1 transcription factors to the Connexin 43 promoter.
Supplementary Material
Acknowledgments
This work was supported by NIH RO1 grant CA76098 (MBE). We would like to thank Didier Trono for kindly providing the lentiviral backbone vectors used to incorporate PAR-1 shRNA and Connexin 43 shRNA. We would also like to thank Shaun R. Coughlin for kindly providing the PAR-1 expression constructs.
References
- 1.Boire A, Covic L, Agarwal A, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Hansen KK, Saifeddine M, Hollenberg MD. Tethered ligand-derived peptides of proteinase-activated receptor 3 (PAR3) activate PAR1 and PAR2 in Jurkat T cells. Immunology. 2004;112:183–90. doi: 10.1111/j.1365-2567.2004.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien PJ, Molino M, Kahn M, et al. Protease activated receptors: theme and variations. Oncogene. 2001;20:1570–81. doi: 10.1038/sj.onc.1204194. [DOI] [PubMed] [Google Scholar]
- 4.Ruf W, Mueller BM. Thrombin generation and the pathogenesis of cancer. Semin Thromb Hemost. 2006;32 (Suppl 1):61–8. doi: 10.1055/s-2006-939555. [DOI] [PubMed] [Google Scholar]
- 5.Trejo J, Connolly AJ, Coughlin SR. The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J Biol Chem. 1996;271:21536–41. doi: 10.1074/jbc.271.35.21536. [DOI] [PubMed] [Google Scholar]
- 6.Villares G, Bar-Eli M. Regulation of Melanoma Progression by the Microenvironment: The Roles of PAR-1 and PAFR. In: Bar-Eli M, editor. The Tumor Microenvironment 2; Regulation of Gene Expression in the Tumor Environment. New York: Springer; 2008. pp. 1–10. [Google Scholar]
- 7.Villares GJ, Zigler M, Wang H, et al. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68:9078–86. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tellez C, Bar-Eli M. Role and regulation of the thrombin receptor (PAR-1) in human melanoma. Oncogene. 2003;22:3130–7. doi: 10.1038/sj.onc.1206453. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal V, Kohli M, Dennis RA, et al. Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2006;66:273–82. doi: 10.1002/pros.20326. [DOI] [PubMed] [Google Scholar]
- 10.Even-Ram S, Uziely B, Cohen P, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–14. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 11.Jin E, Fujiwara M, Pan X, et al. Protease-activated receptor (PAR)-1 and PAR-2 participate in the cell growth of alveolar capillary endothelium in primary lung adenocarcinomas. Cancer. 2003;97:703–13. doi: 10.1002/cncr.11087. [DOI] [PubMed] [Google Scholar]
- 12.Tellez C, McCarty M, Ruiz M, et al. Loss of activator protein-2alpha results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem. 2003;278:46632–42. doi: 10.1074/jbc.M309159200. [DOI] [PubMed] [Google Scholar]
- 13.Massi D, Naldini A, Ardinghi C, et al. Expression of protease-activated receptors 1 and 2 in melanocytic nevi and malignant melanoma. Hum Pathol. 2005;36:676–85. doi: 10.1016/j.humpath.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Tellez CS, Davis DW, Prieto VG, et al. Quantitative analysis of melanocytic tissue array reveals inverse correlation between activator protein-2alpha and protease-activated receptor-1 expression during melanoma progression. J Invest Dermatol. 2007;127:387–93. doi: 10.1038/sj.jid.5700539. [DOI] [PubMed] [Google Scholar]
- 15.Bates DC, Sin WC, Aftab Q, et al. Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia. 2007;55:1554–64. doi: 10.1002/glia.20569. [DOI] [PubMed] [Google Scholar]
- 16.Zhang D, Kaneda M, Nakahama K, et al. Connexin 43 expression promotes malignancy of HuH7 hepatocellular carcinoma cells via the inhibition of cell-cell communication. Cancer Lett. 2007;252:208–15. doi: 10.1016/j.canlet.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Kanczuga-Koda L, Sulkowski S, Lenczewski A, et al. Increased expression of connexins 26 and 43 in lymph node metastases of breast cancer. J Clin Pathol. 2006;59:429–33. doi: 10.1136/jcp.2005.029272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLachlan E, Shao Q, Laird DW. Connexins and gap junctions in mammary gland development and breast cancer progression. J Membr Biol. 2007;218:107–21. doi: 10.1007/s00232-007-9052-x. [DOI] [PubMed] [Google Scholar]
- 19.Haass NK, Smalley KS, Herlyn M. The role of altered cell-cell communication in melanoma progression. J Mol Histol. 2004;35:309–18. doi: 10.1023/b:hijo.0000032362.35354.bb. [DOI] [PubMed] [Google Scholar]
- 20.Toler CR, Taylor DD, Gercel-Taylor C. Loss of communication in ovarian cancer. Am J Obstet Gynecol. 2006;194:e27–31. doi: 10.1016/j.ajog.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Lin JH, Takano T, Cotrina ML, et al. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302–11. doi: 10.1523/JNEUROSCI.22-11-04302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cotrina ML, Lin JH, Nedergaard M. Adhesive properties of connexin hemichannels. Glia. 2008;56:1791–8. doi: 10.1002/glia.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.el-Sabban ME, Pauli BU. Cytoplasmic dye transfer between metastatic tumor cells and vascular endothelium. J Cell Biol. 1991;115:1375–82. doi: 10.1083/jcb.115.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollmann MA, Shao Q, Laird DW, et al. Connexin 43 mediated gap junctional communication enhances breast tumor cell diapedesis in culture. Breast Cancer Res. 2005;7:R522–34. doi: 10.1186/bcr1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.el-Sabban ME, Pauli BU. Adhesion-mediated gap junctional communication between lung-metastatatic cancer cells and endothelium. Invasion Metastasis. 1994;14:164–76. [PubMed] [Google Scholar]
- 26.Elzarrad MK, Haroon A, Willecke K, et al. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 2008;6:20. doi: 10.1186/1741-7015-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang S, Jean D, Luca M, et al. Loss of AP-2 results in downregulation of c-KIT and enhancement of melanoma tumorigenicity and metastasis. Embo J. 1998;17:4358–69. doi: 10.1093/emboj/17.15.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welch DR, Bisi JE, Miller BE, et al. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer. 1991;47:227–37. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz B, Shoseyov O, Melnikova VO, et al. ACTIBIND, a T2 RNase, competes with angiogenin and inhibits human melanoma growth, angiogenesis, and metastasis. Cancer Res. 2007;67:5258–66. doi: 10.1158/0008-5472.CAN-07-0129. [DOI] [PubMed] [Google Scholar]
- 30.Mourad-Zeidan AA, Melnikova VO, Wang H, et al. Expression profiling of Galectin-3-depleted melanoma cells reveals its major role in melanoma cell plasticity and vasculogenic mimicry. Am J Pathol. 2008;173:1839–52. doi: 10.2353/ajpath.2008.080380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan S, Berquin IM, Troen BR, et al. Transcription of human cathepsin B is mediated by Sp1 and Ets family factors in glioma. DNA Cell Biol. 2000;19:79–91. doi: 10.1089/104454900314591. [DOI] [PubMed] [Google Scholar]
- 32.Ng DC, Shafaee S, Lee D, et al. Requirement of an AP-1 site in the calcium response region of the involucrin promoter. J Biol Chem. 2000;275:24080–8. doi: 10.1074/jbc.M002508200. [DOI] [PubMed] [Google Scholar]
- 33.Melnikova VO, Mourad-Zeidan AA, Lev DC, et al. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281:2911–22. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- 34.De Leon JR, Buttrick PM, Fishman GI. Functional analysis of the connexin43 gene promoter in vivo and in vitro. J Mol Cell Cardiol. 1994;26:379–89. doi: 10.1006/jmcc.1994.1047. [DOI] [PubMed] [Google Scholar]
- 35.Echetebu CO, Ali M, Izban MG, et al. Localization of regulatory protein binding sites in the proximal region of human myometrial connexin 43 gene. Mol Hum Reprod. 1999;5:757–66. doi: 10.1093/molehr/5.8.757. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell JA, Lye SJ. Differential activation of the connexin 43 promoter by dimers of activator protein-1 transcription factors in myometrial cells. Endocrinology. 2005;146:2048–54. doi: 10.1210/en.2004-1066. [DOI] [PubMed] [Google Scholar]
- 37.Czyz J. The stage-specific function of gap junctions during tumourigenesis. Cell Mol Biol Lett. 2008;13:92–102. doi: 10.2478/s11658-007-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gershon E, Plaks V, Dekel N. Gap junctions in the ovary: expression, localization and function. Mol Cell Endocrinol. 2008;282:18–25. doi: 10.1016/j.mce.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Shen Y, Khusial PR, Li X, et al. SRC utilizes Cas to block gap junctional communication mediated by connexin43. J Biol Chem. 2007;282:18914–21. doi: 10.1074/jbc.M608980200. [DOI] [PubMed] [Google Scholar]
- 40.Hsu M, Andl T, Li G, et al. Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J Cell Sci. 2000;113 (Pt 9):1535–42. doi: 10.1242/jcs.113.9.1535. [DOI] [PubMed] [Google Scholar]
- 41.Bellafiore M, Sivverini G, Palumbo D, et al. Increased cx43 and angiogenesis in exercised mouse hearts. Int J Sports Med. 2007;28:749–55. doi: 10.1055/s-2007-964899. [DOI] [PubMed] [Google Scholar]
- 42.Zhang W, DeMattia JA, Song H, et al. Communication between malignant glioma cells and vascular endothelial cells through gap junctions. J Neurosurg. 2003;98:846–53. doi: 10.3171/jns.2003.98.4.0846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.